Abstract
Tartrate-resistant acid phosphatase (TRAP) is a lysosomal di-iron protein of mononuclear phagocytes and osteoclasts. Hitherto, no role for the enzyme in immunity has been identified; however, knockout mice lacking TRAP have a skeletal phenotype caused by an intrinsic osteoclast defect. To investigate a putative function for TRAP in macrophages (Mφ), we investigated proinflammatory responses and systemic microbial clearance in knockout mice compared with age- and gender-matched congenic wild-type mice. Phorbol 12-myristate 13-acetate (PMA)-stimulated and interferon-γ (IFN-γ)-induced superoxide formation was enhanced in peritoneal Mφ lacking TRAP; nitrite production in response to stimulation with lipopolysaccharide (LPS) and IFN-γ was also increased. In addition, secretion of the proinflammatory cytokines, tumour necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-12, was significantly greater in TRAP-deficient Mφ when stimulated with LPS, with or without addition of either TNF-α or IFN-γ. The activity of tartrate-sensitive (lysosomal) acid phosphatase was increased in Mφ from the knockout mice but activities of the lysosomal hydrolases N-acetyl β-glucosaminidase and acid β-glucuronidase were unchanged, indicating selective activation of compensatory acid phosphatase activity. Evidence of impaired Mφ function in vivo was obtained in TRAP knockout mice, which showed delayed clearance of the microbial pathogen, Staphylococcus aureus, after sublethal intraperitoneal inoculation. After microbial challenge, peritoneal exudates obtained from TRAP knockout mice had a reduced population of Mφ. As peritoneal Mφ and neutrophils lacking TRAP were able to phagocytose and kill S. aureus normally in vitro, TRAP may directly or indirectly influence recruitment of Mφ to sites of microbial invasion. Our study shows that TRAP participates in the inflammatory response of the Mφ and influences effector signalling pathways in innate immunity.
Introduction
Histochemical and biochemical studies over many years have demonstrated abundant expression of tartrate-resistant acid phosphatase (TRAP) activity in cells of mononuclear phagocytic origin.1,2 The enzyme has broad phosphatase activity against nucleotides, aryl phosphates and phosphoproteins and is a purple iron protein of unknown function alternatively known, on the basis of its electrophoretic properties, as the band 5 isozyme of TRAP, Acp 5 (EC.3.1.3.2).3 The mammalian phosphatase Acp 5 is a member of a class of purple iron-containing proteins that are distributed widely in nature; it contains an iron pair cluster sequestrated in a unique antiparallel spin-coupled binuclear centre. Several members of this protein family participate in oxygen-dependent reactions and the occurrence of interconvertible Fe2+/Fe3+ redox forms appear to mediate this process.4 We and others have shown that mammalian Acp 5 can catalyse peroxidation reactions leading to formation of reactive oxygen species that are important effector molecules in microbial defence; these molecules are also released by the ruffled membrane interface at the bone resorptive surface of osteoclasts.5,6
To characterize further the function of the iron-containing purple phosphatase, we have generated (by targeted disruption of the murine Acp 5 gene in embryonic stem cells) mice lacking TRAP activity.7 Initial examination of these animals, which have an absolute deficiency of TRAP activity, reveals a skeletal phenotype. Homozygous Acp 5 null mice are viable under laboratory conditions but suffer from developmental deformities of the limb and axial skeleton with mild osteopetrosis resulting from defective bone resorption. Osteoclasts isolated from Acp 5 knockout animals demonstrate a cell-intrinsic defect of bone resorption in vitro. As an intermediate skeletal phenotype was observed in mice heterozygous for the null Acp 5 allele, the biological activities of TRAP are evidently critical for normal osteoclastic bone resorption.
Little is known of the function of TRAP outside the skeletal system, although its activity is enhanced in certain activated macrophages (Mφ), such as pulmonary alveolar Mφ, suggesting a possible role in immunity. We have demonstrated that human monocyte-derived Mφ express abundant TRAP activity upon culture with serum-enriched medium.2 TRAP activity is also greatly enhanced in the pathological Mφ of Gaucher's disease and in the phagocytic B-lymphocyte population of myeloid origin in hairy cell leukaemia.8 In Gaucher's disease, serum TRAP activity is used as a surrogate marker of disease and closely reflects beneficial therapeutic interventions including removal of disease bulk by splenectomy and in response to enzyme replacement with Mφ-targeted (mannose-terminated) glucocerebrosidase.9
Mφ share with osteoclasts their origin from myeloid progenitor cells in the bone marrow and it is therefore probable that the Acp 5 protein they express contributes critically to a common phagocytic cell function. Specifically, we propose that this protein participates in the phlogistic response of Mφ and is a component of their effector activities in innate resistance to microbial infections. Accordingly, we report here investigations into the Mφ phenotype of TRAP-deficient mice that examined the ultrastructural features and lysosomal acid hydrolase profile of isolated Mφ, together with their capacity to form reactive oxygen and nitrogen species and secrete proinflammatory cytokines in response to natural activators. In vivo clearance of a bacterial pathogen and the accompanying inflammatory cellular exudates were also examined. These experiments confirm that Acp 5 null mice lacking TRAP activity exhibit an abnormal Mφ inflammatory profile and disturbed responses to microbial challenge.
Materials and methods
Experimental animals
Mice with a targeted disruption of the single Acp 5 gene that maps to murine chromosome 9 were generated as previously described.7 The targeting replacement vector Acp 5 (neo) thymidine kinase (tk) was used to disrupt exon 3 of the murine gene that contains the putative iron-binding domains of the protein. Replacement of this locus by homologous recombination creates the predicted null allele as a result of intragenic disruption. The TRAP-deficient (Acp 5 knockout) animals used for the experiments described here were obtained by multigeneration homozygous inbreeding of Acp 5 null animals in the F2 generation on the germline embryonic stem cell background to obtain congenic 129 Sv strain mutant mice lacking TRAP activity. These animals were compared in age- and gender-matched studies carried out simultaneously in experiments with congenic control animals that were wild type at the Acp 5 locus; the animals were also carefully matched for weight. All animal strains were housed in the same facility. Mouse colonies were housed in polypropylene cages with free access to food and water.
Isolation of peritoneal and bone marrow Mφ
Resident murine Mφ were obtained from the peritoneal cavity by lavage. The Mφ were washed and after lysis of erythocytes were plated at a concentration of 106/ml in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Sigma Chemical Co., Poole, Dorset, UK) supplemented with 10% fetal calf serum (FCS) (First Link UK Limited, Brierly Hill, West Midlands, UK), 1 µg/ml of streptomycin, 100 U/ml of penicillin and l-glutamine to a final concentration of 2 mm.
The Mφ were allowed to adhere overnight and after washing off non-adherent cells, fresh medium was added immediately before stimulation.
Bone marrow Mφ were isolated from freshly removed bone marrow; this was extruded from femoral shafts after epiphyseal section by washing through with supplemented DMEM-containing supernatant conditioned by culture with murine L929 cells.10 Myeloid cells were dispersed by successive passage through 25 and 27 gauge needles and plated in Sterilin (Stone, Staffordshire, UK) petri dishes (one femur per 9-cm diameter dish) and cultured for 5 days at 37° in 5% CO2. Adherent bone marrow Mφ were then washed gently with phosphate-buffered saline (PBS) and retrieved by the addition of EDTA in the same buffer at a final concentration of 10 mm. Cells were then plated at 106/ml in unconditioned DMEM.
Electron microscopy
Cells were fixed in 1% glutaraldehyde, 2% formaldehyde in 0·1 m PIPES buffer for 2 hr at 4° and stained for acid phosphatase activity by using the Gomori method with p-nitrophenol phosphate in the presence or absence of 50 mm sodium tartrate, pH 5·0, to localize intracellular TRAP activity.11 After washing three times in PIPES buffer, cells were incubated for 2 hr at 25° with p-nitrophenol phosphate (3·8 mm) in 0·1 m acetate buffer containing 2·64 mm lead acetate. The reaction was stopped by rinsing in acetate buffer and the cells were then fixed in 1% osmium tetroxide. The cells were dehydrated in ethanol and embedded in Spurr's epoxy resin. Sections of 50–70 nm were mounted on 300-mesh copper grids, then stained with uranyl acetate and lead citrate. Sections were examined in a Philips CM100 transmission electron microscope operated at 80 Kv.
Cell culture
To determine the inflammatory responses of Mφ in culture, the cells were stimulated for 48 hr in the presence or absence of bacterial lipopolysaccharide (LPS) from Escherichia coli serotype 0127 : B8− (Sigma Chemical Company), murine tumour necrosis factor-α (TNF-α) (First Link UK Ltd) or interferon-γ (IFN-γ) (Life Technologies, Paisley, Strathclyde, UK), or combinations of the cytokines with and without the addition of NG-monomethyl-l-arginine (l-NMMA), an established nitric oxide (NO) synthase antagonist (Alexis Corporation, Nottingham, Notts., UK).
Determination of reactive oxygen and nitrogen species
Formation of superoxide was measured spectroscopically by monitoring the reduction of ferricytochrome c.12 After stimulation, the cells were washed twice with Hanks' buffered saline and incubated for 1 hr at 37° with fresh Hanks' solution containing 80 µmol ferricytochrome c (Sigma Chemical Company) and 1 µg/ml of phorbol 12-myristate 13-acetate (PMA) (Alexis). Superoxide dismutase (60 U/ml, Sigma) was added to some wells simultaneously with PMA. The reduction of ferricytochrome c and estimation of superoxide formation was determined spectroscopically at 550 nm. Nitrite production was used as an indicator of formation of NO in cell supernatants and was determined by the Griess assay.13 Secretion of TNF-α, interleukin (IL)-β and IL-12 in supernatants was determined by two-site enzyme-linked immunosorbent assay (ELISA) (R & D Systems Ltd, Oxford, UK).
Lysosomal acid hydrolases
Mφ were lysed by the addition of 0·5% Triton-X-100 (500 µl/106 cells) and several cycles of freeze–thawing. TRAP activity was measured, as previously described,14 using 10 mm 4-nitrophenol phosphate as substrate in 0·4 m sodium acetate buffer, pH 5·6, with the addition of 0·1 m (l +) sodium tartrate. Tartrate-sensitive acid phosphatase was measured similarly but with the omission of tartrate. Acid N-acetyl β-glucosaminidase activity was measured by incubating cell lysates (100 µl) with 1 ml of p-nitrophenyl N-acetyl-β-d-glucosaminide (1·23 mg/ml) in 0·2 m citrate buffer, pH 4·5, for 1 hr at 37°. The reaction was stopped by the addition of 400 µl of 0·2 m sodium borate buffer, pH 9·8, and the absorbance was read at 405 nm. Acid β-glucuronidase activity was determined in the presence of 0·5 mg/ml of phenolphthalein glucuronide (Sigma) in 0·1 m sodium acetate buffer, pH 4·5, for 16–24 hr. Free phenolphthalein was determined spectroscopically at 540 nm after stopping the assay with 0·2 m glycine, pH 10·5, to develop the released chromophore.
Immunohistochemistry
Mφ were plated at a concentration of 106 cells/ml in eight chamber-well slides (Costar Ltd, Cambridge MA), with or without the addition of LPS (1 µg/ml), and stimulated for 48 hr at 37°. Cells were washed twice at room temperature with PBS and then fixed with methanol/acetone (1 : 1) for 5–15 min at −20°. Cells were washed in PBS, permeabilized with 0·1% Triton-X-100 for 5 min and incubated overnight at 4° with immunopurified polyclonal rabbit anti-porcine uteroferrin (to detect the purple phosphatase antigen)15 or with F4/80 antibody (Serotec, Oxford, UK) for 1 hr at room temperature. Immunoreactivity was determined using the streptavidin–biotin–peroxidase system (Daco, Ely, Cambs., UK), with diaminobenzidine as chromogen, and counterstained using haematoxylin.
In vivo challenge with Staphylococcus aureus
Mice were injected intraperitoneally with 0·1 ml of a suspension of 2 × 107 cells of S. aureus strain AKC 25923, which had been grown overnight on 2YT rich-medium plates at 37°. This organism lacks β-lactamase and is the Oxford UK reference strain of pathogenic S. aureus. Peritoneal exudates were obtained after injecting 5 ml of Hanks' buffer; following gentle abdominal massage, 4 ml of fluid was withdrawn for study. At 24, 48 and 72 hr, mice were killed and the peritoneal exudate washed out and plated in 10-fold dilutions overnight on 2YT agar plates to determine the residual number of viable bacterial colony-forming units (CFU).16 No abscesses were apparent on subsequent opening of the peritoneal cavities of any of the infected animals.
Flow cytometry
Peritoneal exudate cells were stained with fluorescein-labelled antibody by using standard methods17 with anti-mouse F7/4 and F4/80 antibodies from Serotec. Three thousand events were collected, and live cells were gated accordingly to their forward- and side-scatter profiles. Subsets within this gate were analysed using a fluorescence-activated cell sorter (FACSort™) flow cytometer (Becton-Dickinson, Cowley, Oxford, UK) and the data were analysed using WinMDI version 1.3.1 (contact: trotter@flosun.salk.edu).
Phagocytosis and intracellular microbial killing by peritoneal Mφ
S. aureus AKC 25923 was cultured overnight, resuspended in DMEM and added to wells containing 106 adherent resident murine peritoneal Mφ that had been plated and cultured for 4 hr to adherence in antibiotic-free medium, at ratios of 10 : 1 and 1 : 1. After incubation for 1 hr at 37°, the Mφ were washed extensively to remove extracellular bacteria and examined for phagocytosis and microbial cytotoxicity.18
Phagocytosis and intracellular microbial killing by peritoneal neutrophils
Cells were isolated from peritoneal exudates obtained from mice 24 hr after inoculation of 2 × 107 heat-killed S. aureus. The cells (105) were plated and incubated for 1 hr at ratios of 1 : 10 and 1 : 40 viable S. aureus strain AKC 25923 in a final volume of 1 ml. Phagocytosis and microbial killing were determined as described above.18 Cellular composition in the exudates was confirmed by staining with Giemsa and with the neurophil marker, CD16.
Statistical evaluation
Statistical analysis of the data was carried out as described below, using the statistical tests discussed in ref. 19. Differences in the means of normally distributed cellular activities were evaluated by using the two-tailed t-test. Where fewer than four samples were available in experimental groups, Lord's mean range test was used. The significance of differences in non-parametric data were determined by using the Wilcoxon Rank Sum Test. Bacterial CFU were analysed, after logarithmic transformation, by two-way analysis of variance.
Results
Immunophenotype of TRAP-deficient and wild-type peritoneal and bone marrow-derived murine Mφ
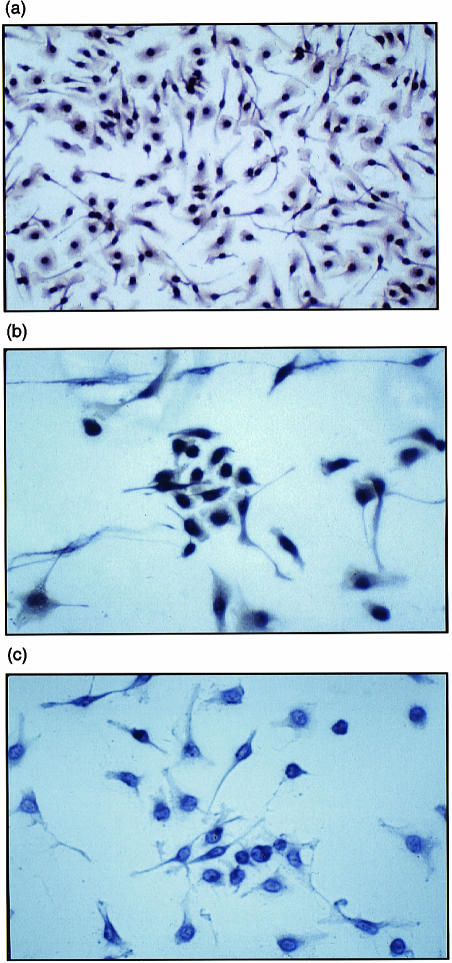
The population of cells isolated from the bone marrow and peritoneal cavity reacted strongly with a monoclonal antibody (mAb) directed against the plasma-membrane differentiation marker, F4/80, which is common to the mononuclear phagocyte system in the mouse (Fig. 1a). These cells have a common lineage from myeloid progenitor cells and appear to be immunologically distinct from Langerhans' and splenic dendritic cells that are non-classical Mφ.20 Immunostaining of Mφ isolated from both sources using immune-specific polyclonal antiuteroferrin antibody showed abundant intracellular staining attributable to TRAP in cells obtained from wild-type animals;15 as expected, immunostaining was absent in cells from Acp 5 null mice lacking TRAP enzymatic activity (Fig. 1b, 1c).7
Figure 1.
Immunophenotyping of murine macrophage (Mφ). F4/80-positive peritoneal Mφ (a) from wild-type mice express abundant intracellular tartrate-resistant acid phosphatase (TRAP) protein when incubated with lipopolysaccharide (LPS) for 48 hr (b). As expected, Mφ isolated from TRAP-deficient mice do not express TRAP under these conditions (c). Bone marrow Mφ behaved identically (results not shown). Peritoneal Mφ were stimulated in chamber slides and fixed in acetone/methanol (1 : 1), as described in the Materials and Methods. Cells were incubated with immunopurified uteroferrin antibody for 12 hr and intracellular TRAP antigen was visualized using a peroxidase-conjugated secondary antibody and developed with diaminobenzidine. Cells were counterstained with Harris hematoxylin.
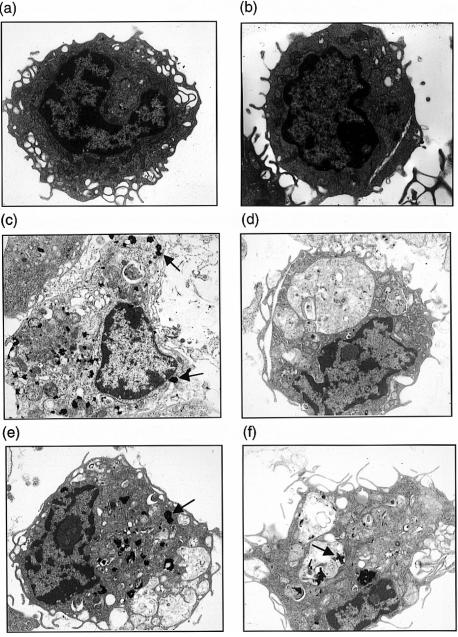
Mφ from TRAP-deficient mice show no evidence of lysosomal storage
Ultrastructural studies were undertaken (Fig. 2) to determine if deficiency of TRAP in homozygous Acp 5 null mice was associated with lysosomal pathology. As TRAP is an acid hydrolase that colocalizes with the lysosomal membrane marker, LAMP-1, in human Mφ, selective deficiency of this enzyme may lead to the accumulation of a natural substrate within the organelle, expansion of the organellar compartment and disturbed intracellular morphology. No differences in overall morphology were observed between resting Mφ obtained from wild-type and TRAP-deficient mice (Fig. 2a, 2b).
Figure 2.
Macrophage (Mφ) ultrastructure and staining reactions for acid phosphatase activities in situ. Resting peritoneal Mφ from wild-type (a) and tartrate-resistant acid phosphatase (TRAP)-deficient mice (b) had similar morphologies. Lipopolysaccharide (LPS)-stimulated wild-type Mφ showed abundant TRAP activity, as exemplified by arrows (c). The acid phosphatase activity in wild-type Mφ was principally caused by TRAP as in the absence of tartrate no additional Gomori product was detectable (e). Mφ from TRAP-deficient mice had no TRAP activity (d) but showed increased reactions with the Gomori stain (arrowed) in the absence of tartrate, which represents the tartrate-sensitive lysosomal acid phosphatase isoenzyme (f).
As also demonstrated in Fig. 2, LPS-stimulated TRAP-deficient peritoneal Mφ gave no appreciable staining for TRAP in the Gomori reaction, and the lysosomal morphology of stimulated Mφ isolated from the two mouse strains was indistinguishable (Fig. 2c, 2d, 2e, 2f). Specifically, no evidence of lysosomal proliferation or expansion was identified and no insoluble storage product was observed within vacuolar spaces. Gomori reactions conducted in the presence and absence of tartrate demonstrated that TRAP is the principal acid phosphatase in wild-type murine Mφ; appreciable tartrate-sensitive phosphatase staining was observed in cells from TRAP-deficient mice (also see Table 1). We conclude that any functional abnormalities identified in Mφ isolated from TRAP-deficient mice cannot be attributed to an overall disturbance in lysosomal structure.
Table 1.
Lysosomal hydrolase activities in lysates of bone marrow macrophages (Mφ) stimulated with lipopolysaccharide (LPS)
| Wild-type mean ± SEM (n) | TRAP-deficient mean ± SEM (n) | |
|---|---|---|
| Tartrate-resistant acid phosphatase | 0·408 ± 0·026 (4) | Not detectable (5) |
| Tartrate-sensitive acid phosphatase | 0·006 ± 0·0026 (5) | 0·186 ± 0·007* (9) |
| N-acetyl βd-glucosaminidase | 0·86 ± 0·11 (5) | 1·32 ± 0·16 (9) |
| β-Glucuronidase | 0·221 ± 0·018 (5) | 0·221 ± 0·008 (7) |
Resident bone marrow Mφ were isolated from wild-type and tartrate-resistant acid phosphatase (TRAP)-deficient mice and incubated with LPS (1 µg/ml) for 48 hr. Supernatants were removed, Triton-X-100 (0·5%) was added to the cells and lysates obtained by freeze–thaw cycles. TRAP and tartrate-sensitive acid phosphatase activities were measured by the hydrolysis of nitrophenylphosphate in the presence and absence of tartrate (100 mm), respectively. Glucosamine was measured by the hydrolysis of nitrophenylglucosaminide. These results are expressed as µmol of substrate hydrolysed/min/106 cells. Acid β-glucuronidase was determined by the hydrolysis of phenolphthalein glucuronide, as described in the Materials and Methods, and is expressed as µmol of substrate hydrolysed/hr/106 cells. Results are representative of three separate experiments.
P < 0·01, Student's t-test. TRAP and tartrate-resistant acid phosphatase only were measured in peritoneal Mφ, with similar findings.
Lysosomal acid hydrolase activities in Mφ obtained from wild-type and TRAP-deficient mice
The activities of TRAP, tartrate-sensitive acid phosphatase, acid N-acetyl β-d-glucosaminidase and acid β-glucuronidase are shown in Table 1. In TRAP-deficient animals there was a significant increase in the activity of the tartrate-sensitive acid phosphatase, which is attributable to the product of the Acp 2 gene, the ubiquitous acid phosphatase of lysosomes. In contrast, the activities of N-acetyl β-glucosaminidase and β-glucuronidase, which are also well-characterized marker enzymes of the lysosome, were not significantly increased in Mφ obtained from TRAP-deficient animals. The increase in tartrate-sensitive acid phosphatase appears to be a reciprocal and selective effect and is not part of a generalized increase in the activity of cognate acid hydrolases that would be expected to accompany a disturbance of lysosomal integrity.
Oxygen and nitrogen free-radical responses in TRAP-deficient Mφ are increased
To investigate the inflammatory response in Mφ obtained from TRAP-deficient mice, PMA-stimulated superoxide production was determined. As shown in Table 2, basal and LPS-stimulated superoxide production was two- to three-fold greater in peritoneal Mφ isolated from TRAP-deficient animals. That the reduction of cytochrome c was attributable to superoxide production was confirmed by addition of superoxide dismutase: this abolished the spectroscopic signal affecting the superoxide product (results not shown).
Table 2.
Enhanced superoxide production in phorbol 12-myristate 13-acetate (PMA)-stimulated peritoneal macrophages (Mφ) from tartrate-resistant acid phosphatase (TRAP)-deficient and age- and gender-matched congenic wild-type mice
| TRAP-deficient | Wild-type | |
|---|---|---|
| Basal | 3·12 ± 0·87** | 0·73 ± 0·26 |
| LPS | 9·69 ± 1·3* | 4·65 ± 0·59 |
| IFN-γ | 8·25 ± 1·85 | 4·56 ± 0·85 |
Resident peritoneal Mφ were isolated and stimulated alone or with lipopolysaccharide (LPS) (1 µg/106 cells) or interferon-γ (IFN-γ) (100 U/106 cells) for 48 hr. Sixty minutes before harvesting, cells were stimulated with PMA (1 µg/106 cells) and ferricytochrome c was added (80 µm). Superoxide, determined as described in the Materials and Methods, is shown as µmol of cytochrome c reduced/106 cells/hr. Results are representative of two separate experiments, each using 10 determinations for each condition, and are expressed as mean±SEM.
P < 0·01;
P < 0·05.
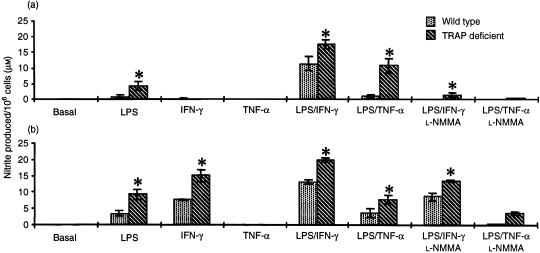
To examine the production of other reactive intermediates in the inflammatory response, NO synthesis was determined. In Fig. 3, production of the stable end-product, nitrite, in LPS-primed Mφ and Mφ activated by the addition of LPS with IFN-γ or TNF-α is shown. Nitrite formation was greatly enhanced in cells obtained from TRAP-deficient Acp 5 null mice when compared with Mφ isolated from wild-type congenic-strain control animals. In experiments in which the NO synthase inhibitor, l-NMMA, was added, there was a significant reduction in nitrite formation, confirming that production of nitrite derived from NO resulted from the activity of NO synthase. Overall, these experiments pointed to an enhanced or compensated inflammatory state in peritoneal and bone-marrow Mφ derived from TRAP-deficient mice, as compared with those derived from congenic-strain wild-type animals. Thus, nitrite formation by primed and activated Mφ from two populations in TRAP-deficient mice was increased.
Figure 3.
Nitrite production is increased in stimulated macrophages (Mφ) from tartrate-resistant acid phosphatase (TRAP)-deficient mice. Mφ obtained from peritoneum (a) and bone marrow (b) were stimulated with lipopolysaccharide (LPS) (1 µg/ml), with or without interferon-γ (IFN-γ) (100 U/ml) or tumour necrosis factor-α (TNF-α) (500 U/ml). Nitrite was determined in cell supernatants by the Griess reaction, as described in the Materials and Methods. The nitric oxide synthase inhibitor, N-monomethyl-l-arginine (l-NMMA) (300 µm), was added to ensure that the nitrite measured was derived from nitric oxide synthase. The data are representative of at least four experiments and expressed as mean ± SEM values. *P < 0·05.
Enhanced proinflammatory secretory responses in Mφ from TRAP-deficient mice
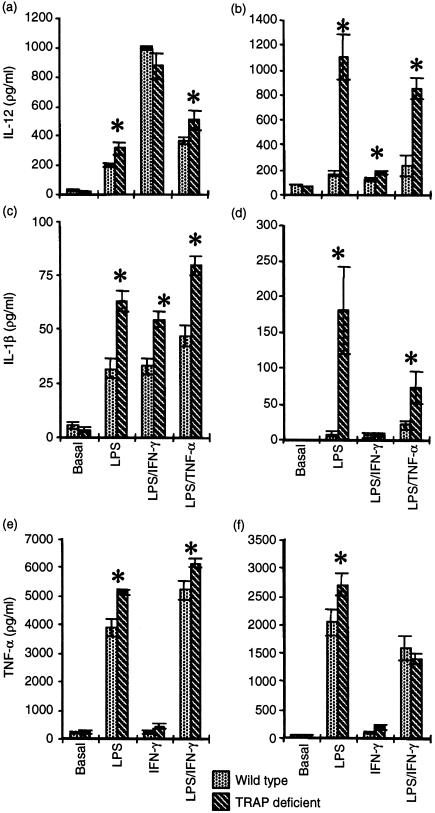
As free radical formation was found to be increased in stimulated Mφ lacking TRAP, the proinflammatory cytokine profile upon priming and stimulation was examined. As depicted in Fig. 4, significant increases in the release of proinflammatory cytokines in TRAP-deficient Mφ were observed on priming with LPS and further stimulation with TNF-α or IFN-γ. Secretion of IL-1-β and IL-12 was greatly increased in TRAP-deficient bone marrow and peritoneal Mφ (Fig. 4a, 4b, 4c, 4d). Secretion of TNF-α in Mφ from TRAP-deficient mice was also enhanced.
Figure 4.
Tartrate-resistant acid phosphatase (TRAP)-deficient macrophages (Mφ) have an abnormal proinflammatory cytokine secretion profile. Peritoneal Mφ (a), (c) and (e), and bone marrow Mφ (b), (d) and (f), from age- and gender-matched mice, were stimulated with lipopolysaccharide (LPS) (1 µg/ml), interferon-γ (IFN-γ) (100 U/ml) or tumour necrosis factor-α (TNF-α) (500 U/ml), alone or in combination, for 48 hr. Supernatants were assayed for interleukin (IL)-12 (a) and (b), IL-1β (c) and (d), and TNF-α (e) and (f). The data shown are representative of two experiments with peritoneal Mφ (using five to 11 determinations for each condition) and two experiments with bone marrow Mφ (three to six determinations for each condition). Results are expressed as mean ± SEM. *P < 0·05.
In summary, priming with LPS led to significantly enhanced proinflammatory cytokine secretion for all modalities studied in both populations of Mφ obtained from TRAP-deficient mice compared with wild-type congenic-strain animals.
In vivo clearance of S. aureus
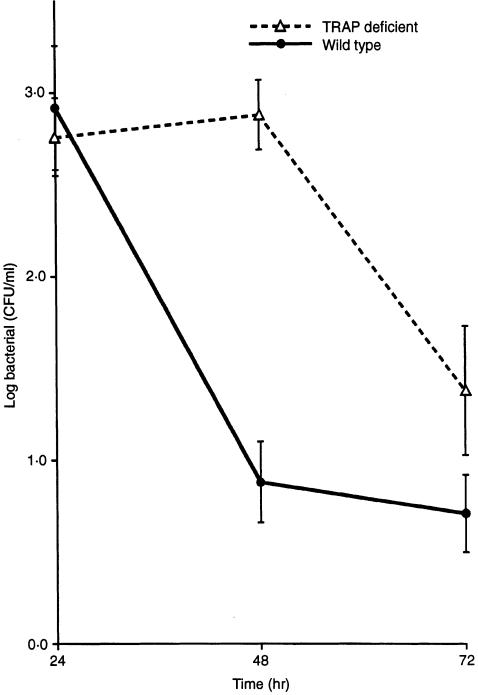
The specific induction of tartrate-sensitive acid phosphatase and the abnormal inflammatory state of Mφ obtained from TRAP-deficient animals, suggested that these cells were compensating for a disabled effector function. We therefore postulated that Acp 5 null mice would show a qualitative defect in microbial clearance consequent upon the loss of an essential component of the Mφ response pathway. To test this hypothesis, 14-week-old female TRAP-deficient and congenic-strain wild-type mice were challenged with a well-characterized virulent strain of the catalase-positive microbial pathogen, S. aureus. As shown in Fig. 5, TRAP-deficient animals showed significantly impaired clearance of viable bacteria following their administration into the intraperitoneal cavity.
Figure 5.
Reduced peritoneal clearance of viable Staphylococcus aureus in tartrate-resistant acid phosphatase (TRAP)-deficient mice. Wild-type and TRAP-deficient mice (n = 8–11 at each time-point) were innoculated intraperitoneally with 2 × 107 cells of the S. aureus, pathogenic strain, AKC 25923. Viable bacterial counts were quantified by determining colony-forming units (CFU) on the peritoneal lavage obtained at each time-point, as described in the Materials and Methods. The figure depicts logarithmically transformed mean± SEM counts/ml of lavage fluid. The two clearance curves were shown to be different by analysis of variance, with a significance value of P < 0·001.
This result suggests that the enhanced proinflammatory cytokine and phlogistic profile of TRAP-deficient Mφ is not sufficient to overcome a defect of microbial clearance in the intact animal. Therefore, despite abnormal secretion of superoxide and NO (as well as IL-1β, TNF-α and IL-12) by their Mφ upon stimulation in vitro, TRAP-deficient animals show increased susceptibility to microbial challenge with reduced capacity to clear bacterial pathogens from the peritoneal cavity in vivo. Comparable results showing delayed microbial clearance were also found in challenge experiments when 14-week-old male mice lacking TRAP activity were compared with wild-type control animals (results not shown).
Examination (using a Neubauer chamber) of peritoneal exudates obtained from mice at 24, 48 and 72 hr after inoculation of 2 × 107 Staphylococcus aureus, showed no differences in cell density between genotypes (0·4–1·3 × 107/ml compared with 0·5–1·4 × 107/ml for TRAP-deficient and wild-type congenic-strain mice, respectively). Microscopy after Trypan Blue staining likewise revealed no clear differences in the number of dead cells in the exudates, as shown by exclusion of the dye.
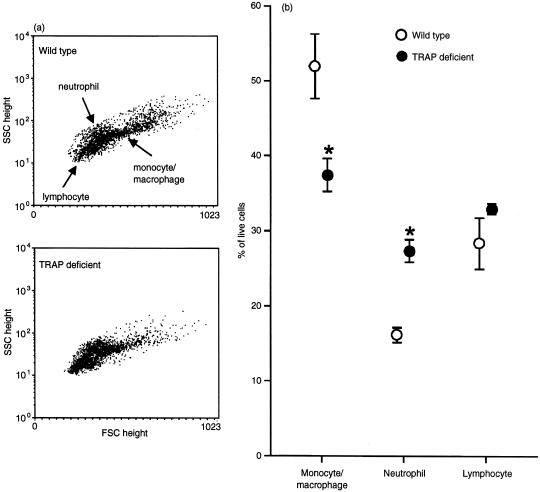
Phenotypic analysis of the peritoneal exudate cells by flow cytometry was carried out by employing their forward- and side-scatter properties, as shown in Fig. 6. The assigned populations of neutrophils and monocyte/Mφ (Fig. 6b) were confirmed with the F7/4 and F4/80 antibody-staining reactions, which are specific for murine neutrophilic polymorphonuclear leucocytes and resident as well as activated monocyte/Mφ, respectively.20,21 No differences in these cellular subsets were observed between Acp 5 genotypes during the first 48 hr after innoculation with S. aureus, the time-period in which influx of neutrophils is known to increase greatly16 (data not shown). However, by 72 hr after infection, the expected increase in the proportion of Mφ22 was significantly impaired in the exudates obtained from TRAP knockout mice compared with congenic wild-type animals. This difference is apparent from the altered cytometric profile (Fig. 6a) that shows representative dot-plots obtained from individual mice at 72 hr. Cell subset analysis (Fig. 6b) indicates that the exudates obtained from infected TRAP-deficient mice contained a significantly greater proportion of neutrophils than those from wild-type control animals at this later stage of infection. As no differences in exudate cell densities were observed between the genotypes, the changes observed in TRAP-deficient mice probably reflect absolute differences in the numbers of Mφ and neutrophils that remain in, and migrate to, the infected peritoneal cavity.
Figure 6.
Reduced monocyte/macrophage (Mφ) complement in peritoneal exudates from tartrate-resistant acid phosphatase (TRAP)-deficient mice after challenge with Staphylococcus aureus in vivo. Peritoneal exudates were obtained as described in the legend to Fig. 5 (and the Materials and Methods). Cells were analysed for their side- and forward-scatter properties (SSC and FSC, respectively), and gated accordingly. These gatings were confirmed by fluorescence staining with F7/4 and F4/80 antibodies for neutrophils and monocyte/Mφ, respectively. Cells not staining for these surface markers and with appropriate forward- and side-scatter parameters were categorized as lymphocytes. (a) Representative dot-plots of exudate cells 72 hr after infection. (b) Cell subset analysis at 72 hr showing data from three to four mice of each genotype, determined in duplicate and expressed as a percentage of live cells ± SEM. *P < 0·05.
Phagocytosis of Staphylococcus aureus by peritoneal Mφ in vitro was not significantly different in cells obtained from animals of different TRAP genotypes: 3·07 ± 0·53 × 105 and 2·5 ± 0·49 × 105 (n = 6) bacteria were phagocytosed in 1 hr by 106 Mφ obtained from wild-type and Acp 5 knockout mice, respectively, at saturating microbial concentrations. Microbial killing of intracellular S. aureus was found to be > 99·5% using pooled Mφ obtained from animals of each genotype. Similar results were obtained in two further experiments carried out to examine phagocytosis and killing. In vitro phagocytosis of S. aureus by neutrophilic exudates obtained 24 hr after elicitation by heat-killed bacteria were not significantly different between pooled wild-type mice (1·3 ± 0·4 × 105, n = 7) and TRAP-deficient mice (2·8 ± 0·9 × 105, n = 8). Subsequent killing of phagocytosed live bacteria was > 99% in all 12 samples tested from each mouse strain. Microscopic examination confirmed that, as expected, > 60% of the peritoneal cells were neutrophilic polymorphonuclear leucocytes and that > 75% of the cells stained positively for the neutrophil marker, CD16.
Discussion
TRAP is a member of a highly conserved class of iron-containing proteins. Lately we have shown that mice lacking TRAP (as a result of a targeted disruption of the murine Acp 5 gene) have a skeletal phenotype, including osteopetrosis with disabled osteoclast function.7 Osteoclasts share a common lineage from myeloid progenitor cells in the bone marrow with other cells of mononuclear phagocytic phenotype. As, when activated, these also express abundant TRAP activity, we examined the function of Mφ obtained from Acp 5 null mice. The present studies were conducted to determine whether TRAP had an extended role common to all cells of phagocytic origin in which it is selectively expressed.
Mφ represent an ancestral component of the immune system in vertebrates that participates in innate and acquired immunity. When intracellular pathogens, bacterial LPS and other microbial products interact with mononuclear phagocytes, the cytokine, IL-12, is released.23 This cytokine appears to be critical for mounting a T helper 1 (Th1) response24 as it promotes the secretion of IFN-γ by natural killer (NK) cells25 which in turn activates Mφ for microbial killing.26 This activation includes stimulation of inducible NO synthase and formation of reactive oxygen species (both of which are central to the microbicidal response) and is necessary for proinflammatory cytokine production.27 Accordingly, we examined the capacity to generate oxygen free radicals and reactive nitrogen species by Mφ upon stimulation in TRAP-deficient and wild-type congenic-strain mice, as well as their capacity to secrete proinflammatory cytokines, including IL-12.
Although ultrastructural studies of resting and LPS-stimulated Mφ from TRAP-deficient mice showed no obvious morphological abnormalities suggesting lysosomal storage as a result of the enzymatic deficiency, studies of the tartrate-resistant isozyme and tartrate-sensitive isozymes clearly showed a selective compensatory increase in the activity of Acp 2, the ubiquitous lysosomal acid phosphatase. This is compatible with studies of lysosomal acid phosphatase-deficient mice, which showed greatly increased expression of TRAP,28 and previous studies of TRAP-deficient osteoclasts, which showed increased activity of alkaline phosphatase and tartrate-sensitive acid phosphatase. It was noteworthy that the levels of other lysosomal hydrolases were not significantly increased in TRAP-deficient Mφ, even though a global increase in hydrolytic enzymes characterizes the phagocytic activation response.29
Upon priming and activation, Mφ obtained from two distinct populations in TRAP-deficient mice had enhanced production of free radicals. The increase in NO, as reflected by nitrite production, was exaggerated to a greater extent in bone marrow-derived Mφ and may reflect different microenvironments, as previously noted.30 The capacity for superoxide formation was also enhanced in PMA-primed TRAP-deficient Mφ as well as primed Mφ activated with LPS or IFN-γ. The effects of LPS on free radical production by Mφ are variable and may depend on the initial concentration studied, because low-level exposure induces tolerance to subsequent high concentrations of this effector.13,31 A single stimulatory exposure to exogenous LPS was used in the current study.32
After priming, with or without further stimulation, TNF-α and IL-1β secretion was greatly increased in TRAP-deficient Mφ when compared with secretion of TNF-α and IL-1β in congenic control mice. The difference in magnitude of cytokine secretion between bone marrow and peritoneal Mφ could be a result of the greater heterogeneity of peritoneal cells and their greater access and exposure to endogenous LPS and other stimulatory molecules in vivo, before isolation.33 Bone marrow Mφ would be expected to be more homogeneous, partly as a consequence of a more prolonged period of culture. However, previous investigators have reported differences in the secretion of TNF-α, antigen presentation and proliferation in vitro between these two populations of Mφ.34–36 Our findings are consistent with TNF-α as the principal mediator of LPS-induced NO production37 and its known synergy with IL-1β in endotoxic responses in vivo.38 The marked increase of IL-12 secretory activity may reflect the increased capacity to generate NO.27 Disturbed IL-12 secretory profiles are also noteworthy as secretion of this T-cell and NK-cell stimulatory factor influences NO-dependent Mφ interactions with other cells that mediate the immune response.27 This suggests that TRAP may have a broader role in inflammation, affecting both innate and acquired immune functions. It is also notable that we have identified TRAP in B-7+ F4/80− cells in murine tissues and isolated murine dendritic cells,39 which are antigen-presenting cells essential for cell-mediated immunity.40 Therefore, TRAP may participate in antigen processing.
Although homozygous Acp 5 null mice exhibit a clear skeletal phenotype associated with mild osteopetrosis and foreshortening of long bones as a result of a defect in endochondral ossification, full blood counts were found to be normal and the mice are viable under laboratory conditions.7 Clearly, having demonstrated enzymatic complementation of acid phosphatase with disturbed Mφ responses in TRAP-deficient mice, it was necessary to test whether a functional defect of microbial disposal occurred. As we have shown that TRAP is an iron-containing phosphatase also capable of biological peroxidation reactions leading to the formation of radical species that are implicated in osteoclastic bone resorption and phagocytic microbicidal activity,5,41 we tested the ability of homozygous Acp 5 null mice to clear a bacterial pathogen in vivo. Exotoxins from S. aureus increase the formation of reactive oxygen intermediates and reactive nitrogen intermediates in vitro in a manner similar to LPS and cytokine administration.42,43 Many of the clinical manifestations of S. aureus toxic shock are indistinguishable from the effects of endotoxaemia.44 In addition, S. aureus is known to reside in the acidic phagolysosomes45 and is a persistent infection in patients with chronic granulomatous disease who lack intrinsic superoxide production.46 Indeed, mice lacking NADPH oxidase exhibit an increased susceptibility to S. aureus in vivo.16
Our experiments showed a markedly delayed in vivo clearance of a virulent strain of S. aureus during the 3-day study period. Disappearance of viable bacteria from the peritoneal cavity in TRAP-deficient animals was significantly delayed over the first 72 hr of infection compared with age- and gender-matched wild-type congenic-strain control animals. Reduced clearance of intraperitoneal S. aureus suggested that TRAP-deficient animals may have defective Mφ recruitment or directly impaired microbicidal activity. We have been unable to demonstrate any defect in phagocytosis or killing in vitro by Mφ or neutrophils obtained from the mutant mouse strain. Examination of peritoneal exudates after infection with S. aureus revealed differences in the later phases of the inflammatory response mounted by TRAP-deficient mice: at 72 hr after inoculation, the infiltrates contained a reduced population of large granular cells of monocyte/Mφ phenotype. These cells may participate in the resolution of the inflammatory response and in the clearance of apoptotic neutrophils and microbial debris that remain in the exudate after the early active period of bacterial killing has been completed.
The reduced Mφ population was accompanied with a reciprocal increase in the proportion of neutrophilic polymorphonuclear leucocytes in the exudates obtained from TRAP knockout animals. As the total cell counts in exudates obtained from mice of each Acp 5 genotype did not differ significantly, the findings reflect changes in the absolute members of the component phagocytes in the inflammatory reaction. We have been unable to demonstrate TRAP in neutrophils and there are several reports that confirm this observation.47,48 Phagocytosis of virulent S. aureus in vitro was apparently unimpaired in Mφ and neutrophils obtained from mice lacking TRAP, and killing of intracellular bacteria exceeded 99% in cells obtained from mice, irrespective of Acp 5 genotype. Although our findings are compatible with a defect in the recruitment of Mφ to the peritoneal cavity in the late phases of the inflammatory response to S. aureus, it remains possible that the impaired clearance of the organism itself is responsible for the altered cellular composition in the exudates from TRAP-deficient animals.
We show here that Mφ derived from homozygous Acp 5 null mice express residual acid phosphatase activity of lysosomal (Acp 2) rather than of the tartrate-resistant type, an enhanced capacity to secrete free radicals and disturbed proinflammatory cytokine secretory profiles. These abnormalities, combined with impaired in vivo clearance of the pathogen S. aureus and a reduced complement of monocyte/Mφ in the inflammatory exudate, provide clear evidence of a Mφ defect caused primarily by the absence of TRAP activity.
Apart from the ability of TRAP to degrade skeletal phosphoproteins, including osteopontin,49 it may influence the effector pathways of phagocytic activation or antigen presentation, perhaps by affecting the phosphorylation of key components that mediate cell signalling. It is thus of great interest that the T-cell cytokine, Eta-1, which interacts with Mφ to induce inflammatory responses, is identical to osteopontin. Eta-1-deficient mice fail to clear pathogens and have defective IL-12 and delayed hypersensitivity responses.50 As Eta-1 is secreted in phosphorylated and non-phosphorylated isoforms,51 and dephosphorylation abolishes Eta-1-mediated IL-12 release, it has been suggested that the phosphorylation status of this molecule regulates the cognate inflammatory pathway.50 We have found that the IL-12 inflammatory pathway is enhanced in TRAP-deficient Mφ and propose that acid phosphatase may be an important regulator of Eta-1 (osteopontin) activity common to both the immune system and skeleton. Our recent studies showing that abundant TRAP activity is expressed in murine (and human) dendritic cells39 are fully compatible with a central role for this protein in pleiotropic defence processes. The findings reported here have implications for the understanding of innate immunity and its molecular interactions with the acquired immune system that promote host responses to micro-organisms.
Acknowledgments
We are indebted to Dr Jeremy Skepper and Mrs Janet Powell for conducting the electron microscopy in the Cambridge Multi-imaging Center; this was made possible by funding from the Wellcome Trust. We are particularly grateful to Dr Joyce Young for expert assistance with the fluorescence-activated cell sorter (FACS) studies. Dr N. Brown kindly provided the S. aurens strain. Mrs Joan Grantham kindly provided excellent secretarial assistance. This work was supported by a grant from the Medical Research Council, UK and EC Biotechnology Program 6.1 (contract B104-CT-98-0385). A. R. Hayman is in receipt of a Research Fellowship from the Arthritis and Rheumatism Research Campaign. M. J. Evans was supported by the Wellcome Trust.
Abbreviations
- IFN-γ
interferon-γ
- l-NMMA
N-monomethyl-l-arginine
- LPS
lipopolysaccharide
- Mφ
macrophage
- NO
nitric oxide
- TNF-α
tumour necrosis factor-α
- TRAP
tartrate-resistant acid phosphatase
References
- 1.Li CY, Lam LT, Lam KW. Acid phosphatase isoenzyme in human leukocytes in normal and pathological conditions. J Histochem Cytochem. 1970;18:473–81. doi: 10.1177/18.7.473. [DOI] [PubMed] [Google Scholar]
- 2.Lord DK, Cross NCP, Bevilacqua MA, et al. Type 5 acid phosphatase. Sequence expression and chromosomal localization of a differentiation-associated protein of the human macrophage. Eur J Biochem. 1990;189:287–93. doi: 10.1111/j.1432-1033.1990.tb15488.x. [DOI] [PubMed] [Google Scholar]
- 3.Li CY, Yam LT, Lam KW. Studies of acid phosphatase isoenzymes in human leukocytes. Demonstration of isoenzyme cell specificity. J Histochem Cytochem. 1970;18:901–10. doi: 10.1177/18.12.901. [DOI] [PubMed] [Google Scholar]
- 4.Que L, Scarrow RC. Active sites of binuclear iron-oxo proteins. Am Chem Soc Symposium. 1988;372:152–78. [Google Scholar]
- 5.Hayman AR, Cox TM. Purple acid phosphatase of the human macrophage and osteoclast. Characterization, molecular properties and crystallization of the recombinant di-iron-oxo protein secreted by baculovirus-infected insect cells. J Biol Chem. 1994;262:59–62. [PubMed] [Google Scholar]
- 6.Sibille J-C, Doi K, Aisen P. Hydroxyl radical formation and iron-binding proteins. Stimulation by the purple acid phosphatases. J Biol Chem. 1987;262:59–62. [PubMed] [Google Scholar]
- 7.Hayman AR, Jones SJ, Boyde A, Foster D, Colledge WH, Carlton MB, Evans MJ, Cox TM. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteoporosis. Development. 1996;122:3151–62. doi: 10.1242/dev.122.10.3151. [DOI] [PubMed] [Google Scholar]
- 8.Ketcham CM, Baumbach GA, Bazer FW, Roberts RM. The type 5 acid phosphatase from spleen of humans with hairy cell leukaemia. Purification properties, immunological characterization and comparison with porcine uteroferrin. J Biol Chem. 1985;260:5768–76. [PubMed] [Google Scholar]
- 9.Robinson DB, Glew RH. Acid phosphatase in Gaucher's disease. Clin Chem. 1980;26:371. [PubMed] [Google Scholar]
- 10.Boltz-Nitulescu G, Wiltschke C, Holzinger C, Fellinger A, Scheiner O, Gessl A, Forster O. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J Leukoc Biol. 1987;41:83–91. doi: 10.1002/jlb.41.1.83. [DOI] [PubMed] [Google Scholar]
- 11.Gomori G. Histochemical methods for acid phosphatase. J Histochem Cytochem. 1956;4:453–61. doi: 10.1177/4.5.453. [DOI] [PubMed] [Google Scholar]
-
12.Johnston RB. Measurement of
 secreted by monocytes and macrophages. Methods Enzymol. 1982;105:365–9. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
secreted by monocytes and macrophages. Methods Enzymol. 1982;105:365–9. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar] - 13.Ding A, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates for mouse peritoneal macrophages. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 14.Hayman AR, Warburton MJ, Pringle JAS, Coles B, Chambers TJ. Purification and characterization of a tartrate-resistant acid phosphatase from human osteoclastomas. Biochem J. 1989;261:601–9. doi: 10.1042/bj2610601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Echetebu ZO, Cox TM, Moss DW. Antibodies to porcine uteroferrin used in the measurement of human tartrate-resistant acid phosphatase. Clin Chem. 1987;33:1832–6. [PubMed] [Google Scholar]
- 16.Pollock JD, Williams DA, Gifford MA, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–9. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 17.Robinson JP. Handbook of Flow Cytometry Methods. New York: A. A. Wiley-Liss; 1993. [Google Scholar]
- 18.Nilsson I-M, Lee JC, Bremell Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicaemia and septic arthritis. Infect Immun. 1997;65:4216–21. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snedecor GW, Cochran WG. Statistical Methods. 7. Vol. 140. Ames: Iowa State University Press; 1980. pp. 143–6. 255–333. [Google Scholar]
- 20.Gordon S, Lawson L, Rabinowitz S, Croker PR, Morris L, Perry VH. Antigen markers of macrophage differentiation in murine tissues. In: Russell S, Gordon S, editors. Macrophage Biology and ActivationCurrent Topics in Microbiology and Immunology. Vol. 181. Berlin: Springer-Verlag; 1992. pp. 1–37. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by a monoclonal antibody F7/4. Immunogenetics. 1983;18:229–39. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 22.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–50. doi: 10.1016/0022-1759(96)00138-x. 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 24.Hseih C-S, Macatonia SE, Tripp CS, Wolff SF, Garra AO, Murphy KM. Development of Th1 CD 4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–49. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 25.Altare F, Durandy A, Lammas D, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–35. doi: 10.1126/science.280.5368.1432. 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Kawakami K, Quereshi MH, Okamura H, Kuinoto N, Sato A. Interleukin-12 and IL-18 synergistically induce the fungicidal activity of murine exudate cells against Cryptococcus neoforms through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–9. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diefenbach A, Schindler H, Röllinghoff M, Yokoyama WM, Bogdanm C. Requirement for type 2 NO synthase for IL-12 signalling in innate immunity. Science. 1999;284:951–5. doi: 10.1126/science.284.5416.951. 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 28.Saftig P, Hartmann D, Lullmann-Rauch R, et al. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J Biol Chem. 1997;272:18628–35. doi: 10.1074/jbc.272.30.18628. [DOI] [PubMed] [Google Scholar]
- 29.Musson RA, Shafran H, Henson PM. Intracellular levels and stimulated release of lysosomal enzymes from human peripheral blood monocytes and monocyte-derived macrophages. J Reticuloendothelial Soc. 1980;28:249–64. [PubMed] [Google Scholar]
- 30.Wenzel SE, Trudeau JB, Riches DW, Westcott JY, Henson PM. Peritoneal lavage fluid alters patterns of eicosanoid production in murine bone marrow derived and peritoneal macrophages: dependency on the inflammatory state of the peritoneum. Inflammation. 1993;17:743–56. doi: 10.1007/BF00920478. [DOI] [PubMed] [Google Scholar]
- 31.Severn A, Xu D, Doyle J, Leal LM, O'donnell CA, Brett SJ, Moss DW, Liew FY. Pre-exposure of murine macrophages to lipopolysaccharide inhibits induction of nitric oxide synthase and reduces leishmanicidal activity. Eur J Immunol. 1993;23:1711–4. doi: 10.1002/eji.1830230747. [DOI] [PubMed] [Google Scholar]
- 32.Johnston RB, Kitagawa S. Molecular basis for the enhanced respiratory burst of activated macrophages. Federation Proc. 1985;44:2927–31. [PubMed] [Google Scholar]
- 33.Phillips WA, Hamilton JA. Phorbol ester-stimulated superoxide production by murine bone marrow derived macrophages requires pre-exposure to cytokines. J Immunol. 1989;142:2445–9. [PubMed] [Google Scholar]
- 34.Bradbury MG, Moreno C. Effect of lipoarabinomannan and mycobacteria on tumour necrosis factor production by different populations of murine macrophages. Clin Exp Immunol. 1993;94:57–63. doi: 10.1111/j.1365-2249.1993.tb05977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Blanc PA, Katz MR, Russell SW. A discrete population of mononuclear phagocytes depleted by monoclonal antibody. Infect Immun. 1980;29:520–5. doi: 10.1128/iai.29.2.520-525.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanagasundaram V, Christy E, Hamilton JA, Jawarowski A. Different pathways of colony-stimulating factor 1 degradation in macrophage populations revealed by Wortmannin sensitivity. Biochem J. 1998;330:197–202. doi: 10.1042/bj3300197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harbrecht BG, Di Silvo M, Demetris AJ, Simmons RL, Billar TR. Tumour necrosis factor-alpha regulates in vivo nitric oxide synthesis and induces liver injury during endotoxemia. Hepatology. 1994;20:1055–60. doi: 10.1002/hep.1840200439. [DOI] [PubMed] [Google Scholar]
- 38.Okusawa S, Gelfsand SA, Kejama T, Connolly RJ, Dinarello CA. Interleukin-1 induces a shock like state in rabbits. J Clin Invest. 1988;81:1162–72. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayman AR, Bune AJ, Bradley JR, Rashbass J, Cox TM. Osteoclastic tartrate-resistant acid phosphatase (Acp 5). Its localization to dendritic cells and diverse murine tissues. J Histochem Cytochem. 2000;48:219–27. doi: 10.1177/002215540004800207. [DOI] [PubMed] [Google Scholar]
- 40.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 41.Halleen JM, Raisenen S, Salo JJ, et al. Intracellular fragmentation of bone resorption products by reactive oxygen species generated by osteoclastic tartrate-resistant acid phosphatase. J Biol Chem. 1999;274:22907–10. doi: 10.1074/jbc.274.33.22907. [DOI] [PubMed] [Google Scholar]
- 42.Hattor Y, Kasai K, Akimoto K, Thiemermann C. Induction of NO synthesis by lipoteichoic acid from Staphylococcus aureus in J774 macrophages: involvement of a CD14 dependent pathway. Biochim Biophys Res Commun. 1997;233:375–9. doi: 10.1006/bbrc.1997.6462. 10.1006/bbrc.1997.6462. [DOI] [PubMed] [Google Scholar]
- 43.Busam K, Gieringer C, Freudenberg M, Hohmann H-P. Staphylococcus aureus and derived exotoxins induce nuclear factor κB-like activity in murine bone marrow macrophages. Infect Immun. 1992;60:2008–15. doi: 10.1128/iai.60.5.2008-2015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wardle EN. Bacteraemic and endotoxic shock. Br J Hosp Med. 1979;21:223–31. [PubMed] [Google Scholar]
- 45.Maurin M, Raoult D. Phagolysosomal alkalinisation and intracellular killing of Staphylococcus aureus by Amikacin. J Infect Dis. 1994;169:330–6. doi: 10.1093/infdis/169.2.330. [DOI] [PubMed] [Google Scholar]
- 46.Gallin J. Recent advances in chronic gramulomatous diseases. Ann Intern Med. 1983;99:657–74. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- 47.Drexler HG, Gigac SM. Characterization and expression of tartrate-resistant acid phosphatase (TRAP) in haematopoietic cells. Leukaemia. 1994;8:354–68. [PubMed] [Google Scholar]
- 48.Janckila AJ, Yaziji H, Lear SC, Martin AW, Yam LT. Localization of tartrate-resistant acid phosphatase in human placenta. Histochem J. 1994;28:195–200. doi: 10.1007/BF02331443. [DOI] [PubMed] [Google Scholar]
- 49.Ek-Rylander B, Flores M, Wendel M, Heinegård D, Andersson G. Dephosphorylation of osteopontin and bone sialoprotein by osteoclastic tartrate-resistant acid phosphatase. Modulation of osteoclast adhesion in vitro. J Biol Chem. 1994;269:14853–6. [PubMed] [Google Scholar]
- 50.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:859–64. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 51.Kubota T, Zhang Q, Wrana JL, Ber R, Aubin JE, Butler WT, Sodek J. Multiple forms of Spp 1 (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochem Biophys Res Commun. 1989;162:1453–57. doi: 10.1016/0006-291x(89)90837-1. [DOI] [PubMed] [Google Scholar]