Abstract
Whether intermediate TCR (TCRint) cells and natural killer T (NKT or NK1.1+TCRint) cells are extrathymically generated remains controversial. This arises from the fact that there are few of these T cells in athymic nude mice and neonatally thymectomized mice. However, when athymic mice were provided with appropriate microenvironments or stimulation, many TCRint cells (mainly NK1.1−) were found to arise in the liver. NKT cells are known to be positively selected by monomorphic major histocompatibility complex (MHC) -like antigens (e.g. CD1d). This is true even if they are CD4+. In other words, a MHC class I-like antigen is restricted to CD4 antigen. This rule is somewhat different from that seen in conventional T cells (i.e. the restriction of class II with CD4 and that of class I and CD8). In the case of NK1.1−TCRint cells, they were selected by polymorphic MHC antigens, but their MHC restriction to CD4 or CD8 antigen was incomplete. This was revealed by experiments of bone marrow transfer with class I (bm 1) or II (bm 12) disparity. Depending on the disparity, a unique cytokine profile in sera was detected. These results suggest that the development of T lineage lymphocytes and MHC restriction to CD4 and CD8 might have occurred in parallell as a phylogenic event, and that NK1.1− extrathymic T cells (i.e. NK1.1−TCRint) are at an intermediate position between NKT cells and conventional T cells in phylogeny.
Introduction
Unusual T cells, such as double-negative (DN) CD4– CD8– T cells or T cells coexpressing a natural killer (NK) marker (NK1.1), i.e. natural killer T (NKT) cells, have been found in the thymus of mice,1–3 although these T cells are also present in other immune organs. In contrast to conventional T cells, which are generated through the mainstream of T-cell differentiation in the thymus, these DN (and CD4+) NKT cells are generated by an alternative intrathymic pathway. Subsequent studies have revealed that NKT cells recognize some antigens in the context of monomorphic major histocompatibility complex (MHC) -like (or MHC) antigens (e.g. CD1d and TL)4,5 and use a restricted T-cell receptor (TCR) repertoire of Vα14Jα281 and Vβ8.2 (or Vβ7 or Vβ2).6 On the other hand, we have independently characterized intermediate TCR (TCRint) cells which, similarly to NK cells, consistently express interleukin-2 receptor β (IL-2Rβ).7 These T cells are abundant in the liver and are considered to be of extrathymic origin.
A subsequent study has shown that IL-2Rβ+ TCRint cells in the liver comprise both NK1.1+ and NK1.1– subsets at a ratio of approximately 1:1.8 Moreover, similar NK1.1+ TCRint (i.e. NKT cells) and NK1.1– TCRint populations are found even in the thymus. This raises two questions as follows: whether all NK1.1+ TCRint and NK1.1– TCRint cells in the liver are supplied by the thymus and how NK1.1+ TCRint and NK1.1– TCRint cells are regulated by MHC antigens in their differentiation. The first question arises from the fact that there are few of these unusual T cells in the liver of athymic nude mice9 and neonatally thymectomized mice.10 The second question stems from the fact that there is little information about the regulation of NK1.1– TCRint cells by MHC antigens. On the other hand, NKT cells are characterized as being regulated by non-classical MHC-like antigens.4,5 In detail, NKT cells recognize some peptide antigens11 or glycolipid antigens (e.g. α-galactosylceramide) in the context of CD1d.12 However, some NKT cells appear to recognize some antigens in the context of non-CD1d molecules.13
In this study, we examined what conditions are appropriate for the induction of extrathymic T cells in athymic nude mice. We then examined what types of MHC antigens regulate the differentiation of such extrathymic T cells. For this purpose, we investigated whether IL-2Rβ+ TCRint cells (including the NK1.1+ and NK1.1– subsets) recognize the point mutations on polymorphic MHC antigens (i.e. bm 1 and bm 12 mutations). In bm 1 mice, there are three point mutations on the polymorphic MHC class I antigen (i.e. H-2Kbm1),14 while in bm 12 mice there are three such mutations on the polymorphic MHC class II antigen (i.e. I-Abm12).15 Intensive studies have thus far indicated that the disparity of bm 1 is recoginzed by CD8+ conventional T cells and results in acute graft-versus-host disease (GVHD) after bone marrow transplantation (BMT; bm 1 → B6 and vice versa).16 The disparity of bm 12 is recognized by CD4+ conventional T cells and results in chronic GVHD after BMT (bm12 ↔ B6). We can thus expect to determine whether extrathymic T cells recognize the MHC antigen disparity of bm 1 or bm 12.
Materials and methods
Mice
C57BL/6 (B6) and B6-nu/nu mice were used at the age of 8–40 weeks. These mice were originally obtained from Charles River Japan, Tokyo, Japan and were maintained in the animal facility of Niigata University under specific pathogen-free conditions. In some experiments, B6-C-H-2bm1 (bm 1) mice (i.e. H-2Kbm1) and B6-C-H-2bm12 (bm 12) mice (i.e. I-Abm12) were used at the age of 10–12 weeks. These mice were Ly5.2+. F1 mice were also prepared from bm 1 or bm 12 mice in conjunction with B6.Ly5.1 mice. These (bm 1 × B6.Ly5.1) F1 or (bm 12 × B6.Ly5.1) F1 mice were used at the age of 10–12 weeks.
Administration of interleukin-12
At the age of 40 weeks, the nu/nu mice were intraperitoneally injected with murine interleukin-12 (IL-12; 103 U/mouse) (Genzyme Corporation, Cambridge, MA)
Immunofluorescence tests
The phenotypes of cells were determined by two- or three-colour immunofluorescence tests.8 For flow cytometric analysis, anti-CD3 (145-2C11), anti-IL-2Rβ (TM-β1), anti-NK1.1 (PK136), anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-CD8β (53-5.8), anti-TCRαβ (H57-597), and anti-TCRγδ (GL3) monoclonal antibodies (mAb) were purchased from PharMingen (San Diego, CA). Mouse anti-Ly5.1 (A20.1.7) mAb was provided by Dr T. Kina (Kyoto University, Japan). All mAbs were used in a fluorescein isothiocyanate- (FITC), phycoerythrin- (PE), or biotin-conjugated form. Biotinylated reagents were developed with TRI-COLOR-conjugated streptavidin (Caltag Lab., San Francisco, CA). Cells were analysed by fluorescence-activated cell sorter (FACScan; Becton Dickinson Co., Mountain View, CA). To prevent non-specific binding of mAb, CD32/16 (2·4G2) was added before staining with labelled mAb.
Cell transfer experiments
B6-nu/nu mice were irradiated with a sublethal dose (4 Gy), and 107 bone marrow cells, obtained from mutant mice, were injected 1 day after the irradiation. Bone marrow cells were isolated from B6, bm 1, bm 12 (bm 1 × Ly5.1) F1, and (bm 12 × Ly5.1) F1 mice and were depleted of Thy-1+ cells by the complement-mediated cell lysis. These bone marrow cells were injected intravenously.
Identification of serum levels of interferon-γ and IL-4 by ELISA
Sera were harvested and levels of interferon-γ (IFN-γ) and IL-4 were determined by enzyme-linked immunosorbent assay (ELISA) as described previously.17,18
Results
Induction of NK1.1– CD3int cells in the liver of athymic nude mice, with aging or by the injection of IL-12
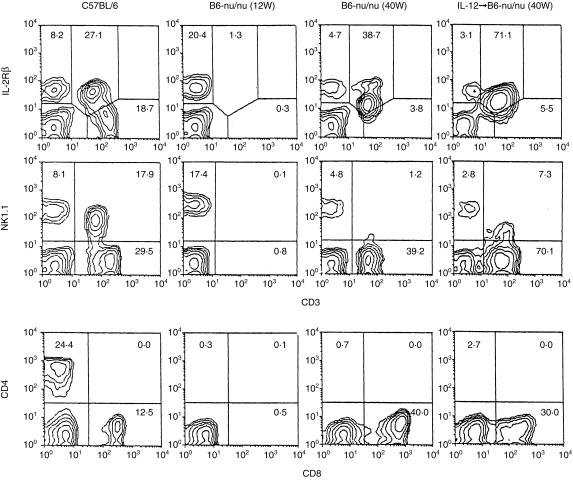
To determine whether TCRint or CD3int cells are generated in athymic nude mice, young (12-week-old) and old (40-week-old) B6-nu/nu mice, and old nu/nu mice injected with IL-12 (103 U/mouse), were examined in parallel with young B6 mice (Fig. 1). Two-colour staining for CD3 and IL-2Rβ, for CD3 and NK1.1 and for CD4 and CD8 was conducted.8 Although the proportion of IL-2Rβ+ CD3int cells was small (1·3%) in the liver of young nu/nu mice, it increased (38·7%) in the liver of old nu/nu mice and such mice injected with IL-12 (71·1%). Two-colour staining for CD3 and NK1.1 and for CD4 and CD8(α) showed that the majority of CD3int cells in old nu/nu mice were CD8+ NK1.1–. A few NK1.1+ subsets of CD3int cells appeared in the expanding CD3int cells in old nu/nu mice injected with IL-12. They were CD8+ (data not shown). On the other hand, NK1.1– CD3int cells in these mice were a mixture of CD8+ (30%) and DN CD4– CD8– (40%).
Figure 1.
Athymic B6-nu/nu mice have the ability to produce IL-2Rβ+ CD3int cells. B6 and B6-nu/nu mice were used at the age of 12 weeks. The nu/nu mice at the age of 40 weeks were intraperitoneally injected with murine IL-12 (103 U/mouse) and were examined on day 3. Two-colour staining for CD3 and IL-2Rβ, for CD3 and NK1.1 and for CD4 and CD8 were conducted. Numbers in the figure represent the percentages of fluorescence-positive cells. The data shown here are representative of three experiments.
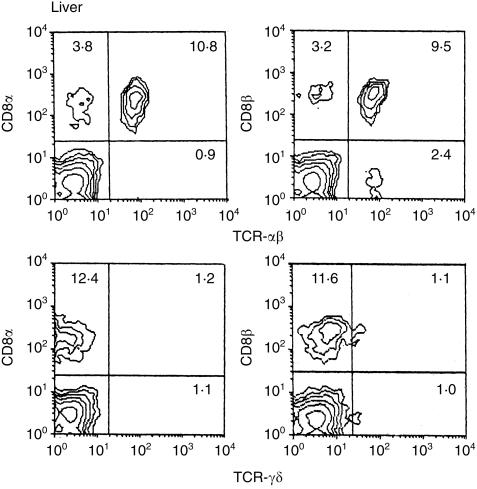
We further examined the phenotypes of these cells, in terms of TCR and CD8, in the liver of adult (25-week-old) nu/nu mice (Fig. 2). The majority were CD8α+β+ αβTCR+ cells, while there were few γδTCR+ cells.
Figure 2.
Further phenotypic characterization of CD3int cells. Two-colour staining for αβTCR and CD8α (or CD8β) and for γδTCR and CD8α (or CD8β) were conducted. In this experiment, nu/nu mice at the age of 25 weeks were used and lymphocytes in the liver were examined.
Prominent expansion of NK1.1– CD3int cells in the liver, when athymic mice were sublethally irradiated and were subjected to BMT with MHC disparity
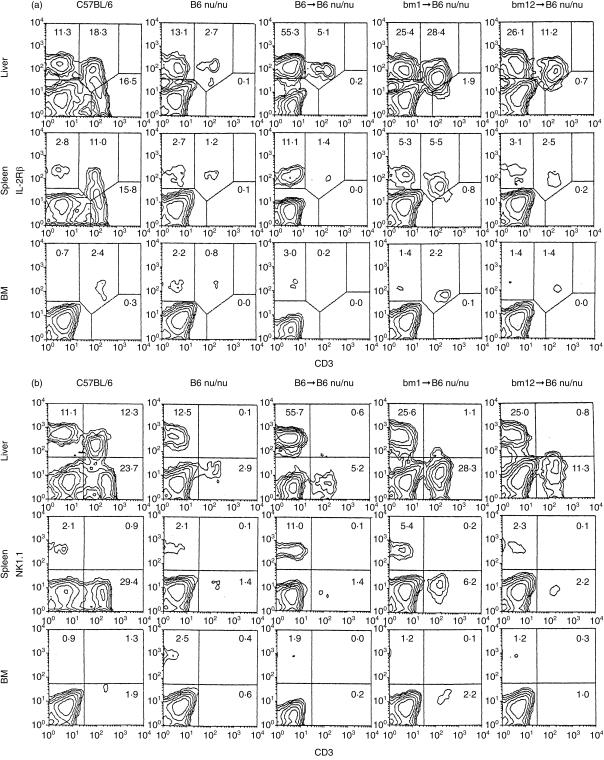
We conducted BMT from B6, bm 1, or bm 12 mice (depleted of Thy-1+ cells) into sublethally irradiated (4 Gy) nu/nu mice (8-week-old). Under these experimental conditions, either donor or recipient cells were recovered and gave rise to lymphocytes. Two weeks after BMT, lymphocytes were obtained from the liver, spleen and bone marrow and their phenotype was identified (Fig. 3). Two-colour staining for CD3 and IL-2Rβ showed that the expansion of NK cells and CD3int cells had begun in the liver of these BMT mice (Fig. 3a). Although the expansion of NK cells was prominent, that of CD3int cells was not so prominent in B6 → B6 nu/nu mice. In sharp contrast, the expansion of CD3int cells was prominent in bm 1 → B6 nu/nu mice and in bm 12 → B6 nu/nu mice. Similar to the case of control nude mice, these BMT mice lacked IL-2Rβ– TCRhigh conventional T cells which were seen in control euthymic B6 mice.
Figure 3.
Generation of NK1.1– CD3int cells by positive selection of the mutation on polymorphic MHC antigens. (a) Two-colour staining for CD3 and IL-2Rβ. (b) Two-colour staining for CD3 and NK1.1. B6 and B6-nu/nu mice were used at the age of 10 weeks as controls. B6-nu/nu mice at the age of 8 weeks were irradiated (4 Gy) and were injected with 107 bone marrow cells of bm 1 or bm 12 origin. Immunofluorescence tests of lymphocytes in the liver, spleen and bone marrow (BM) were performed on day 14 after BMT. The data shown here are representative of five experiments.
We examined which subset of CD3int cells expanded in these BMT (bm 1 and bm 12) nu/nu mice (Fig. 3b). In both groups of mice, the majority of CD3int cells were NK1.1–. In the case of control B6 mice, 67% (12·3/18·3) of CD3int cells were NK1.1+.
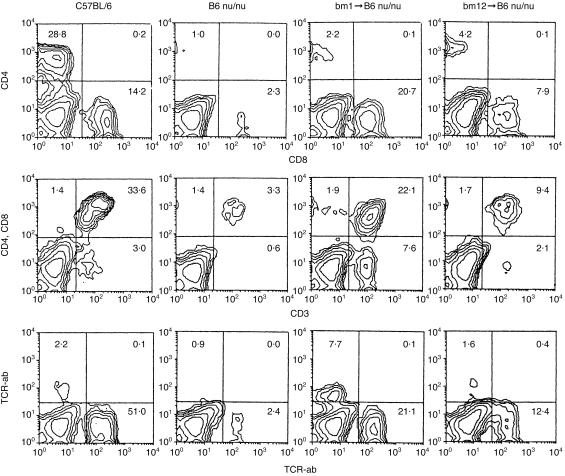
Further characterization of expanding T cells in the liver was conducted by two-colour staining for the indicated combinations (Fig. 4). In the case of bm 1 BMT mice, the major expanding cells were CD8+ (20·7%) and DN (7·6%). On the other hand, a mixture of CD4+ (4·2%) and CD8+ (7·9%) cells expanded in bm 12 BMT mice. Both αβT (21·1%) and γδT (7·7%) cells expanded in bm 1 BMT mice, whereas only αβT cells expanded in bm 12 BMT mice.
Figure 4.
Further phenotypic characterization of lymphocytes in nude mice subjected to BMT with the disparity of MHC class I or II antigens was performed. Two-colour staining for various combinations as indicated in the figure was conducted. Immunofluorescence test of lymphocytes in the liver was performed on day 14 after BMT. The data shown here are representative of five experiments.
In a final set of experiments, we determined the distribution of CD4+, DN CD4– CD8– and CD8+ cells among expanding T cells in the liver (Table 1). Normal B6 mice and B6 nude mice were examined in parallel. It was found that the number of CD3+ cells (0·09 × 106/mouse) in the liver of nude mice was fewer than that (0·66 × 106/mouse) in the liver of control B6 mice. However, bm 1 BMT nude (1·68 × 106/mouse) and bm 12 BMT (0·75 × 106/mouse) nude mice showed a high level of the number of CD3+ cells (all CD3int cells) in the liver.
Table 1.
Distribution of CD3+ cells with CD4+, DN CD4–CD8– and CD8+ phenotypes in the liver
| % Positive cells among CD3+ cells | ||||
|---|---|---|---|---|
| Mouse strain | Absolute no. of CD3int cells ( × 106) | CD4+ | CD4– CD8– | CD8+ |
| B6a | 0·66 ± 0·07 | 64·2 ± 2·3 | 5·9 ± 0·8 | 30·2 ± 1·1 |
| B6-nu/nub | 0·09 ± 0·06 | 27·4 ± 2·4 | 20·4 ± 9·4 | 52·1 ± 7·5 |
| bm1 → B6-nu/nu | 1·68 ± 1·0 | 6·9 ± 1·9 | 26·6 ± 5·6 | 67·7 ± 4·6 |
| bm12 → B6-nu/nu | 0·75 ± 0·67 | 30·5 ± 4·3 | 13·9 ± 9·9 | 55·6 ± 5·7 |
Liver lymphocytes in B6 mice were a mixture of CD3int and CD3high cells.
Liver lymphocytes in nude mice were all CD3int cells.
Four mice were used to produce the mean and one SD in each experiment.
It was also confirmed that CD8+ and DN cells mainly expanded in the liver of bm 1 BMT nude mice while CD4+ and CD8+ cells mainly expanded in the liver of bm 12 BMT nude mice. In this experiment, the proportion of DN CD4– CD8– cells was evaluated by calculation, using the values of CD3+ cells, CD4+ cells and CD8+ cells which were produced by two-colour staining. Since the absolute number of NK1.1– CD3int cells yielded by the liver of bm 1 and bm 12 BMT mice was apparently greater than that by the liver of control B6 nude mice, the numbers of CD8+ and DN cells in bm 1 BMT nude mice and of CD4+ and CD8+ cells in bm 12 BMT nude mice were substantial.
Determination of the recipient or donor origin in the expanding T cells
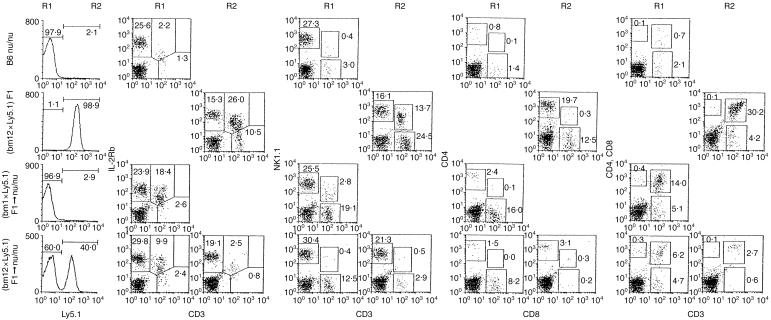
To determine the interaction of lymphocytes between donor and recipient origins, we prepared (bm 1 × Ly5.1) F1 and (bm 12 × Ly5.1) F1 mice.19 When bone marrow cells from these F1 mice were injected into irradiated nu/nu mice, we were able to distinguish the origins of recovering lymphocytes by the expression of Ly5.1 or Ly5.2 alloantigen. We conducted Ly5.1 staining in the liver and spleen of control and BMT mice (Fig. 5). Lymphocytes of nu/nu mice (Ly5.2+) lacked Ly5.1 while all those of (bm 12 × Ly5.1) F1 and (bm 1 × Ly5.1) F1 mice (data not shown here) expressed Ly5.1. There was a big difference in the expression of Ly5.1 between (bm 1 × Ly5.1) F1 → nu/nu mice and (bm 12 × Ly5.1) F1 → nu/nu mice. These stainings were done on day 21 after BMT. The majority of recovering cells were of recipient origin in (bm 1 × Ly5.1) F1 → nu/nu mice. In contrast, the recovering cells were a half-and-half mixture of donor and recipient origins in (bm 12 × Ly5.1) F1 → nu/nu mice.
Figure 5.
Three-colour staining of Ly5.1, CD3 and IL-2Rβ and those of the other indicated combinations in the liver of mice. B6 nu/nu, (bm 12 × Ly5.1) F1 mice, (bm 1 × Ly5.1) F1 → B6 nu/nu mice, and (bm 12 × Ly5.1) F1 → B6 nu/nu mice were used.
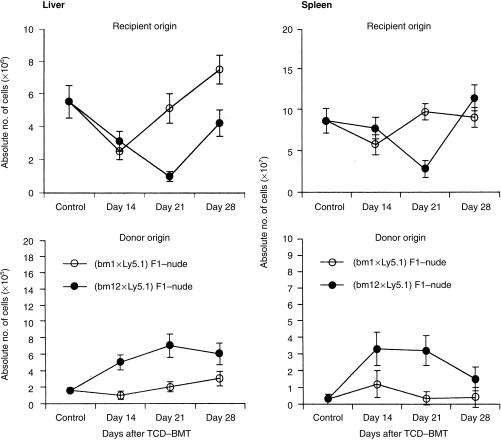
We also performed a time-kinetic study by repeated experiments as indicated in Fig. 5 (Fig. 6). In the liver and spleen, a similar pattern was observed. In the case of (bm 1 × Ly5.1) F1 → nu/nu mice, the recovery of recipient cells was rapid but that of donor cells was suppressed. In the case of (bm 12 × Ly5.1) F1 → nu/nu mice, the recovery of recipient cells was rather suppressed and that of donor cells was substantial.
Figure 6.
A time-kinetic study on the recovery of recipient or donor cells after BMT. Bone marrow cells (depleted of Thy-1+ cells, 107/mouse) from either (bm 1 × Ly5.1) F1 or (bm 12 × Ly5.1) F1 mice were injected into 4 Gy-irradiated nu/nu mice at the age of 8 weeks. The origin of lymphocytes was determined by the gated analysis of Ly5.1. This result was representative of day 21. The repeated experiments of Fig. 5. were conducted on days 14, 21 and 28. The mean and one SD at each time-point are produced from four mice.
Serum levels of IFN-γ and IL-4 in athymic nude mice after the subjection to BMT of bm 1 or bm 12 origin
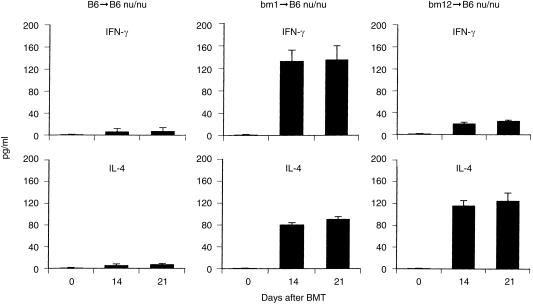
In a final element of the present experiments, we examined what types of cytokines were released into the sera by the interaction of NK1.1– CD3int cells with class I or class II disparity cells (Fig. 7). In the case of bm 1 BMT (bm 1 → B6-nu/nu), high titres of both IFN-γ and IL-4 were detected on days 14 and 21 in the sera of nude mice. On the other hand, a high titre of only IL-4 was induced in the sera of nude mice subjected to bm 12 BMT (bm 12 → B6-nu/nu). Since control mice (B6 → B6-nu/nu) did not show any positive levels of IFN-γ and IL-4 in the sera, the positive results of bm 1 → B6-nu/nu and bm 12 → B6-nu/nu mice were suggested to be derived from the interaction of bm 1 and bm 12 mutations with the recipient mice.
Figure 7.
Serum levels of IFN-γ and IL-4 in B6-nu/nu mice with BMT of bm 1 origin (bm 1 → B6-nu/nu) and of bm 12 origin (bm 12 → B6-nu/nu). Sera were obtained from four mice at the indicated time-points. The mean and one SD are represented.
Discussion
In the present study, new evidence regarding several points emerged. Euthymic mice carried a large number of IL-2Rβ+ TCRint and NK1.1+ TCRint cells (i.e. NKT cells) in the liver. On the other hand, athymic nude mice, especially in youth, produced neither IL-2Rβ+ TCRint cells nor NK1.1+ TCRint cells even in the liver. However, with aging, athymic mice produced IL-2Rβ+ TCRint cells but still did not produce NK1.1+ TCRint cells in the liver. Namely, the majority of TCRint cells were the NK1.1– subset (CD8α+β+ or DN). The microenvironments in the liver or a lack of thymic influence might be associated with this unique profile seen in athymic nude mice. The absence of IL-2Rβ– TCRhigh conventional T cells was confirmed in athymic mice under all tested conditions. In the case of NKT cells, a few CD8+ NKT cells emerged, but CD4+ (or DN) NKT cells did not emerge in athymic mice. In all tested conditions, the usage of an invariant chain of Vα14Jα281 was not detected in these athymic mice by reverse transcription-polymerase chain reaction (data not shown).
We then investigated whether the precursors of extrathymic T cells could recognize polymorphic MHC class I or II antigens. Even in young athymic mice, many IL-2Rβ+ TCRint cells, especially the NK1.1– subset, expanded in the liver but not in the bone marrow or the spleen. There was proof of the recognition of polymorphic MHC class I and II antigens by extrathymic T cells during differentiation. Firstly, the disparity of class I antigens induced the expansion of CD8+ and DN cells of recipient origin, while that of class II antigens induced the expansion of CD4+ and CD8+ cells, and secondly, the disparity of class I antigens suppressed the expansion of lymphocytes of donor origin, while that of class II did not do so.
Young athymic mice carried very few TCRint cells. Several reasons for this are possible. Firstly, TCRint cells are generated by an alternative intrathymic pathway20 and TCRint cells in the thymus consistently migrate into the liver of euthymic mice but not athymic mice. Secondly, TCRhigh conventional T cells migrate to the liver and stimulate the expansion of TCRint cells21 and thirdly, the microenvironments which support the differentiation of TCRint and NKT cells are defective in the liver of athymic mice.
There is a possibility that all three of these causes are involved. However, based on the present results, we emphasize the third reason. If appropriate microenvironments or stimulation had existed at the site for the differentiation (e.g. the stimulation with polymorphic MHC antigens), athymic mice would have begun to produce these TCRint cells. Cumulative evidence has suggested that extrathymic T cells seen in the liver might be more primordial than conventional T cells in phylogeny.22 Such primordial properties include a consistent expression of IL-2Rβ, similar to the case of NK cells, the expression of a NK marker (NK1.1) on a restricted population, the incompleteness of negative selection and the existence of forbidden clones estimated by the minor lymphocyte stimulating (MIS) system,23 and the use of monomorphic MHC-like antigens (e.g. CD1d) for their antigen recognition.4,5
Under all conditions tested in this study, CD4+ NKT cells, which use an invariant chain of Vα14Jα281, were not generated in athymic nude mice. Reflecting the fact of the lack of Vα14+ CD4+ NKT cells in nude mice, no response was induced by the in vivo injection of α-galactosylceramide (our unpublished observation). One possibility is that all CD4+ NKT cells are supplied from the thymus.24 Another possibility is that some CD4+ NKT cells with Vα14Jα281 are generated extrathymically in the liver of euthymic mice.6 This subject remains to be further investigated.
The limited expansion of NK1.1– TCRint cells from the precursors when there was a disparity of polymorphic class I and II antigens taught us that the recognition of polymorphic MHC antigens might be an event mainly involving NK1.1– TCRint cells among non-conventional T cells. However, the restriction of polymorphic class I and CD8+ cells and that of polymorphic class II and CD4+ cells were incomplete in the case of NK1.1– TCRint cells. Namely, the disparity of polymorphic class I accompanied DN cells while that of polymorphic class II accompanied CD8+ cells. This phenomenon is unique when compared with their complete restriction in conventional T cells.16 It should also be mentioned that the mutations on polymorphic class I antigens were recognized by γδT cells as well as by αβT cells, while those on polymorphic class II antigens were recognized by only αβT cells.
The present results may suggest the possible nature of NK1.1– TCRint cells seen in autoimmune-prone mice such as MRL-lpr/lpr mice,25 NOD mice26,27 and neonatally thymectomized mice.28,29 It is speculated that NK1.1– TCRint cells might be generated extrathymically (or by an alternative intrathymic pathway) and have the ability to induce certain autoimmune diseases when the regulatory function of NKT cells decreases.26,27,30 SJL mice, which lack NKT cells, also carry a large proportion of NK1.1– TCRint cells.31,32
To determine the physiological significance of the cell interaction in vivo, we examined the cytokine profile of the sera in nude mice subjected to BMT. The production of both IFN-γ and IL-4 in the case of class I disparity (bm 1 BMT → B6-nu/nu) resembled the T helper 0 (Th0) type of cytokine profile. In contrast, only the production of IL-4 in the case of class II disparity (bm 12 BMT → B6-nu/nu) resembled the Th2 type of cytokine profile. An ability to produce IFN-γ and/or IL-4 by NK1.1– TCRint cells depending on stimulation resembles such ability ascribed to NKT cells.33,34
In any case, we report herein that NK1.1– extrathymic T cells have the ability to recognize polymorphic MHC antigens. Since the strongest recognition of polymorphic MHC antigens is that of conventional T cells, a population of extrathymic T cells might acquire a similar recognition system during phylogenic development. In other words, NK1.1– TCRint cells stand at an intermediate position between NKT cells and conventional T cells in phylogeny.
We could ignore neither the presence of CD8+ NK1.1– TCRint cells (of extrathymic origin) nor their function. In an earlier study, it was reported that some unusual T-cell populations recognize alloantigens and simultaneously induce self-reactive cytotoxicity.35 In this paper, the expression of NK1.1 antigen was not determined. The function of CD8+ NK1.1– TCRint cells might be intimately related to this phenomenon. In future studies, we aim to ascertain whether such CD8+ NK1.1– TCRint cells in the liver are responsible for the induction of the autoimmune-like disorder seen in (C57BL/6 × DBA/2) F1 mice injected with T cells of DBA/2 mice. These F1 mice fall victim to autoimmune liver disease by CD8+ NK1.1– TCRint cells which are activated by injected allogeneic T cells (manuscript in preparation).
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research and Cancer Research from the Ministry of Education, Science and Culture, Japan. We wish to thank Mrs Yuko Kaneko for preparation of the manuscript.
Abbreviations
- BMT
bone marrow cell transfer
- DN
double-negative
- IL-2Rβ
interleukin-2 receptor β-chain
- NKT cells
natural killer T cells
- TCRhigh cells
high T cells
- TCRint cells
intermediate T cells
References
- 1.Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, Pardoll DM. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly expresses a single Vβ gene family. Nature. 1987;329:251–4. doi: 10.1038/329251a0. [DOI] [PubMed] [Google Scholar]
- 2.Budd RC, Miescher GC, How RC, Lees RK, Bron C, MacDonald HR. Developmentally regulated expression of T cell receptor β chain variable domains in immature thymocytes. J Exp Med. 1987;166:577–82. doi: 10.1084/jem.166.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearse M, Gallagher P, Wilson A, Wu L, Fisicaro N, Miller JFAP, Scollay R, Shortman K. Molecular characterization of T-cell antigen receptor expression by subsets of CD4–CD8– murine thymocytes. Proc Natl Acad Sci USA. 1988;85:6082–6. doi: 10.1073/pnas.85.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 5.Joyce S, Negishi I, Boesteanu A, DeSilva AD, Sharma P, Chorney MJ, Loh DY, Kaer LV. Expansion of natural (NK1+) T cells that express αβ T cell receptors in transporters associated with antigen presentation-1 null and thymus leukemia antigen positive mice. J Exp Med. 1996;184:1579–84. doi: 10.1084/jem.184.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makino Y, Yamagata N, Sasho T, Adachi Y, Kanno R, Koseki H, Kanno M, Taniguchi M. Extrathymic development of V α14 -positive T cells. J Exp Med. 1993;177:1399–1408. doi: 10.1084/jem.177.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato K, Ohtsuka K, Hasegawa K, et al. Evidence for extrathymic generation of intermediate TCR cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, Iwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+T cells in various immune organs. NK1.1+T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 9.Emoto M, Emoto Y, Kaufmann SHE. CD8αβ+ TCRαβintermediate lymphocytes expressing skewed TCRVβ repertoire in the liver of aged athymic nu/nu mice. J Immunol. 1997;158:1041–50. [PubMed] [Google Scholar]
- 10.Hammond K, Cain W, Driel IV, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998;10:1491–9. doi: 10.1093/intimm/10.10.1491. 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- 11.Tangri S, Brossay L, Burdin N, Lee DJ, Corr M, Kronenberg M. Presentation of peptide antigens by mouse CD1 requires endosomal localization and protein antigen processing. Proc Natl Acad Sci USA. 1998;95:14314–9. doi: 10.1073/pnas.95.24.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα 14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 13.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NKT cells. J Immunol. 1999;162:6410–19. [PubMed] [Google Scholar]
- 14.Mathenson SG, Geliebter J, Pfaffenbach GM, Zeff RA. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- 15.Flavell RA, Allen H, Huber B, Wake C, Widera G. Organization and expression of the MHC of the C57 black/10 mouse. Immunologic Rev. 1985;84:29–50. doi: 10.1111/j.1600-065x.1985.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 16.Gleichmann E, Pals ST, Rolink AG, Radaszkiewicz T, Gleichmann H. Graft-versus-host reaction: clues to the etiopathology of a spectrum of immunological disease. Immunol Today. 1984;5:324–32. doi: 10.1016/0167-5699(84)90126-9. [DOI] [PubMed] [Google Scholar]
- 17.Kawamura T, Takeda K, Mendiratta SK, Kawamura H, Van Kaer L, Yagita H, Abo T, Okumura K. Critical role of NK1+ T cells in IL-12-induced immune responses in vivo. J Immunol. 1998;160:16–9. [PubMed] [Google Scholar]
- 18.Toyabe S, Seki S, Iiai T, et al. Requirement of IL-4 and liver NK1+ T cells for concanavalin a induced hepatic injury in mice. J Immunol. 1997;159:1537–42. [PubMed] [Google Scholar]
- 19.Suzuki S, Sugahara S, Shimizu T, et al. Low level of mixing of partner cells seen in extrathymic T cells in the liver and intestine of parabiotic mice: Its biological implication. Eur J Immunol. 1998;28:3719–29. doi: 10.1002/(SICI)1521-4141(199811)28:11<3719::AID-IMMU3719>3.0.CO;2-O. 10.1002/(sici)1521-4141(199811)28:11<3719::aid-immu3719>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 20.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 21.Tsukahara A, Moroda T, Iiai T, Suzuki S, Tada T, Hatakeyama K, Abo T. Absolute dependence of TCRhi cell generation and relative dependence of TCRint cell generation on the thymus. Eur J Immunol. 1997;27:361–7. doi: 10.1002/eji.1830270204. [DOI] [PubMed] [Google Scholar]
- 22.Abo T, Watanabe H, Sato K, Iiai T, Moroda T, Takeda K, Seki S. Extrathymic T cells stand at an intermediate phylogenetic position between natural killer cells and thymus-derived T cells. Natural Immunity. 1995;14:173–87. [PubMed] [Google Scholar]
- 23.Kawachi Y, Watanabe H, Moroda T, Haga M, Iiai T, Hatakeyama K, Abo T. Self-reactive T cell clones in a restricted population of IL-2 receptor β+ cells expressing intermediate levels of the T cell receptor in the liver and other immune organs. Eur J Immunol. 1995;25:2272–8. doi: 10.1002/eji.1830250824. [DOI] [PubMed] [Google Scholar]
- 24.Tilloy F, Santo JPD, Bendelac A, Lantz O. Thymic dependence of invariant Vα14+ natural killer-T cell development. Eur J Immunol. 1999;29:3313–8. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. 10.1002/(sici)1521-4141(199910)29:10<3313::aid-immu3313>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Ohteki T, Seki S, Abo T, Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4–8– double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990;172:7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach J-F, Monteiro RC. Overexpression of natural killer T cells protects Vα14-Jα281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammond KJL, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. α/β-T cell receptor (TCR) +CD4–CD8– (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD) /Lt mice by the influence of interleukin (IL) -1 and /or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones LA, Chin LT, Merriam GR, Nelson LM, Kruisbeck AM. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990;172:1277–85. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonomo A, Kehn PJ, Payer E, Rizzo L, Cheever AW, Shevach EM. Pathogenesis of post-thymectomy autoimmunity role of syngeneic MLR-reactive T cells. J Immunol. 1995;154:6602–11. [PubMed] [Google Scholar]
- 30.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1-positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–64. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beutner U, Launois P, Oheki T, Louis JA, MacDonald HR. Natural killer-like T cells develop in SJL mice despite genetically distinct defects in NK1.1 expression and in inducible interleukin-4 production. Eur J Immunol. 1997;27:928–34. doi: 10.1002/eji.1830270419. [DOI] [PubMed] [Google Scholar]
- 32.Murakami M, Paul WE. Age-dependent appearance of NK1.1+ T cells in the livers of β2-microglobulin knockout and SJL mice. J Immunol. 1998;160:2649–54. [PubMed] [Google Scholar]
- 33.Yoshimoto T, Paul WE. CD4+, NK1.1+ T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bendelac A, Schwartz RH. Th0 cells in the thymus: the question of T helper lineages. Immunol Rev. 1991;123:169–88. doi: 10.1111/j.1600-065x.1991.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 35.Glas R, Öhlen C, Höglund P, Kärre K. The CD8+ T cell repertoire in β2-microglobulin-deficient mice is biased towards reactivity against self-major histocompatibility class I. J Exp Med. 1994;179:661–72. doi: 10.1084/jem.179.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]