Abstract
Despite strong evidence for CD8+ T-cell function in murine mycobacterial infections, their corresponding role in human tuberculosis has proven more difficult to demonstrate. We have evaluated the human macrophage (Mφ) cell line U937 as an in vitro model for human leucocyte antigen (HLA) class I-restricted presentation of mycobacterial antigens, as HLA class I is constitutively expressed at high levels by U937 cells in the absence of detectable HLA class II or CD1 molecules. U937 cells were evaluated for their ability to phagocytose Mycobacterium tuberculosis and for their ability to present mycobacterial antigens to human HLA class I-matched cytotoxic T lymphocytes (CTLs). Differentiated U937 cells were capable of efficient phagocytosis of M. tuberculosis but did not generate a subsequent respiratory burst response, and were permissive for intracellular growth of both bacillus Calmette–Guérin (BCG) and the virulent M. tuberculosis H37Rv strain. CTL activity was restricted to live mycobacterial organisms and was shown to be mediated by M. tuberculosis-specific, HLA class I-matched, purified CD8+ CTL lines and CD8+ T-cell clones. Furthermore, M. tuberculosis-infected U937 targets were more rapidly and strongly lysed by CD8+ CTLs than were infected autologous Mφ. Finally, M. tuberculosis-infected U937 cells simultaneously provided a sensitive indicator for detection of mycobacterial-specific, HLA-unrestricted γδ+ CTL activity.
Introduction
Cell-mediated cytotoxic activity in mycobacterial infections is mediated by an array of effector cells, which include human leucocyte antigen (HLA) class II-restricted1–3 CD4+ and HLA class I-restricted4–14 CD8+ cytotoxic T lymphocytes (CTLs), as well as non-HLA-restricted γδ+ CTLs,15 natural killer (NK) cells and lymphokine-activated killer (LAK) cell mediators.2 Evidence for CD8 T-cell involvement from murine models of tuberculosis included the demonstration that CD8+ CTL lines were cytolytic towards macrophages (Mφ) infected with Mycobacterium tuberculosis,4,5 that β2-microglobulin-deficient mice were reported to be more susceptible to mycobacterial infection than their wild-type littermates,6 and that mice with a targeted disruption in the gene for TAP1 (necessary for HLA class I presentation) were found to be highly susceptible to infection with M. tuberculosis.7
Until recently, little success had been achieved in determining the role of HLA class I-restricted CD8+ T cells in human immunity to tuberculosis, with mycobacteria-responsive CD8+ T cells only rarely being isolated from patients with tuberculosis.16 With the emergence of more sensitive immunological techniques and a corresponding renewed interest in the role of CD8+ CTLs in tuberculosis, a number of studies have recently been published, demonstrating the existence of classical HLA class I-restricted M. tuberculosis-responsive human CD8+ T cells.8–14 Many of these studies have focused on the ability of CD8+ T cells to produce cytokines or proliferate in response to stimulation with defined mycobacterial peptides.10–12 Those studies that demonstrated the existence of cytotoxic CD8+ T cells which are capable of lysing M. tuberculosis-infected target cells, also reported a requirement for specific priming conditions to facilitate the generation of CD8+ CTLs. Tan et al.9 reported that mycobacterial-specific CD8+ CTL activity was only demonstrable following in vitro co-culture with specific growth factors, while Mohagheghpour et al.12 made use of mycobacterial peptide-pulsed dendritic cells to efficiently prime CD8+ CTL effector cells.
This study describes a relatively simple, robust and easily adaptable in vitro model that makes use of the human Mφ cell line, U937,17 to present mycobacterial antigens to human HLA class I-restricted CD8+ CTL. U937 cells were selected because they constitutively express high levels of cell-surface HLA class I molecules, while HLA class II is undetectable and uninducible, both at the mRNA level and at the cell surface.18 U937 cells have also been shown to have a relatively efficient phagosome-to-cytosol pathway for delivery of exogenous antigens to the HLA class I-processing pathway.19 We demonstrate here that M. tuberculosis-infected U937 target cells are more rapidly and strongly lysed by CD8+ CTL than infected autologous Mφ. Not only do U937 cells provide a useful in vitro human Mφ model for evaluation of HLA class I-restricted CD8+ CTL function in mycobacterial infections, but they are also shown to be a sensitive and selective indicator of γδ+ CTL activity, in the absence of significant LAK cell mediators.
Materials and methods
Cell lines and culture conditions
U937 cells were maintained in suspension culture in RPMI-1640 (Flow Laboratories, Irvine, UK) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS; Delta Bioproducts, Kempton Park, South Africa), 2 mm l-glutamine and 10 mm HEPES, at 37° in a humidified atmosphere of 5% CO2. U937 cells were induced to differentiate by exposing the cells (5 × 105 cells/ml) to 5 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO), 200 IU/ml of recombinant interferon-γ (IFN-γ) (Biomedical PBL Laboratories, New Brunswick, NJ) or 10−7 m 1,25-dihydroxyvitamin D3 (VD3) (Roche Pharmaceuticals, Nutley, NJ), for 48 hr.
Mycobacterial growth conditions
M. tuberculosis H37Rv and M. bovis bacillus Calmette–Guérin (BCG) were grown in Middlebrook 7H9 broth (Difco Laboratories, Detroit, MI) supplemented with 10% Oleic Acid Albumin Dextran Catalase (OADC) (State Vaccine, Cape Town, South Africa) and 0·02% Tween-80 (Merck, Darmstadt, Germany) at 37° in an atmosphere of 5% CO2. Mid-log phase cultures were snap-frozen in liquid nitrogen and stored at −80°.
Phagocytosis of M. tuberculosis by U937 cells
For evaluation of mycobacterial binding, Zeihl–Neilsen (ZN) staining and light microscopy were used. M. tuberculosis H37Rv was either untreated or precoated with serum opsonins by culture for 30 min at 37° in the presence of an equal volume of fresh human serum. Immediately before infection, both untreated and serum-coated M. tuberculosis were washed once in phosphate-buffered saline (PBS). Untreated or differentiated (PMA, IFN-γ or VD3) U937 cells were infected with M. tuberculosis H37Rv (10 colony-forming units [CFU]/cell) for 90 min in the absence of additional serum. Alternatively, mycobacterial phagocytosis was assessed by transmission electron microscopy (TEM), according to the method described by Schaible et al.20 In these experiments, PMA-differentiated U937 cells were infected with serum-opsonized M. tuberculosis (50 CFU/cell) for 90 min.
Following the finding in Fig. 1(a) that the presence of serum opsonins increased the ability of U937 cells to bind M. tuberculosis, precoating of M. tuberculosis with human serum was used for all subsequent experiments.
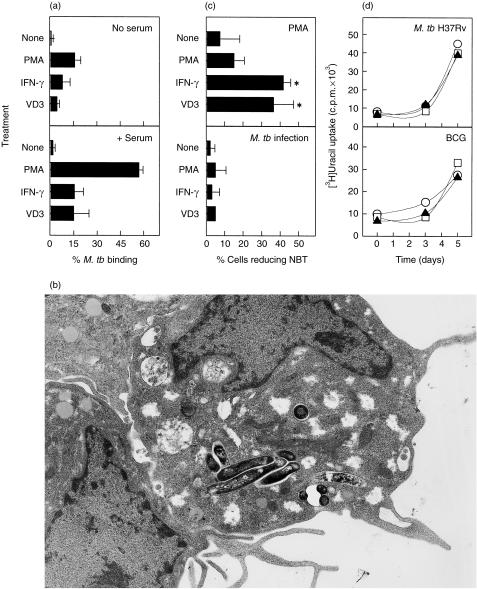
Figure 1.
The effect of differentiation with phorbol 12-myristate 13-acetate (PMA), interferon-γ (IFN-γ) or 1,25-dihydroxyvitamin D3 (VD3) on the ability of U937 cells to bind (a) and phagocytose (b) Mycobacterium tuberculosis, generate a respiratory burst response following infection (c) and control intracellular mycobacterial growth (d). (a) The ability of untreated, PMA- (5 ng/ml), IFN-γ- (200 IU/ml), or VD3- (10−7 m) differentiated U937 cells to bind M. tuberculosis (10 colony-forming units [CFU]/cell) in the absence (top panel) or presence (bottom panel) of fresh human serum was investigated using Zeihl–Neilson (ZN) staining and light microscopy. M.tb, Mycobacterium tuberculosis. (b) Transmission electron micrograph (TEM) photomicrograph of M. tuberculosis contained in membrane-bound phagosomes within PMA-differentiated U937 cells (× 5000 magnification). (c) Untreated, PMA-, IFN-γ- or VD3-differentiated U937 cells were assessed for their ability to mount a respiratory burst by Nitro-blue tetrazolium (NBT) reduction following stimulation with PMA (20 µg/ml; top panel) or infection with M. tuberculosis H37Rv (50 CFU/cell; bottom panel). *Represents significantly (P < 0·05) increased ability to reduce NBT following differentiation. (d) The permissiveness of PMA-differentiated (○), IFN-γ-differentiated (▴) or VD3-differentiated (□) U937 for intracellular growth of M. tuberculosis H37Rv (0·1 CFU/cell; top panel) or bacillus Calmette–Guérin (BCG) (0·1 CFU/cell; bottom panel) was assessed over a period of 5 days. Each bar/data point represents the mean percentage (± SD) of at least three independent experiments.
Nitro-blue tetrazolium (NBT) reduction assay
Respiratory burst activity was assessed by NBT reduction, according to the method described by Roberts et al.21 U937 cells (either untreated or differentiated with PMA, IFN-γ or VD3 for 48 hr) were suspended in RPMI-1640 containing 10% FCS and 0·05% (w/v) NBT (Sigma, St. Louis, MO), and primed with PMA (20 µg/ml, Sigma), infected with M. tuberculosis (50 CFU/cell) or left untreated, for 60 min at 37°. NBT reduction was assessed microscopically and at least 200 cells from duplicate experiments were counted for each of the treatments.
Permissiveness of U937 cells for intracellular mycobacterial growth
PMA-, IFN-γ- or VD3-differentiated U937 cells were infected with either M. tuberculosis H37Rv or BCG (0·1 CFU/cell) in the presence of 10% FCS. On days 0 (90-min postinfection), 3 and 5, the cells were lysed using 0·25% sodium dodecyl sulphate (SDS). The cell lysate was diluted 10-fold in 10% OADC Middlebrooks 7H9 broth (Difco), plated into a 96-well plate (100 µl/well; replicates of six per treatment) and pulsed with [3H]uridine (1 µCi/well; Amersham Pharmacia Biotech, Buckingham, UK) for 10–12 days at 37°. Bacterial cell-associated radioactivity was measured using a liquid scintillation counter (Tricarb 4640; Packard, Downers Grove, IL).
Isolation of human peripheral blood mononuclear cells (PBMC)
PBMC were obtained from healthy adult volunteers by centrifugation of heparinized venous blood over Ficoll–Hypaque density gradients (Sigma).
Tissue typing
HLA typing of U937 cells and human PBMC was determined according to phenotype, as described by Bodmer et al.22
Flow cytometry
An Epics Profile II flow cytometer (Coulter, Miami, FL) was used to perform immunophenotyping. Monoclonal antibodies (mAbs) were from Coulter (Hialeah, FL) or Becton-Dickinson (San Jose, CA).
Stimulation of mycobacterial-specific CTLs
PBMC (1 × 106 cells/ml) were stimulated with purified-protein derivative (PPD) (3 µg/ml; Central Veterinary College, Weybridge, UK), M. tuberculosis H37Rv or BCG (1 CFU/cell), for 6 days at 37°. Where indicated, specific T-cell subsets were isolated using the Minimacs magnetic bead separation system (Miltenyi Biotec, Auburn, CA). The purity of the positively selected T-cell subsets ranged from 95 to 99%. On day 6 of stimulation, the primed CTLs were adjusted to the concentration required for the cytotoxicity assays.
To investigate the effect of initial CTL priming on CD8 cytolytic activity, in some experiments the PBMC were: (i) primed with M. tuberculosis (1 CFU/ml) in the presence of recombinant human interleukin-2 (rhIL-2) (10 IU/ml; Cetus, Chiron Corporation, Emeryville, CA) (Tan et al.)9 and CD8+ cells were isolated after 6 days; (ii) primed with M. tuberculosis for 24 hr, then the CD8+ CTLs were isolated and cultured in the presence of rhIL-2 (50 IU/ml) for the remaining 5 days; or (iii) primed with M. tuberculosis-infected, ‘osmotically shocked’ Mφ (Moore et al.)23 and then the CD8+ T cells were isolated after 6 days.
Generation of T-cell clones
T-cell clones were generated by limiting dilution from M. tuberculosis-primed bulk PBMC cultures or from short-term CD8+ or γδ+ T-cell lines. T-cell subpopulations were seeded at 0·2 cells/well into Terasaki wells in the presence of irradiated (40 Gy) autologous PBMC feeders (1 × 106 cells/ml), phytohaemagglutinin (PHA; 1·13 × 10−2 mitogenic units/ml) (Murex Biotec, Dartford, UK) and recombinant interleukin-2 (rIL-2; 100 IU/ml), for 10–12 days. Mycobacterial-specific cytolytic CD8+ or γδ+ T-cell clones were restimulated every 7 days with PHA (1·13 × 10−2 mitogenic units/ml) or M. tuberculosis (1 CFU/cell) and rhIL-2 (100 IU/ml), in the presence of autologous irradiated PBMCs.
Cytotoxicity assays
The non-adherent target cytotoxicity assay for determining CD8+, NK- and LAK-mediated cytotoxicity against U937 targets was adapted from Ratcliffe et al.24 PMA-differentiated U937 cells were either infected with M. tuberculosis H37Rv (5 CFU/cell) or pulsed with the irrelevant streptococcal antigen streptokinase-streptodornase (SK-SD; 250 IU/ml of SK; 62·5 IU/ml of SD) (Lederle Laboratory, Wayne, NJ) for 16 hr at 37°. Suspension cells were then labelled with 250 µCi of chromium-51 (51Cr) for 90 min, washed three times and adjusted to the desired concentration.
The adherent target cytotoxicity assay for determining cytolysis against autologous Mφ targets has been described previously by Lorgat et al.2 Monocyte-derived Mφ were infected with M. tuberculosis H37Rv (5 CFU/cell) or pulsed with SK-SD (250 IU/ml of SK; 62·5 IU/ml of SD) (Lederle Laboratory) and concurrently labelled with 51Cr (6 µCi/well; Amersham) for 16 hr at 37°.
M. tuberculosis-primed CTL effector cells were then added to either non-adherent (U937) or adherent (Mφ) target cells, at various effector : target ratios, for either 4 or 16 hr (as indicated in the figure legends) at 37° in an atmosphere of 5% CO2.
Lymphoproliferative assay
T-cell clones (1 × 106 cells/ml) were stimulated with fresh irradiated autologous feeders (1 × 106 cells/ml) in the presence of PHA (1·1 × 10−2 mitogenic units/ml), PPD (3 µg/ml), or M. tuberculosis H37Rv (1 CFU/feeder), for 40–48 hr at 37° in an atmosphere of 5% CO2. [3H]Thymidine (1 µCi/well) (Amersham) was added to triplicate wells for the last 8 hr of the assay. Radioactivity was measured (in counts per minute [c.p.m.]) using a liquid scintillation counter (Tricarb 4640; Packard).
Statistical analysis
Statistical analyses were performed using the Student's t-test with the commercial statistical software package Statistica®.
Results
Phagocytosis and intracellular growth of mycobacteria
Undifferentiated U937 cells were unable to bind M. tuberculosis H37Rv, but differentiation using PMA, IFN-γ or VD3 significantly enhanced their ability to bind mycobacteria (Fig. 1a), particularly in the presence of serum opsonins. PMA-induced U937 cells showed the greatest ability to bind M. tuberculosis (57 ± 3% binding one or more bacilli in the presence of serum). TEM of PMA-treated U937 cells infected with M. tuberculosis revealed that the bacilli were intracellular and contained within vacuoles (Fig. 1b). Quantitative analysis of the infected cells by TEM confirmed that ≈ 53% of PMA-treated U937 cells became infected.
Differentiation of U937 cells with IFN-γ or VD3, but not with PMA, significantly (P = 0·02, P = 0·03 and P = 0·25, respectively) increased their ability to reduce NBT (Fig. 1c). However, despite showing moderate-to-strong respiratory burst activity following stimulation with PMA, differentiated U937 cells showed poor oxidative burst activity following infection with M. tuberculosis.
Differentiated U937 cells were found to be permissive to the growth of both virulent M. tuberculosis H37Rv and, to a lesser extent, non-virulent BCG, regardless of the agent used to induce differentiation (Fig. 1d).
Surface expression of HLA class I, HLA class II and CD1
U937 cells do not express cell-surface HLA class II but do express significant levels of HLA class I (Table 1).18 HLA class II expression was not inducible using rhIFN-γ (101−103 units/ml), PMA (5 ng/ml), VD3 (10−5−10−9 m), granulocyte–macrophage colony-stimulating factor (GM-CSF) (5–10 ng/ml), or a combination of these agents (data not shown). Although mycobacterial infection was found to have no effect on the number of cells expressing HLA class II or on the level of cell-surface expression, the level of HLA class I on the M. tuberculosis-infected, but not the BCG-infected, cells was significantly up-regulated (P < 0·05) compared with uninfected cells (Table 1).
Table 1.
Effect of mycobacterial infection on human leucocyte antigen (HLA) expression by U937 cells
| Percentage (± SD) of cells expressing surface antigen | MFI (± SD) of cells expressing surface antigen | ||||
|---|---|---|---|---|---|
| HLA | Treatment | Isotypic Ab* | Specific Ab | Isotypic Ab | Specific Ab |
| HLA class II | Uninfected | 2·0 (± 1·0) | 3·2 (± 2·6) | 34·4 (± 20·9) | 30·3 (± 18·1) |
| BCG infected | 2·1 (± 0·6) | 2·5 (± 1·3) | 27·5 (± 11·2) | 29·7 (± 15·9) | |
| M. tb infected | 2·0 (± 0·7) | 2·2 (± 1·2) | 35·0 (± 10·7) | 32·8 (± 9·4) | |
| HLA class I | Uninfected | 2·1 (± 1·9) | 96·6 (± 2·6) | 12·6 (± 13·4) | 27·0 (± 12·1) |
| BCG infected | 2·5 (± 1·7) | 96·7 (± 2·1) | 18·2 (± 19·5) | 28·2 (± 8·9) | |
| M. tb infected | 2·3 (± 0·4) | 94·3 (± 1·3) | 23·8 (± 3·5) | 59·5 (± 9·7)† | |
These results are presented as the mean percentage (± SD) or mean fluorescence intensity (MFI) (± SD) of phorbol 12-myristate 13-acetate (PMA)-differentiated U937 cells expressing cell surface HLA class I or class II.
Isotypic controls were used in all cases to set cursors to allow 2% false positives. Ab, antibody.
HLA class I expression was significantly up-regulated (P < 0·05) following infection with Mycobacterium tuberculosis (M. tb) H37Rv but not with bacillus Calmette–Guérin (BCG).
We also found that U937 cells do not express measurable levels of CD1a, CD1b or CD1c following conventional differentiation or stimulation with GM-CSF/interleukin-4 (IL-4) (data not shown).25
Selection of HLA class I-matched donors
U937 cells were found to express HLA-A3, -B18, -B51 and -Cw1 (Table 2). Twelve healthy employees of the Groote Schuur Hospital were recruited for this study on the basis of their HLA typing. Six of the donors were HLA class I-matched to U937. The remaining donors were HLA class I-mismatched to U937 cells. All of the selected donors showed strong proliferative responses to PPD.
Table 2.
Human leucocyte antigen (HLA) typing of HLA class I-matched and -mismatched donors
| HLA compatibility: | Donor | SI | HLA class I typing | HLA class II typing | ||||
|---|---|---|---|---|---|---|---|---|
| Cell line: | U937 | – | A3,– | B18,51 | Cw1,– | – | – | |
| HLA class I matched | TS | 125·4 | A3*,2 | B35,44 | Cw4,5 | DR1,7 | DQ1,2 | |
| PB | 77·3 | A3*,28 | B7,42 | Cw7,– | DR2,11 | DQ1,7 | ||
| ES | 89·1 | A3*,28 | B35,7 | Cw4,– | DR2,– | DQ1,– | ||
| EC | 91·8 | A3*,30 | B7,65 | Cw7,8 | DR2,11 | DQ1,7 | ||
| RG | 58·8 | A2,31 | B51*,62 | Cw3,– | DR4,11 | DQ3,– | ||
| BR | 7·0 | A2,24 | B51*,7 | Cw3,6 | DR2,13 | DQ1,– | ||
| HLA class I mismatched | MH | 12·3 | A1,28 | B37,62 | Cw3,6 | DR2,13 | DQ1,– | |
| NP | 22·9 | A11,30 | B13,58 | Cw6,7 | DR2,17 | DQ1,7 | ||
| SG | 52·3 | A28,34 | B16,40 | Cw–,– | DR2,14 | DQ1,– | ||
| SJ | 23·4 | A24,31 | B7,8 | Cw7,– | DR2,17 | DQ1,2 | ||
| MF | 40·5 | A26,33 | B58,– | Cw3,6 | DR4,17 | DQ2,8 | ||
| WM | 193·5 | A30,68 | B42,53 | Cw4,– | DR8,18 | DQ4,7 | ||
HLA class I match to U937 cells.
Lymphoproliferative responses of selected donors to purified protein derivative (PPD) are expressed as stimulation index (SI) in counts per minute (c.p.m.) and were calculated as follows: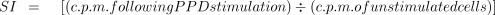
NK and LAK activity against U937
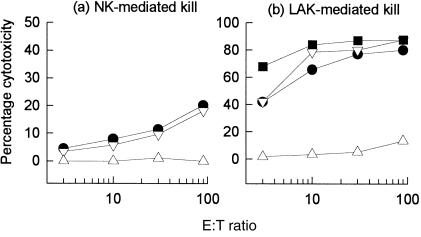
We initially evaluated the relative contribution of NK- or LAK-cell cytotoxicity against U937 cells before and following differentiation (Fig. 2). Using freshly isolated PBMC effector cells, undifferentiated U937 cells were found to be as susceptible to NK activity as K562 (the classical NK-indicator cell line). Following rIL-2 stimulation of PBMC to generate LAK effectors (6 days), U937 cells were killed with an efficiency similar to that of Daudi cells (the classical LAK indicator cell line). Differentiation of U937, however, significantly abrogated their sensitivity to both NK- and LAK-mediated cytotoxicity.
Figure 2.
Natural killer (NK) (a) and lymphokine-activated killer (LAK) (b) cell-mediated cytotoxicity against untreated U937 cells (▿), phorbol 12-myristate 13-acetate (PMA)-differentiated U937 cells (▵), K562 (•) and Daudi (▪). Cytotoxicity was determined after 4 hr. Each data point represents the mean percentage kill of duplicate experiments.
Cytolysis of U937 and autologous Mφ
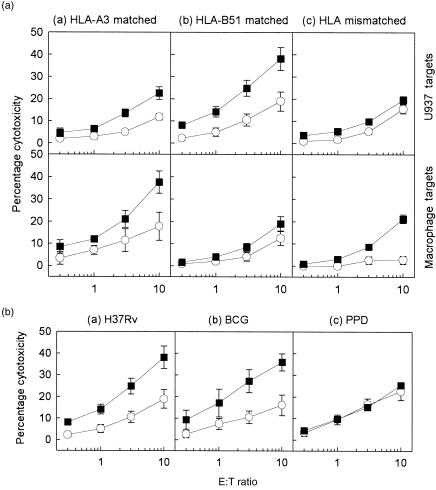
Figure 3(a) shows the susceptibility of M. tuberculosis-infected U937 compared with autologous Mφ targets to mycobacterial antigen-specific CTL activity. Both HLA-A3- (n = 4; Table 2) and HLA-B51-matched CTLs (n = 2) showed significantly greater ability (P < 0·05) to lyse M. tuberculosis-infected U937 cells than SK-SD-pulsed cells. Mycobacterial-specific CTLs generated from donors who were HLA mismatched to U937 cells (n = 6) did not demonstrate any significant ability to lyse U937 targets (P > 0·2), but showed cytolytic activity against autologous Mφ targets.
Figure 3.
(A) Mycobacterium tuberculosis-specific cytolysis generated by the (a) human leucocyte antigen (HLA)-A3-matched (n = 4; TS, PB, ES and EC), (b) HLA-B51-matched (n = 2; RG and BR), and (c) HLA-mismatched (n = 6; MH, NP, SG, SJ, MF and WM) donor cytotoxic T lymphocytes (CTL) against U937 (top panel) and autologous macrophage (Mφ) targets (bottom panel). (B) Mycobacterial antigen-specific cytolysis generated by HLA-B51-matched donor CTLs (n = 1; RG) against U937 targets infected with (a) M. tuberculosis H37Rv (5 colony-forming units [CFU]/cell) (b) bacillus Calmette–Guérin (BCG) (5 CFU/cell), or (c) pulsed with the soluble mycobacterial extract purified-protein derivative (PPD) (3 µg/ml). Target cells were infected with M. tuberculosis (5 CFU/cell) (▪) or pulsed with an irrelevant antigen, streptokinase-streptodornase (SK-SD) (○). Cytotoxicity against U937 and autologous Mφ targets was measured after 4 hr. Each data point represents the mean percentage cytolysis (± SEM) of at least three independent experiments.
Lysis by the HLA class I-matched CTL populations was found to be restricted to M. tuberculosis H37Rv- and BCG-infected U937 targets; no significantly enhanced lysis was found in cells pulsed with the soluble mycobacterial extract, PPD (Fig. 3b). The same M. tuberculosis-primed CTLs were capable of lysing autologous Mφ targets pulsed with PPD (data not shown). The inability of PPD-pulsed U937 target cells to be recognized in this context is consistent with the absence of a functional HLA class II-processing pathway in the U937 cell line.
T-cell subset purification
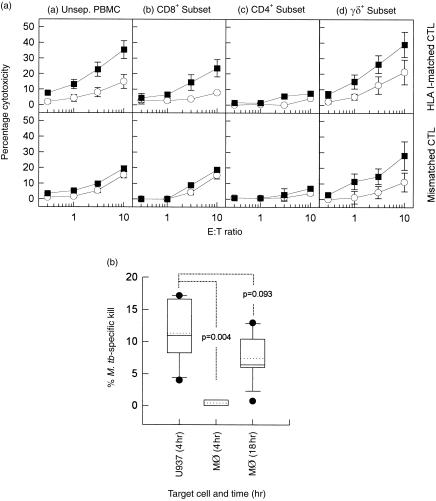
To directly determine the contribution of the different T-cell subsets to mycobacterial antigen-specific cytolysis generated against U937 cells, CD8+, CD4+ and γδ+ T cells were isolated from M. tuberculosis-primed PBMC. As shown in Fig. 4(a) (top panel), both unseparated effectors and M. tuberculosis-activated, purified CD8+ T cells (from HLA-B51-matched donors RG and BR) were cytolytic towards HLA class I-matched M. tuberculosis-infected U937 but not cells pulsed with the irrelevant streptococcal antigen, SK-SD (15·7 ± 5·0% mycobacterial-specific cytolysis, P < 0·05). The CD8+ CTL activity was HLA class I-restricted, as CD8+ cells from HLA class I-mismatched donors (MF and WM) showed no significant ability to lyse M. tuberculosis-infected U937 targets (P > 0·1). No cytolysis was obtained with purified CD4+ T cells from the HLA class I-matched and HLA-mismatched donors. The γδ+-enriched CTL population showed the most significant ability to lyse M. tuberculosis-infected U937 target cells (17·9 ± 9·3% mycobacterial-specific cytolysis, P < 0·02). Although clearly mycobacterial antigen specific, these cells were found not to be restricted to the classical HLA class I or class II molecules because γδ+ CTLs from both HLA class I-matched and the HLA-mismatched donors showed strong cytolytic activity against infected U937 targets.
Figure 4.
(A) Characterization of cytolytic T-cell subsets generating mycobacterial-specific cytolysis against U937 target cells. Mycobacterium tuberculosis-primed human leucocyte antigen (HLA)-B51-matched cytotoxic T lymphocytes (CTL) (RG and BR; top panel) and the HLA-mismatched CTL (MF and WM; bottom panel) populations were either (a) not separated, or fractionated into (b) CD8+ (c) CD4+ or (d) γδ+ T-cell subsets, and assessed for their respective cytolytic abilities against U937 target cells. U937 target cells were either infected with M. tuberculosis (5 colony-forming units [CFU]/cell) (▪) or pulsed with an irrelevant antigen, streptokinase-streptodornase (SK-SD) (○). Each data point represents the mean percentage cytolysis (± SEM) of at least three independent experiments. (B) Comparison of CD8+ CTL cytolytic activity generated by HLA-B51-matched donors (n = 2; RG and BR) against U937 versus macrophage (Mφ) targets. Mycobacterial-specific cytolysis was calculated according to the following equation: Cytotoxicity was measured after 4 hr for U937 targets, and after 4 and 18 hr for Mφ targets (as indicated). Each box-and-whisker plot shows the distribution, median (solid line), mean (dotted line), 10th and 90th percentile of six independent experiments. M.tb, Mycobacterium tuberculosis.
U937 and autologous Mφ as antigen-presenting cells (APCs)
Differentiated U937 cells were more rapidly and strongly lysed by CD8+ cells than autologous Mφ targets (Fig. 4b, P = 0·004). Although all target cells were infected with M. tuberculosis for the same period of time and under identical culture conditions, Mφ required an extended 18-hr culture period to be efficiently lysed by CD8+ CTL.
CD8+ T-cell activation
Phenotypic characterization of the CD8+ T-cell population showed that under conventional priming conditions (M. tuberculosis priming of bulk PBMC in the absence of additional growth factors) only 24·3% (± 18·2%) of the CD8 cells expressed CD25 (IL-2R) and 15·3% (± 8·4%) expressed HLA-DR. Although CD8+ CTL were not selectively expanded following exposure to mycobacterial antigens (relative expansion ratio of 1 : 0·9 [day 0 : day 7]), there was no significant reduction in the proportion of CD8 T cells following activation.
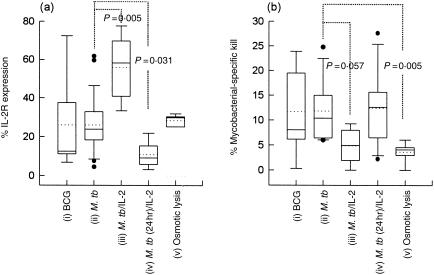
Because it has been well established that conventional mycobacterial priming preferentially activates and expands CD4+ and, possibly, γδ+ T cells, it has been proposed that more specific culture conditions may be necessary for optimal activation of M. tuberculosis-specific CD8+ CTLs ex vivo.9,12 In order to define the optimal conditions necessary for CD8+ CTL priming in mycobacterial infections, the effect of alternative strategies on CD8 activation marker expression and cytolytic ability were investigated (Fig. 5). The results showed that priming of bulk PBMC with the virulent M. tuberculosis H37Rv did not result in any significant enhancement in CD8+ CTL activation or cytolytic function compared with stimulation by attenuated BCG (P = 0·465). In contrast, PBMCs cultured together with a combination of M. tuberculosis and low doses of rhIL-2 (10 IU/ml) showed significantly increased expression of CD25 (IL-2 receptor [IL-2R]) on CD8+ CTLs (P = 0·005) but no concomitant enhancement of mycobacterial-specific CD8 T-cell cytolytic activity. Short-term bulk stimulation of PBMCs with M. tuberculosis (24 hr) followed by CD8+ CTL-subset isolation and rIL-2 (50–100 IU/ml) activation was used to investigate whether early exclusion of CD4+ T cells enhanced mycobacterial-specific CD8+ CTL activity. We found significantly reduced CD8+ CTL activation (P = 0·031) and no significant difference in cytolytic activity compared with conventional M. tuberculosis priming used throughout this study. We also investigated the use of ‘osmotic shock’ to facilitate the release of exogenous antigens into the HLA class I processing pathway.23 Although marginally increased expression of CD25 (IL-2R) on CD8+ T cells was observed (P = 0·246), these CD8 cells demonstrated reduced cytolytic ability compared with conventional priming (P = 0·005).
Figure 5.
The effect of priming conditions on mycobacterial-specific CD8+ cytotoxic T lymphocyte (CTL) cytolytic function. (a) CD25 (interleukin-2 receptor [IL-2R]) expression and (b) mycobacterial-specific cytolytic activity against monocyte-derived macrophages (Mφ) was assessed following different CTL priming procedures. Mycobacterial-specific CTLs were generated from peripheral blood mononuclear cells (PBMC) stimulated with: (i) bacillus Calmette–Guérin (BCG) (1 colony-forming unit [CFU]/ml) (ii), Mycobacterium tuberculosis (M.tb) H37Rv (1 CFU/ml) or (iii) M. tuberculosis H37Rv (1 CFU/ml) in the presence of additional recombinant human IL-2 (M.tb/IL-2) (10 IU/ml) and CD8+ cells isolated after 6 days. Alternatively, CTLs were generated by (iv) stimulating PBMC with M. tuberculosis for 24 hr, isolating the CD8+ CTLs and then culturing these in the presence of rhIL-2 (50 IU/ml) for the remaining 5 days [M.tb (24 hr)/IL-2]; or (v) primed with M. tuberculosis-infected, osmotically lysed Mφ and then isolating the CD8+ T cells after 6 days. Mycobacterial-specific cytolysis was calculated according to the following equation: Cytotoxicity was measured after 18 hr. Each box-and-whisker plot shows the distribution, median (solid line), mean (dotted line), 10th and 90th percentile of six independent experiments.
It is probable that the low cytolytic capacity demonstrated by CD8+ CTLs in mycobacterial infections may be the result of the combined effects of inadequate initial priming of this T-cell subset, a phenomenon recently described in the context of viral infections,26 together with the relative inefficiency of the pathway allowing HLA class I presentation of exogenously derived bacterial antigens.27
T-cell clones
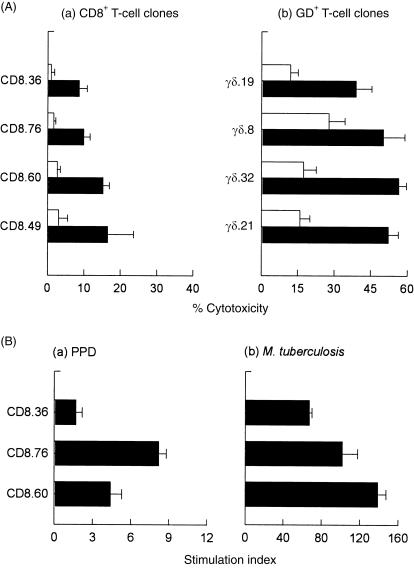
T-cell clones were generated from M. tuberculosis-primed HLA-B51-matched PBMC (RG; Table 2) and selected on the basis of their cytolytic activity against M. tuberculosis-infected U937 target cells (Fig. 6a). Although the T-cell clones that demonstrated cytolytic activity against infected U937 targets were found to be predominantly CD8+ (48/56), all the CD8 T-cell clones tested demonstrated only moderate-to-weak cytolytic activity against M. tuberculosis-infected U937 targets (13·2 ± 8·1% mycobacterial antigen-specific). By comparison, these CD8+ clones showed poor cytolytic activity towards M. tuberculosis-infected autologous Mφ targets (5·4 ± 3·3%). All of the CD8+ T-cell clones tested proliferated strongly to live M. tuberculosis but much less so to soluble PPD (Fig. 6b). On the other hand, the T-cell clones from the HLA-B51-matched donor (RG, Table 2), from whom the CD8 clones were generated, demonstrated strong proliferative responses to PPD (SI = 58·8) using bulk PBMCs. A small proportion (eight of 56) of cytolytic clones expressed the γδ+ T-cell receptor (CD8–). Despite being present in lower numbers compared with the CD8 CTL clones, these γδ+ T-cell clones generated strong cytolytic activity against infected U937 target cells.
Figure 6.
Cytotoxic and proliferative responses of mycobacterial antigen-specific cytotoxic T-lymphocyte (CTL) clones generated from a human leucocyte antigen (HLA)-B51-matched donor (RG). (A) Both CD8+ (a) and γδ+ (b) CTL clones mediate mycobacterial-specific cytolytic activity against U937 target cells. U937 target cells were either infected with Mycobacterium tuberculosis (5 colony-forming units [CFU]/cell) (filled bars) or pulsed with an irrelevant antigen, streptokinase-streptodornase (SK-SD) (open bars). Cytotoxicity was measured after 4 hr. Each data point represents the mean percentage cytolysis (± SD) of at least four independent experiments. (B) Proliferative responses of CD8+ T-cell clones to (a) soluble purified-protein derivative (PPD) or (b) M. tuberculosis. CD8+ T-cell clones were unstimulated, or stimulated with PPD (3 µg/ml) or M. tuberculosis (5 CFU/cell) in triplicate wells in the presence of autologous irradiated peripheral blood mononuclear cells (PBMCs). After 40 hr, cultures were pulsed with [3H]thymidine (1 µCi/well) for 8 hr. Results are expressed as stimulation indices (SI) (in counts per minute [c.p.m.]) and were calculated as follows: Each bar represents the mean SI (± SD) of triplicate wells.
Discussion
The U937 cell line has been used extensively as an in vitro model for human Mφ differentiation28,29 and effector function in various infectious diseases, including Legionella,30 Leishmania31 and Salmonella spp.32 U937 cells have been shown to be capable of Mφ-like differentiation without concomitant induction of HLA class II18 and are able to efficiently process antigens for presentation by HLA class I molecules.19
In the present study, we have established the utility of the U937 cell line as an in vitro model for mycobacterial antigen presentation to non-CD4+ human cytolytic T cells (CD8+ and γδ+) because of the following:
the fact that differentiated U937 cells phagocytosed M. tuberculosis efficiently without generating a respiratory burst response, together with their ability to strongly support the intracellular growth of attenuated (BCG) and virulent (M. tuberculosis H37Rv) mycobacterial strains, probably ensured high antigenic load and HLA class I processing;33
differentiation of U937 cells virtually abrogated their susceptibility to both NK- and LAK-mediated anomalous cytotoxicity;
M. tuberculosis-infected U937 cells were efficiently lysed by M. tuberculosis-specific HLA class I-matched, but not HLA-mismatched, human CTLs, while lack of HLA class II expression by U937 target cells precluded killing by CD4+ CTLs.1,2
CTL activity was shown to be restricted to live mycobacterial organisms (M. tuberculosis H37Rv and BCG) that are capable of access to the HLA class I pathway34,35 but was not detected against PPD-pulsed target cells that are lysed by CD4+ CTL;2 and
CTL activity was found to be mediated by purified CD8+ CTL lines and CD8+ T-cell clones only from HLA class I-matched donors.
Given the absence of HLA class II (Table 1)18 and CD125 expression on U937 cells, the data presented here further supports the conclusion that antigens derived from M. tuberculosis can access the HLA class I processing pathway.7,13,34,35
The mechanism by which mycobacterial antigens derived from intracellular, but phagosomally situated, M. tuberculosis might gain access to the HLA class I-presentation pathway, is slowly emerging. The ability of exogenous bacterial and particulate antigens to gain access to the HLA class I-processing pathway has been described previously36,37 and there is now evidence to suggest that infection with M. tuberculosis is able to facilitate such an exchange of antigens between phagosomes and the cytoplasm.34,35 Furthermore, a recent report by Canaday et al.13 has demonstrated that M. tuberculosis-derived antigens are capable of accessing the HLA class I pathway by an alternative route that does not require proteosomal processing or trafficking through the endoplasmic reticulum. The phagosome-to-cytosol HLA class I-processing pathway for exogenous antigens does not, however, seem to be a constitutive characteristic of all professional APCs.27
Although U937 target cells shared only a single HLA class I haplotype match (either HLA-A3 or -B51) with the CTL donors investigated in this study, they were more rapidly and strongly lysed than autologous Mφ targets. Several possibilities could account for this interesting observation. One possible explanation may be that a more efficient phagosome-to-cytosol antigen delivery system in U937 cells could result in enhanced surface antigen presentation to CTL effectors. Although additional research is needed to confirm this, a recent report by Harris and colleagues19 lends supports to this interpretation. These authors directly isolated and identified HLA class I-associated peptides from human immunodeficiency virus (HIV) nef-transfected U937 cells and found that, in addition to the anticipated HIV nef- and endogenously derived peptides, a significant proportion of peptides isolated from the HLA class I pools were derived from identifiable exogenous proteins. Although Harris et al.19 showed relatively good yields of exogenously derived HLA class I-associated peptides, they calculated that HLA class I presentation of endogenously derived peptides was still 200-fold more efficient than presentation of peptides derived from exogenous proteins.
In addition to HLA class I-restricted CD8 CTL responses, we also found that M. tuberculosis-infected U937 target cells were sensitive to non-HLA-restricted, but mycobacterial-specific, CTL activity mediated by purified γδ+ CTL lines and γδ+ T-cell clones. Previous reports have confirmed that antigen-specific responses of γδ T cells are not restricted by classical HLA class I or II, or non-classical CD1a, CD1b or CD1c antigen-presenting molecules and that antigens may be presented directly on the surface of infected cells as no apparent antigen processing appears to be necessary.38 The present finding that differentiation of U937 cells abrogated their susceptibility to both NK- and LAK-mediated anomalous cytotoxicity, thereby permitting selective distinction of γδ+ CTL from LAK activity, may indicate a useful role for this Mφ model in the study of γδ+ CTL responses in mycobacterial infections.
In summary, this study has demonstrated that the human monocytic cell line, U937, is a suitable in vitro model for HLA class I-restricted presentation of mycobacterial antigens to human cytolytic T cells. In addition, infected U937 cells simultaneously provided a sensitive indicator for detection of mycobacterial-specific, HLA-unrestricted γδ+ CTL activity.
Acknowledgments
The authors wish to thank Darren Martin for proof-reading the final manuscript; Dereck Taljaard and Lorraine Hope of the Department of Tissue Immunology, University of Cape Town, for tissue typing U937 cells and CTL donors; and Mohammed Jaffer of the Electron Microscopy Unit, University of Cape Town, for preparing the TEM samples and assisting with analysis of the results. We gratefully acknowledge funding provided by GlaxoWellcome Action TB Initiative.
References
- 1.Kaleab B, Ottenoff T, Converse P, Halapi E, Tadesse G, Rottenberg M, Kiessling R. Mycobacterial-induced cytotoxic T lymphocyte as well as non-specific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990;20:2651. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- 2.Lorgat F, Keraan MM, Lukey PT, Ress SR. Evidence for in vivo generation of cytotoxic T cells. PPD-stimulated lymphocytes from tuberculosis pleural effusions demonstrate enhanced cytotoxicity with accelerated kinetics of induction. Am Rev Respir Dis. 1992;145:418. doi: 10.1164/ajrccm/145.2_Pt_1.418. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PF, Modlin RL, Ellner JJ. T cell responses and cytokines. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection and Control. Washington: ASM Press; 1994. p. 417. [Google Scholar]
- 4.De Libero G, Flesch I, Kaufmann SHE. Mycobacteria reactive Lyt2+ T cell lines. Eur J Immunol. 1988;18:59. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 5.Serbina NV, Liu CC, Scanga CA, Flynn JL. CD8+ CTL from lungs of M. tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J Immunol. 2000;165:353. doi: 10.4049/jimmunol.165.1.353. [DOI] [PubMed] [Google Scholar]
- 6.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium infection. Proc Natl Acad Sci USA. 1992;89:12013. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behar S, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1d or TAP1 to infection with M. tuberculosis. J Exp Med. 1999;189:1973. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner J, Dockrell HM. Stimulation of human peripheral blood mononuclear cells with live M. bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JS, Canaday DH, Boom WH, Balaji KN, Schwander SK, Rich EA. Human alveolar T lymphocyte responses to M. tuberculosis antigens. J Immunol. 1997;159:290. [PubMed] [Google Scholar]
- 10.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and IFN-γ-secreting CD8+ T lymphocytes specific for M. tuberculosis. Proc Natl Acad Sci USA. 1998;95:270. doi: 10.1073/pnas.95.1.270. 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with M. tuberculosis-infected antigen presenting cells. J Exp Med. 1998;187:1633. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohagheghpour N, Gammon D, Kawamura LM, van Vollenhoven A, Benike CJ, Engleman EG. CTL response to M. tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400. [PubMed] [Google Scholar]
- 13.Canaday DH, Ziebold C, Noss EH, Chervenak KA, Harding CV, Boom WH. Activation of human CD8+ αβ TCR+ cells by M. tuberculosis via an alternate class I MHC antigen processing pathway. J Immunol. 1999;162:372. [PubMed] [Google Scholar]
- 14.Smith SM, Malin AS, Lukey PT, Atkinson SE, Content J, Huygen K, Dockrell HM. Characterization of human M. bovis BCG-reactive CD8+ T cells. Infect Immun. 1999;67:5223. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsukaguchi K, Balaji KN, Boom WH. CD4+ αβ T cell and γδ T cell responses to M. tuberculosis: similarities and differences in antigen recognition, cytotoxic effector function, and cytokine production. J Immunol. 1995;154:1786. [PubMed] [Google Scholar]
- 16.Rees A, Scoging A, Mehlert A, Young DB, Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988;18:1881. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- 17.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 18.Peterlin BM, Gonwa TA, Stobo JD. Expression of HLA-DR by a human monocyte cell line is under transcriptional control. J Mol Cell Immunol. 1984;1:191. [PubMed] [Google Scholar]
- 19.Harris PE, Colovai AI, Maffei A, Liu Z, Suciu Foca N. MHC class I presentation of exogenous and endogenous protein-derived peptides by a transfected human monocyte cell line. Immunology. 1995;86:606. [PMC free article] [PubMed] [Google Scholar]
- 20.Schaible UE, Sturgill-Koszycki S, Schlesinger PH, Russell DG. Cytokine activation leads to acidification and increases maturation of M. avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290. [PubMed] [Google Scholar]
- 21.Roberts PJ, Devalia V, Faint R, Pizzey A, Bainton AL, Thomas NSB, Pilkington GR, Linch DC. Differentiation-linked activation of the respiratory burst in monocytic cell line (U937) via FcRII. A study of activation pathways and their regulation. J Immunol. 1991;147:3104. [PubMed] [Google Scholar]
- 22.Bodmer JG, Pickbourne P, Richards S. Joint report on Ia serology. In: Bodmer WR, editor. Histocompatibility Testing. Copenhagen: Munksgaard; 1977. p. 35. [Google Scholar]
- 23.Moore MW, Carbone FR, Bevan MJ. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988;54:777. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 24.Ratcliffe LT, MacKenzie CR, Lukey PT, Ress SR. Reduced NK cell activity in multi-drug resistant pulmonary tuberculosis. Scand J Immunol (suppl.) 1992;11:167. doi: 10.1111/j.1365-3083.1992.tb01643.x. [DOI] [PubMed] [Google Scholar]
- 25.Kasinrerk W, Baumruker T, Majdic O, Knapp W, Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte–macrophage colony-stimulating factor. J Immunol. 1993;150:579. [PubMed] [Google Scholar]
- 26.Spencer JV, Braciale TJ. Incomplete CD8+ T lymphocyte differentiation as a mechanism for subdominant cytotoxic T lymphocyte responses to a viral antigen. J Exp Med. 2000;191:1687. doi: 10.1084/jem.191.10.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reis e Sousa C, Germain RN. MHC class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minta JO, Pambrun L. In vitro induction of cytologic and functional differentiation of monocyte-like cell line U937 with phorbol myristate acetate. Am J Pathol. 1985;119:111. [PMC free article] [PubMed] [Google Scholar]
- 29.Taimi M, Chateau MT, Cabane S, Marti J. Synergistic effect of retinoic acid and 1,25-dihydroxyvitamin D3 on differentiation of the human monocytic cell line U937. Leuk Res. 1991;15:1145. doi: 10.1016/0145-2126(91)90183-t. [DOI] [PubMed] [Google Scholar]
- 30.Abu Kwaik Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect Immun. 1998;66:203. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arena A, Capozza AB, Delfino D, Iannello D. Production of TNF-α and interleukin-6 by differentiated U937 cells infected with Leishmania major. New Microbiol. 1997;20:233. [PubMed] [Google Scholar]
- 32.Chateau MT, Caravano R. Infection of differentiated U937 cells by Salmonella typhimurium: absence of correlation between oxidative burst and antimicrobial defense. FEMS Immunol Med Microbiol. 1993;7:111. doi: 10.1111/j.1574-695X.1993.tb00389.x. [DOI] [PubMed] [Google Scholar]
- 33.Kurts C, Miller JFAP, Subramaniam RM, Carbone FR, Heath WR. MHC class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzaccaro RJ, Gedde M, Jensen ER, Van Santen HM, Ploegh HL, Rock KL, Bloom BR. MHC class I presentation of soluble antigen facilitated by M. tuberculosis infection. Proc Natl Acad Sci USA. 1996;93:11786. doi: 10.1073/pnas.93.21.11786. 10.1073/pnas.93.21.11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teitelbaum R, Cammer M, Maitland ML, Freitag NE, Condeelis J, Bloom BR. Mycobacterial infection of macrophages results in membrane-permeable phagosomes. Proc Natl Acad Sci USA. 1999;96:15190. doi: 10.1073/pnas.96.26.15190. 10.1073/pnas.96.26.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA. 1993;90:4942. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 38.Morita CT, Beckman EM, Bukowski JF, et al. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity. 1995;3:495. doi: 10.1016/1074-7613(95)90178-7. [DOI] [PubMed] [Google Scholar]