Abstract
Reperfusion of ischaemic tissue initiates an inflammatory reaction that increases tissue injury. Complement activation at the endothelium contributes to this inflammation. This study investigated the mechanism of complement activation following reoxygenation of hypoxic human umbilical vein endothelial cells (HUVEC) as a model for complement activation observed on endothelium in reperfused ischaemic tissue. HUVEC cultured in 1% oxygen followed by reoxygenation activated the classical complement pathway resulting in C3 deposition. There was an increase in apoptotic cells in these cultures that was demonstrated by binding of fluorescein isothiocyanate-Annexin V and staining for hypodiploid nuclei. To determine if apoptotic HUVEC activate complement, uniformly apoptotic cells were produced by serum and growth factor deprivation. These cells, but not the control HUVEC, activated the classical complement pathway in the absence of antibody or other serum factors. To determine if apoptotic cells in the reoxygenated cultures were activating complement, fluorescent analysis was done. Annexin V binding and C3d deposition on cells from reoxygenated cultures showed complete concordance on the subpopulation of apoptotic cells. In addition, complement activation following reoxygenation of HUVEC was eliminated by treatment of the cultures with a caspase inhibitor during reoxygenation. These results suggest that oxidative damage to endothelial cells during reoxygenation initiates apoptosis with exposure of phosphatidylserine. Apoptotic cells directly activate the classical pathway of complement by binding C1. Activation of complement at the endothelium may contribute to the inflammatory response as well as clearance and repair.
Introduction
Reperfusion of ischaemic tissue induces an inflammatory response that results in damage to vascular endothelium and underlying tissue. Reperfusion injury can increase the extent of vascular and tissue damage beyond that created by the initial ischaemia and is an important factor in the pathogenesis of tissue injury following myocardial infarction, stroke and other acute ischaemic events.
Experimental evidence indicates that complement activation is critically involved in neutrophil infiltration and vascular leakage in many types of reperfusion injury. During myocardial infarction, complement fragments can be identified bound to infarcted tissue and levels of complement split products are elevated in serum.1,2 The injury observed in experimental ischaemia/reperfusion is decreased by prior depletion of complement or neutrophils.3 Complement inhibition prior to coronary artery ligation significantly reduced the extent of myocardial injury and neutrophil infiltration following reperfusion.1,4,5 In a hindlimb ischaemia/reperfusion model, 50% less vascular leakage was seen in mice deficient in C3 or C4 compared to controls.6 In a model of intestinal ischaemia/reperfusion injury, administration of a C5a receptor antagonist reduced both local and remote tissue injury.7
Complement proteins are deposited on endothelium early in the course of skeletal muscle and myocardial reperfusion injury.4,6 The stimulus for this complement activation is unknown. However, Collard et al. showed complement activation by endothelial cells in vitro in response to hypoxia/reoxygenation.8 In this model, human umbilical vein endothelial cells (HUVEC) cultured in 1% oxygen for 12–24 hr then reoxygenated for 3 hr in the presence of human serum activated complement. Further studies showed that complement activation in these cultures was inhibited by compounds that inhibit reactive oxygen species.9
The present study was undertaken to determine the stimulus for complement activation in HUVEC cultures exposed to low oxygen and reoxygenation as a model for endothelial cells in reperfused ischaemic tissue. The results show that complement activation by endothelial cells exposed to hypoxia/reoxygenation is induced by a subpopulation of apoptotic cells in these cultures. Complement activation by apoptotic endothelial cells is associated with exposure of phosphatidylserine, is mediated by the classical pathway, does not require antibody or other serum proteins, and is prevented by treatment with a caspase inhibitor.
Materials and methods
Reagents
The following buffers were used: GVB (0·1% gelatin, 5 mm Veronal-buffered saline, pH 7·4), GVB+ + (GVB, 0·5 mm MgCl2, 0·15 mm CaCl2), DGVB+ + (GVB diluted 1 : 1 with 5% dextrose, 0·5 mm MgCl2, 0·15 mm CaCl2), and HBSS (Hanks' balanced salt solution, Sigma, St Louis, MO). Normal human serum (NHS) was stored at −70°, heat-inactivated for 30 min at 56°, or depleted of factor D and C1q (DHS) by passage over BioRex 70 (BioRad, Richmond, CA).10 DHS was reconstituted for the classical and alternative pathways by addition of 100 µg/ml purified C1q (Sigma) or 5 µg/ml purified factor D.11 Depletion and reconstitution of the classical and alternative pathways was confirmed by haemolytic assays. Purified C1 was kindly provided by Dr M. E. Medof (Case Western Reserve University, Cleveland, OH).
The following were purchased: C2 and C4 (Advanced Research Technologies, San Diego, CA); human immunoglobulin M (IgM; Sigma); horseradish peroxidase (HRP) –goat anti-human C3 (Cappel, Durham, NC); mouse monoclonal anti-C1q and C3d (Quidel, San Diego, CA); fluorescein isothiocyanate (FITC)-Annexin V (Pharmingen, San Diego, CA); FITC-goat anti-human IgM, FITC-goat anti-human IgG and phycoerythrin (PE)-F(ab′)2 goat anti-mouse IgG (Caltag, Burlingame, CA); allophycocyanin (APC) -F(ab′)2 goat anti-mouse IgG (Accurate Antibodies, Westbury, NY); and caspase inhibitor I, Z-Val-Ala-Asp-fluoromethylketone (Z-VAD) (Calbiochem, San Diego, CA).
Cell culture
HUVEC (BioWhittaker, Walkersville, MD) were grown in complete endothelial growth medium (EGM) on 0·1% gelatin-coated wells or flasks. EGM consists of basal medium supplemented with 2% fetal bovine serum, 12 µg/ml bovine brain extract, 0·01 µg/ml human epidermal growth factor, 1 µg/ml hydrocortisone and antibiotics. Cells were used at passages 2–4. Low oxygen culture was in a humidified, heated chamber designed for hypoxia experiments and purchased from Coy Laboratory Products Inc. (Grass Lake, MI). The chamber was flushed with nitrogen until the measured oxygen levels within the chamber were less than 1% prior to starting the hypoxic culture which was carried out in 1% oxygen, 5% CO2 with the balance being N2. Normal culture (21% oxygen) was in a CO2 incubator with 5% CO2.
Enzyme-linked immunosorbent assay (ELISA) for complement activation
HUVEC were cultured overnight in 21% or 1% oxygen in 96-well plates. HUVEC were then either washed and tested for complement activation, incubated for 1 hr in HBSS in 1% or 21% oxygen, or incubated for 1 hr in HBSS and cultured for 3 hr in EGM. Plates were washed with GVB++ and incubated with 10% NHS in GVB++ for 30 min at 37°. Cells were washed, fixed with 1% paraformaldehyde (15 min, room temperature), washed again and blocked with GVB++. Plates were developed with HRP-anti-C3 and substrate (0·005% H2O2, 1 mg/ml 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid in 0·1 m Na2HPO4, 50 mm citric acid, pH 4·6). Absorbance was read at 405 nm. Control wells used to determine background values were incubated with heat-inactivated NHS or DHS. The background values using heat-inactivated serum and DHS are shown in Figs 1 and 2. The background values using buffer (GVB) were similar to these values.
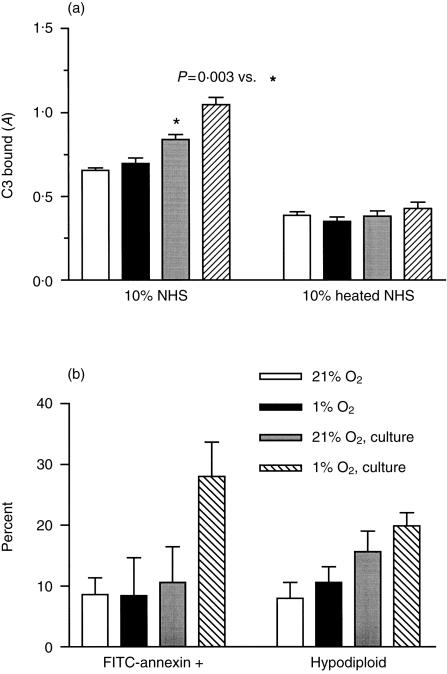
Figure 1.
C3 binding and apoptosis in HUVEC cultures following hypoxia/reoxygenation. HUVEC were cultured overnight in 21% oxygen or 1% oxygen. Cells were then analysed for C3 binding and apoptosis (O2) or incubated with HBSS for 1 hr in 21% oxygen and cultured an additional 3 hr in 21% oxygen (O2, culture). (a) Following treatment, cells were incubated with 10% NHS for 30 min at 37°, washed and fixed. C3 binding was then measured by a C3-specific ELISA. The means± SEM of six wells from one of three experiments are shown as well as the heat-inactivated NHS control. P values were determined by unpaired two-tailed t-tests. (b) HUVEC cultured as described above were released by trypsinization and analysed for apoptosis. The bars on the left indicate the percentage of cells that stained with FITC-Annexin V and excluded PI. The bars on the right indicate the percentage of hypodiploid nuclei. The mean results from two experiments are shown.
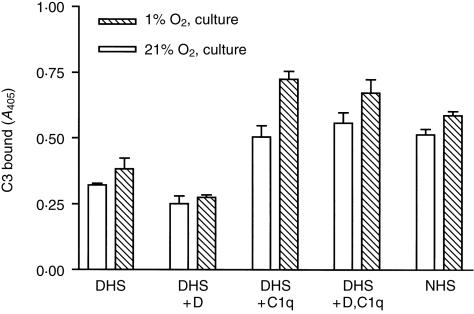
Figure 2.
C3 binding by HUVEC following hypoxia/reoxygenation requires C1q. HUVEC were cultured overnight in 21% oxygen or 1% oxygen, incubated with HBSS for 1 hr in 21% oxygen and cultured in 21% oxygen for 3 hr. Following treatment, HUVEC were incubated with 10% serum for 30 min at 37°, washed and fixed C3 binding was then measured by a C3-specific ELISA. The serum used was NHS, DHS, DHS reconstituted with C1q, factor D or both. The means± SEM of triplicate wells from one of four experiments are shown.
Flow cytometric assay for complement activation
HUVEC were cultured overnight in gelatin-coated flasks in 21% or 1% oxygen or in uncoated flasks in the absence of serum and growth factors (starved). HUVEC released from the flasks in starved cultures were used directly. Control cells and low oxygen cultures were released by brief trypsinization [0·05% trypsin, 1 mm ethylenediamine tetraacetic acid (EDTA) in phosphate-buffered saline]. Cells were washed with GVB++ and incubated with 10% serum in GVB++ for 30 min at 37°. Cells were stained with 2 µg monoclonal antibodies (mAb) to C1q or C3d and second antibody (PE- or APC-conjugated goat anti-mouse IgG), and analysed on a FACSCalibur flow cytometer with Cell Quest Software (Becton-Dickinson, Mountain View, CA). Fluorescence was measured on a log scale and expressed as geometric mean channel fluorescence (GMFI). Where indicated propidium iodide (PI, 5 µg/ml) was added prior to analysis and cells stained with PI were excluded.
Flow cytometric assays for apoptosis
Cells were washed and resuspended in 10 mm HEPES-buffered saline containing 2·5 mm CaCl2, then incubated with 0·5 µg/ml FITC-Annexin V and 5 µg/ml PI for 15 min in the dark. Cells with permeable membranes stain brightly with PI. Early apoptotic cells exclude PI, but stain with FITC-Annexin V.12
Nuclear changes associated with apoptosis were determined by quantification of the subdiploid peak after staining permeabilized cells with PI.13 Cells were stained for 1 hr on ice with 50 µg/ml PI in 0·1% sodium citrate, 0·1% Triton-X-100 prior to analysis.
Complement activation on HUVEC using purified components
Cells were incubated sequentially with complement components at 10% NHS concentrations using DGVB++ as the dilution and washing buffer. Control or starved HUVEC were incubated for 30 min on ice with 150 µg/ml IgM or buffer, then washed once. Cells were incubated for 15 min at 30° with 8 µg/ml C1, washed once, incubated for 20 min at 30° with 50 µg/ml C4, washed once, and incubated for 5 min at 30° with 25 µg/ml C2, followed by a 30-min incubation at 37° with C3–9 reagent (10% NHS in GVB−40 mm EDTA). The absence of IgM or IgG in the purified C1 was confirmed by incubating starved HUVEC with C1 and staining with FITC-anti-human IgM or FITC-anti-human IgG.
Data analysis
P-values were determined by unpaired two-tailed t-tests using GraphPad Prism v2·0 (GraphPad Software, San Diego, CA).
Results
Complement activation by HUVEC following hypoxia/reoxygenation is induced by apoptotic cells and mediated by the classical pathway
HUVEC cultured overnight in 1% oxygen and reoxygenated for 1 hr in buffer showed an increase in C3 deposition following incubation in NHS, but not DHS. Low oxygen culture and reoxygenation were both required for this increased C3 deposition which was measured by ELISA. For one representative experiment (of four), the mean ± SEM A405 values (absorbance at 405 nm) were 0·467 ± 0·046 for cells cultured in normal oxygen, 0·321 ± 0·081 for cells cultured in 1% oxygen without reoxygenation and 0·632 ± 0·064 for cells cultured in 1% oxygen with reoxygenation. There was no increase in cell lysis measured using an assay for released lactate dehydrogenase (LDH). However, a small increase (5–10%) in the number of FITC-Annexin V-positive cells was observed in the hypoxia/reoxygenated cultures.
To determine if the increase in apoptotic cells was associated with the increased C3 deposition, the culture conditions were modified to allow apoptosis to proceed. After overnight culture and exposure to oxygen for 1 hr in HBSS, cells were cultured for an additional 3 hr in 21% oxygen. This protocol resulted in an increase in FITC-Annexin V staining, an increase in the number of hypodiploid nuclei (Fig. 1b) and an increase in C3 binding following incubation in NHS, but not heat-inactivated serum (Fig. 1a). C3 binding was not increased by overnight culture in 1% oxygen without reoxygenation.
To confirm that the C3 binding measured by ELISA reflected complement activation and to determine the pathway of complement activation by HUVEC following hypoxia/reoxygenation, DHS lacking C1q and factor D was reconstituted with purified C1q, factor D, or both. C1q was required for C3 deposition on HUVEC and no additional activation was found with factor D, indicating that the increased C3 deposition in reoxygenated cultures was entirely dependent on classical pathway complement activation (Fig. 2).
To determine if apoptosis and complement activation in reoxygenated cultures were related, HUVEC were cultured overnight in 1% or normal oxygen and reoxygenated in the presence of the caspase inhibitor Z-VAD to block apoptosis. C3 ELISA readings for wells incubated in NHS after subtracting the heated serum control were 0·647 ± 0·051 for reoxygenated hypoxic cultures compared to 0·458 ± 0·022 for normoxic cultures (P < 0·01). This increased C3 binding was eliminated in cultures treated with Z-VAD during reoxygenation (A405 0·510 ± 0·031 for reoxygenated hypoxic cultures compared to 0·449 ± 0·026 for normoxic cultures, P > 0·1). The results shown are for one of three experiments with six replicate wells.
The association between apoptosis and complement activation was further assessed using flow cytometry. Following culture and rexogyenation, cells were incubated with 10% NHS or DHS, stained with PI, FITC-Annexin V, mAb to C3d and APC-goat anti-mouse IgG. Cells that stained with PI were excluded. FITC-Annexin V-positive cells (10–15% of the total) accounted for virtually all of the specific C3d binding (anti-C3d staining after incubation in NHS compared to DHS). In three experiments, the ΔGMFI for specific anti-C3d staining of FITC-Annexin V-positive cells was 60·4 ± 1·1 compared to 1·4 ± 1·4 for FITC-Annexin V-negative cells. When NHS-treated cells were analysed based on C3d staining, the GMFI for FITC-Annexin V staining was 234 ± 117 for C3d-positive cells compared to 8 ± 2 for C3d-negative cells. These experiments demonstrate that the cells that activate complement following culture in 1% oxygen and reoxygenation are a subpopulation with membrane changes characteristic of early apoptosis.
Complement activation by apoptotic HUVEC occurs through direct activation of the classical pathway
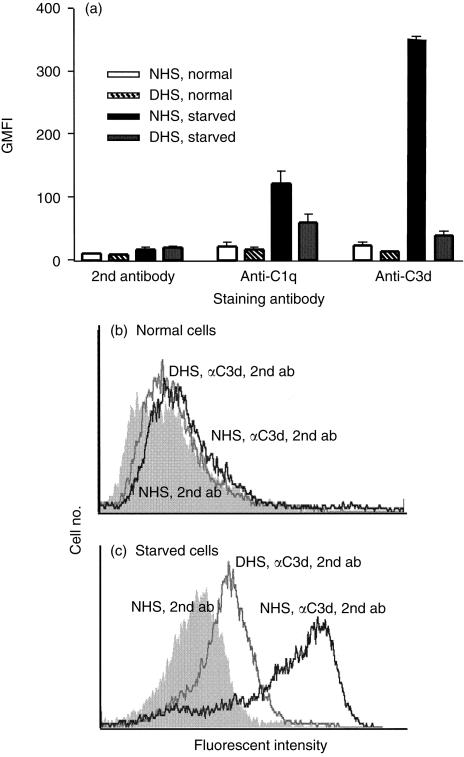
To characterize further the complement activation by apoptotic cells, a uniformly apoptotic population of HUVEC was prepared by serum and growth factor deprivation. Between 60 and 80% of starved cells excluded PI and more than 80% of the PI-negative cells stained with FITC-Annexin V. More than 95% of normal cells excluded PI and less than 5% were FITC-Annexin V-positive. Starved cells and normal cells were incubated with NHS or DHS and complement activation was determined by flow cytometry. Starved HUVEC activated complement and bound anti-C1q and anti-C3d mAb, whereas normal cells did not (Fig. 3). In Fig. 3 the GMFI for mAb staining and second antibody alone is shown for two experiments. Figure 3(b, c) shows representative histograms comparing normal and starved HUVEC incubated with NHS or DHS and stained with anti-C3d and second antibody to cells incubated with NHS and stained with second antibody alone.
Figure 3.
Complement activation by apoptotic HUVEC. HUVEC were cultured overnight in complete EGM (normal) or EGM without serum or growth factors (starved). Cells were incubated with 10% NHS or DHS for 30 min at 37°, washed and stained with mAb specific for C1q or C3d and PE-conjugated goat anti-mouse IgG (second antibody). (a) The GMFI values for cells treated with NHS or DHS and of cells stained with second antibody only are shown. The results shown are the means ± SEM of two experiments. (b,c) Representative histograms of normal and starved cells incubated with NHS (dark line) or DHS (grey line) and stained with anti-C3d and second antibody compared to cells incubated with NHS and stained with second antibody alone (shaded histogram).
The pathway of complement activation was determined by testing the ability of purified C1q, factor D, or both to restore complement activation in DHS (data not shown). C1q was required for C3d deposition on starved HUVEC and no additional activation was found with the addition of factor D indicating that, as in the reoxygenated cultures, complement activation was entirely dependent on the classical pathway.
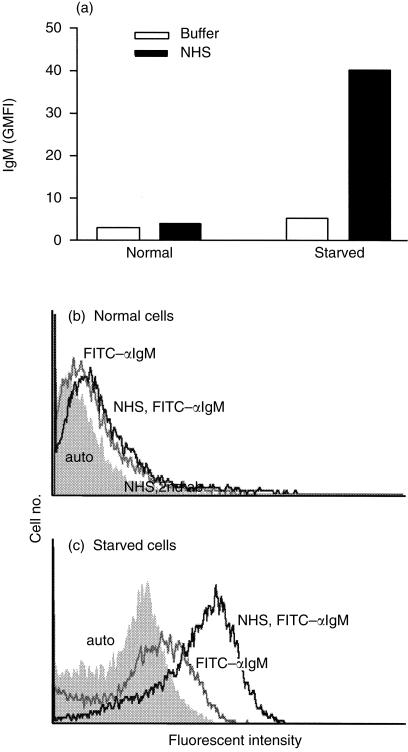
Results using RAG2–/– and C4–/– mice6 suggested that complement activation following reperfusion injury is mediated by natural antibody and the classical pathway. Therefore we tested IgM binding to normal and starved HUVEC by staining with FITC-anti-human IgM after incubation of cells in NHS. IgM binding to starved cells but not normal cells was observed (Fig. 4). Only cells that excluded PI were included in the analysis. IgM binding to early apoptotic cells was confirmed using 150 µg/ml purified IgM in place of NHS (not shown).
Figure 4.
IgM binds to apoptotic HUVEC. HUVEC were cultured overnight in complete EGM (normal) or EGM without serum or growth factors (starved). Cells were incubated with 10% NHS or GVB++ (buffer) for 30 min at 37°, washed and stained with FITC-anti-IgM and PI. PI-positive cells were excluded from the analysis. Representative histograms of normal (a) and starved (b) cells incubated with NHS (dark line) or buffer (grey line) and stained with FITC-anti-IgM compared to unstained cells (shaded histogram).
To determine the role of IgM in classical pathway activation by apoptotic HUVEC, starved HUVEC were incubated with IgM or buffer, and then with purified C1, C4 and C2 to form a C3 convertase. All cells were then treated with C3–9 reagent to allow C3 deposition and C3d binding was determined. C3d binding was similar following incubation in 10% NHS or C142, regardless of the addition of normal human IgM (data not shown). Normal HUVEC did not bind anti-C3d after incubation with 10% NHS, or purified components (data not shown). These experiments demonstrate direct classical pathway activation by apoptotic HUVEC prior to loss of membrane integrity and are consistent with the complement activation observed following hypoxia/reoxygenation. Direct binding of purified C1q to apoptotic but not control HUVEC was confirmed by flow cytometry (data not shown). Together these results show that apoptotic HUVEC directly activate C1 as well as binding IgM. IgM is not required for C3 deposition, but could play a role in generation of inflammatory mediators.
Discussion
HUVEC cultured in 1% oxygen and reoxygenated were studied as a model for the complement activation observed on endothelium in reperfused ischaemic tissue. These findings extend previous results9,15 by demonstrating that induction of apoptotic membrane changes in HUVEC provide the stimulus for complement activation. Since both hypoxia and reoxygenation of HUVEC were carried out prior to the addition of serum, possible effects of oxidants on complement or other serum proteins were eliminated. Complement activation was restricted to the subpopulation of Annexin-binding cells in the cultures.
It has been proposed that activation of xanthine oxidase during ischaemia of endothelium generates reactive oxygen metabolites upon reperfusion, which cause initial damage to the microvascular endothelium.14 Adherence and activation of neutrophils to the endothelium leads to a second wave of oxidant release and tissue destruction. This adhesive interaction is greatly enhanced by stimulation of neutrophils with chemotactic factors, including C5a.16,17 In vivo inhibition of complement activation with soluble complement regulatory proteins or other agents significantly decreases the recruitment of neutrophils to the site of injury.1,3,4 Our results and those of Collard et al.9 suggest that endothelial cells damaged by oxidants directly activate the complement system, providing a possible link between endothelial oxidant generation and neutrophil adhesion and activation.
Both apoptotic and necrotic endothelial cells are found at sites of reperfusion injury. Our results suggest that the changes in HUVEC resulting from oxidant damage in culture are characteristic of apoptosis and that these changes are sufficient to initiate complement activation. The same treatment that led to complement activation by HUVEC also induced apoptosis in 10–25% of the cells. Two findings directly implicate these apoptotic cells in the complement activation. First, addition of Z-VAD during reoxygenation at concentrations that block HUVEC apoptosis,18 completely prevented the increased C3 binding observed in hypoxic cultures. Second, analysis by flow cytometry indicated that C3d was bound exclusively to the 10–25% Annexin V-binding cells in the population of HUVEC following hypoxia/reoxygenation.
A uniformly apoptotic population of HUVEC was generated by serum and growth factor deprivation so that the characteristics of complement activation could be compared to those of reoxygenated cultures. These studies demonstrate direct classical pathway activation by apoptotic HUVEC consistent with the findings in the reoxygenated cultures. Our findings are also consistent with an earlier report of C1q binding to apoptotic blebs on UV-irradiated keratinocytes following incubation in serum or with purified C1q.21 This study did not assess complement activation beyond C1q binding.
Apoptosis is characterized by the exposure of inner leaflet phospholipids, including phosphatidylserine, on the outer membrane of cells. Apoptotic cells as well as erythrocytes with similar phospholipid changes activate complement.19,20 We hypothesize that acidic phospholipids on the surface of apoptotic cells initiate classical pathway activation. Phosphatidylserine and other acidic phospholipids in liposomes have been shown to activate the classical pathway in human serum.22 Although cardiolipin, an acidic phospholipid found in mitochondrial membranes, is a direct activator of C1,23 it is unlikely that release of internal cell membranes or other cellular constituents induced complement activation, because exclusion of PI-permeable cells from the analysis of C3d deposition did not significantly alter the results. LDH release was not increased in cultures after hypoxia/reoxygenation.
Other serum proteins that bind damaged cells could potentially contribute to complement activation. Collard et al. recently reported a possible role for the mannose-binding lectin pathway in complement activation by oxidatively damaged cells.8 However, our results demonstrate a loss of complement activation following C1q and factor D depletion that could be restored completely by purified C1q, indicating that mannose-binding lectin is not required. In addition, we have shown for the first time that serum proteins other than complement are not required for classical pathway activation by apoptotic cells, since C3 deposition occurred following incubation with purified C1, C4 and C2. In a mouse skeletal muscle ischaemia–reperfusion model, IgM was found co-localized with C3 on endothelium and it was proposed that antiphospholipid antibodies induced complement activation.6 Our results demonstrate IgM binding to apoptotic endothelial cells, but show that this binding does not affect the amount of C3 binding in vitro. It is, however, possible that IgM or other serum proteins contribute to the generation of the later inflammatory complement components [C5a and membrane attack complex (MAC)] that are critical for tissue injury. Further studies will be needed to determine the role of antibody and other serum proteins in activation of the terminal part of the complement pathway and regulation of complement activation by injured cells.
Together these studies describe a mechanism for initiation of complement activation on endothelium following reperfusion of ischaemic tissue. In this model membrane changes in response to oxidant damage of endothelial cells are sufficient to induce complement activation directly. Localized complement activation along with induction of cytokine and adhesion molecule synthesis is likely to contribute to the recruitment and activation of neutrophils leading to reperfusion injury.
Abbreviations
- APC
allophycocyanin
- C3-C9
reagent
- normal
human serum chelated with 40 mm EDTA used as a source of C3-C9
- DHS
normal human serum depleted of C1q and factor D
- EGM
endothelial growth medium
- GMFI
geometric mean channel fluorescence
- LDH
lactate dehydrogenase
- NHS
normal human serum
- PE
phycoerythrin
- PI
propidium iodide
- Z-VAD
caspase inhibitor I, Z-Val-Ala-Asp-fluoromethylketone.
References
- 1.Crawford MH, Grover FL, Kolb WP, McMahan A, O'Rourke RA, McManus LM, Pinckard RN. Complement and neutrophil activation in the pathogenesis of ischemic myocardial injury. Circulation. 1988;78:1449–58. doi: 10.1161/01.cir.78.6.1449. [DOI] [PubMed] [Google Scholar]
- 2.Kilgore KS, Friedrichs GS, Homeister JW, Lucchesi BR. The complement system in myocardial ischaemia/reperfusion injury. Cardiovasc Res. 1994;28:437–44. doi: 10.1093/cvr/28.4.437. [DOI] [PubMed] [Google Scholar]
- 3.Pemberton M, Anderson G, Vetvicka V, Justus DE, Ross GD. Microvascular effects of complement blockade with soluble recombinant CR1 on ischemia/reperfusion injury of skeletal muscle. J Immunol. 1993;150:5104–13. [PubMed] [Google Scholar]
- 4.Weisman HF, Bartow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 5.Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion. Role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–67. doi: 10.1161/01.cir.97.22.2259. [DOI] [PubMed] [Google Scholar]
- 6.Weiser MR, Williams JP, Moore Fd, Jr, Kobzik L, Ma M, Hechtman HB, Carroll MC. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183:2343–8. doi: 10.1084/jem.183.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heller T, Hennecke M, Baumann U, et al. Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol. 1999;163:985–94. [PubMed] [Google Scholar]
- 8.Collard CD, Lekowski R, Jordean JE, Agah A, Stahl GL. Complement activation following oxidative stress. Mol Immunol. 1999;36:941–8. doi: 10.1016/s0161-5890(99)00116-9. 10.1016/s0161-5890(99)00116-9. [DOI] [PubMed] [Google Scholar]
- 9.Collard CD, Agah A, Stahl GL. Complement activation following reoxygenation of hypoxic human endothelial cells: role of intracellular reactive oxygen species, NF-κB and new protein synthesis. Immunopharmacology. 1998;39:39–50. doi: 10.1016/s0162-3109(97)00096-9. 10.1016/s0162-3109(97)00096-9. [DOI] [PubMed] [Google Scholar]
- 10.Praz F, Barreira MC, Lesavre P. A one-step procedure for preparation of classical pathway (C1q) and alternative pathway (factor D) depleted human serum. J Immunol Meth. 1982;50:227–31. doi: 10.1016/0022-1759(82)90229-0. [DOI] [PubMed] [Google Scholar]
- 11.Catana E, Schifferli JA. Purification of human complement factor D from the peritoneal fluid of patients on chronic ambulatory peritoneal dialysis. J Immunol Meth. 1991;138:265–71. doi: 10.1016/0022-1759(91)90175-f. [DOI] [PubMed] [Google Scholar]
- 12.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Meth. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 13.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Meth. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 14.Bulkley GB. Physiology of reactive oxidant-mediated signal transduction: an overview. Biochem Soc Trans. 1997;25:804–12. doi: 10.1042/bst0250804. [DOI] [PubMed] [Google Scholar]
- 15.Collard CD, Väkevä A, Büküsoglu C, Zünd G, Sperati CJ, Colgan SP, Stahl GL. Reoxygenation of hypoxic human umbilical vein endothelial cells activates the classic complement pathway. Circulation. 1997;96:326–33. doi: 10.1161/01.cir.96.1.326. [DOI] [PubMed] [Google Scholar]
- 16.Birdsall HH, Green DM, Trial J, et al. Complement C5a, TGF-β1, and MCP-1 in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–92. doi: 10.1161/01.cir.95.3.684. [DOI] [PubMed] [Google Scholar]
- 17.Kukielka GL, Youker KA, Michael LH, Kumar AG, Ballantyne CM, Smith CW, Entman ML. Role of early reperfusion in the induction of adhesion molecules and cytokines in previously ischemic myocardium. Mol Cell Biochem. 1995;147:5–12. doi: 10.1007/BF00944777. [DOI] [PubMed] [Google Scholar]
- 18.Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J Biol Chem. 1998;273:35371–80. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- 19.Wang RH, Phillips G, Jr, Medof ME, Mold C. Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. J Clin Invest. 1993;92:1326–35. doi: 10.1172/JCI116706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes. Complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 22.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–41. [PubMed] [Google Scholar]
- 23.Kovacsovics T, Tschopp J, Kress A, Isliker H. Antibody-independent activation of C1, the first component of complement, by cardiolipin. J Immunol. 1985;135:2695–700. [PubMed] [Google Scholar]