Abstract
CD14 is a pattern-recognition receptor implicated in the inflammatory response to microbial components such as lipopolysaccharide, peptidoglycan and lipoarabinomannan. In this work, we made use of CD14-deficient (CD14−/−) mice to evaluate the relative importance of CD14 in response to infection with viable, intact cells of Mycobacterium avium in vitro and in vivo. Following co-incubation of either bone marrow-derived macrophages (Mφ) or thioglycollate-elicited peritoneal Mφ from CD14−/− mice with viable M. avium, tumour necrosis factor (TNF) production was significantly reduced and delayed compared to TNF secretion by infected CD14+/+ Mφ. However, following intravenous infection with a M. avium strain of either high virulence (TMC724) or intermediate virulence (SE01), there was no difference in the bacterial loads of lungs, livers or spleens at 3, 5 and 8 weeks postinfection in CD14−/− mice when compared with syngeneic CD14+/+ mice. At these time-points, TNF and interferon-γ (IFN-γ) mRNA expression in the liver was similar in infected CD14+/+ and CD14−/− mice, and granuloma formation and expression of inducible nitric oxide synthase within granuloma Mφ was the same in both mouse groups. In conclusion, although the absence of CD14 results in significantly reduced and delayed TNF production in response to stimulation with M. avium in vitro, there is no evidence that CD14 plays a significant role in either the antibacterial defence or the chronic granulomatous reaction to M. avium infection in vivo.
Introduction
CD14 is a cell-surface glycosyl-phosphatidylinositol (GPI)-anchored 55 000-molecular weight (MW) glycoprotein that is predominantly expressed on myelomonocytic cells.1–3 CD14 is a major acceptor molecule for lipopolysaccharide (LPS) of Gram-negative bacteria, but can also interact with lipoteichoic acids and peptidoglycan of Gram-positive bacteria, and with lipoarabinomannan, a structural component of mycobacteria.4–7 Because of this variety of structurally diverse ligands, CD14 has been termed a ‘pattern-recognition receptor’.8 Engagement of microbial products by CD14 ultimately leads to cellular activation and cytokine secretion, presumably via transducer elements such as toll-like receptors.9,10
CD14 has a pivotal role in the induction and manifestation of septic shock induced by LPS. In vitro, co-incubation of human monocytes with anti-CD14 monoclonal antibodies (mAbs) completely abrogated tumour necrosis factor (TNF), interleukin (IL)-6 and IL-1β secretion following LPS stimulation.6,7 In vivo, transgenic mice overexpressing human CD14 were hypersensitive to LPS.11 Conversely, mice with a targeted disruption of the CD14 gene were resistant to a lethal challenge with LPS.12
CD14 is also involved in the cytokine response triggered by components of the cell wall of Gram-positive bacteria. In vitro, peptidoglycan, lipoproteins, lipopeptides and whole organisms (such as streptococci), triggered TNF and IL-10 production from human monocytes in a CD14-dependent manner.5,13–15 However, in vivo, CD14-deficient (CD14−/−) mice challenged with live Staphylococcus aureus had increased, rather than decreased, TNF serum levels and did not survive significantly longer than infected CD14+/+ mice.16
With respect to mycobacterial infections, CD14 was shown to be a mediator of the chemotactic response of human macrophages (Mφ) to lipoarabinomannan (LAM; see ref. 17). In monocytic cell lines stimulated with LAM, TNF and IL-1β release were completely inhibited by mAbs to CD14.18 The role of CD14 in response to viable mycobacteria in vitro and to mycobacterial infection in vivo has thus far not been established.
Mycobacterium avium is the most prevalent opportunistic infection in patients with acquired immune deficiency syndrome (AIDS), but also infects immunocompetent individuals, in particular children and patients suffering from pulmonary disorders.19 Experimental infection with M. avium in mice is a highly suitable model for investigating both the virulence and persistence of the micro-organism and the immunopathology induced in the course of infection.20,21 Indeed, inflammatory lesions in M. avium-infected mice reflect the full spectrum of granuloma differentiation known to occur in human patients.20,22
TNF has been shown to be critically involved in both the granulomatous and the protective host response in murine models of mycobacterial infection, particularly with M. tuberculosis and M. bovis bacillus Calmette–Guérin (BCG).23,24 Following infection with M. avium, TNF neutralization resulted in a small, but significant, increase in organ bacterial load.25 Importantly, in severe combined immunodeficiency (SCID) mice infected with M. avium, anti-TNF treatment was associated with a complete abrogation of granuloma formation.26
We sought to establish the role of CD14 in cytokine production necessary for the antibacterial protection against, and the chronic inflammatory response to, mycobacterial infection. We therefore measured TNF levels induced in Mφ from CD14−/− mice stimulated with viable M. avium, and infected CD14−/− mice with M. avium to compare the bacterial growth and histopathology of infected organs to the course of infection in CD14+/+ mice. We now report that CD14−/−mice are as capable of mounting a protective and granulomatous response to mycobacterial infection as infected, syngeneic controls.
Materials and methods
Mice
CD14−/− mice were obtained as described previously.12,27 The mice used in these studies are sixth generation backcrosses onto a BALB/c background and were genotyped to confirm the presence of the gene disruption and the presence of the susceptible allele of the NRAMP1 gene, as previously described.28 CD14−/− and syngeneic BALB/c (CD14+/+) mice (Harlan-Sprague Dawley, Indianapolis, IN) were housed under specific pathogen-free conditions in the animal facilities of the North Shore University Hospital (Manhasset, NY). For the course of M. avium infection, age- and gender-matched CD14+/+ and CD14−/− mice were housed in isolator cages under barrier conditions at the Borstel Research Center (Borstel, Germany). Experiments involving living animals were performed in accordance with local ethical guidelines.
Bacteria
M. avium TMC724 (originally obtained from Dr F. Collins, Trudeau Institute, Saranac Lake, NY) and M. avium SE01 (an isolate from the blood culture of an AIDS patient) were cultured to mid-logarithmic phase in Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI) supplemented with OADC (oleic acid, albumin, dextrose, catalase; Becton-Dickinson, Heidelberg, Germany). Aliquots were frozen at −70° until required.
Stimulation of bone marrow-derived Mφs
Bone marrow-derived Mφs were obtained by culturing bone marrow cells from BALB/c or CD14−/− mice, obtained by flushing the femurs with Hanks' balanced salt solution (HBSS) (Life Technologies, Karlsruhe, Germany). To remove fibroblasts, the cells were cultured overnight in cell culture dishes (Nunc, Wiesbaden, Germany) in Dulbecco's modified Eagle's minimal essential medium (DMEM) (Life Technologies) supplemented with 10 mm HEPES, 1 mm sodium pyruvate, 10 mm glutamine, 10% of heat-inactivated Myoclone calf serum (Life Technologies) and 50 ng/ml of Mφ colony-stimulating factor (M-CSF; PeproTech, Princeton, NJ). The non-adherent cells were collected with warm HBSS medium and cultured for 9 days in the presence of 50 ng/ml of M-CSF. Each well, containing 0·5 × 106 Mφ, was infected with 0·5 ml of DMEM containing 0·5–50 × 106 colony-forming units (CFU) of M. avium TMC724 or SE01. After infection, the cultures were washed with HBSS to remove unphagocytosed bacteria. Bacterial LPS of Salmonella enterica, serotype friedenau H909, was kindly provided by Dr H. Brade (Research Center Borstel, Germany) and used as a control stimulus at 10 ng/ml. Cell-free supernatants were collected at 6 and 24 hr postinfection, and mouse TNF concentrations in the supernatants were measured by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's instructions (R & D Systems, Abingdon, UK). The lower limit of detection for TNF was 30 pg/ml.
Stimulation of thioglycollate-elicited murine peritoneal Mφ
Eight-week-old female CD14−/− mice or control BALB/c mice were injected intraperitoneally with 3 ml of 3% (wt/vol) Brewer thioglycollate broth (Difco). Four days later, cells were harvested by peritoneal lavage with 10 ml of RPMI-1640 (Gibco BRL, Gaithersburg, MD) containing 2 mm l-glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin. The cells were washed twice in RPMI-1640, resuspended in the above medium supplemented with 1% autologous serum and then added to the wells (5 × 105 Mφ/well) of a 24-well tissue culture plate (Nunc Inc., Naperville, IL). The cells were incubated for 3 hr at 37° in a humidified 5% CO2 incubator to allow the Mφ to adhere to the plates. The wells were then washed twice with 1 ml of medium before treatment with different concentrations of mycobacteria. Briefly, aliquots of M. avium TMC724 and SE01 were thawed, washed twice in RPMI-1640 and diluted in the above media to the indicated concentrations and then added to adherent Mφ (0·5 ml/well); 1% autologous serum was added to each well simultaneously. Following a 4-hr incubation, cell-free supernatants were collected and assayed by ELISA for both mouse TNF (Genzyme, Cambridge, Massachusetts) and mouse IL-6 (Endogen, Cambridge, Massachusetts), according to the manufacturer's instructions. TNF production was also measured after 24 hr of incubation. The lower limit of detection was 30 pg/ml for TNF and 15 pg/ml for IL-6.
Infection
An inoculum of bacteria was prepared by thawing an aliquot and diluting it in phosphate-buffered saline (PBS). Mice were infected intravenously via the lateral tail vein with an inoculum of bacteria in 0·2 ml of PBS. Mice were anaesthetized and killed at the indicated time-points to follow the course of infection. After the mice were killed, their organs were removed aseptically and homogenized in 10 ml of distilled water; bacterial loads in organs were evaluated by plating serial 10-fold dilutions of whole-organ homogenates on nutrient Middlebrook 7H10 agar (Difco) supplemented with OADC. Bacterial colony numbers (CFUs) were determined after 14–21 days of incubation at 37° in humidified air. The natural course of infection and the kinetics of granuloma formation in mice infected with both TMC724 (the highly virulent strain) and SE01 (the strain of intermediate virulence) have been described previously.20,29
Histology and immunohistology
One cranial and one caudal liver lobe per mouse were fixed in 4% formalin-PBS, set in paraffin blocks, sectioned (2–3 µm) and stained using haematoxylin and eosin (H & E). For immunohistology, tissue sections were deparaffinized, placed in 10 mm sodium citrate buffer (pH 6·0) and then pressure-cooked for exactly 1 min.30 After blocking for 20 min in 1% H2O2 solution, slides were incubated with appropriately diluted polyclonal rabbit anti-mouse inducible nitric oxide synthase (iNOS) (Biomol, Hamburg, Germany) in Tris-buffered saline (TBS) /10% fetal calf serum (FCS), for 30 min in a humid chamber. Appropriately diluted goat anti-rabbit immunoglobulin G (IgG) (bridging antibody) (Dianova, Hamburg, Germany) and diluted rabbit anti-goat IgG–peroxidase conjugate (tertiary antibody) (Dianova) were used in sequential incubations of 30 min each. Development was performed using 3,3-diaminobenzidine (Sigma, Deisenhofen, Germany) and urea superoxide (Sigma), and hemalum was used to counterstain the slides.
Reverse transcription–polymerase chain reaction (RT–PCR)
RT–PCR was performed essentially as described previously.31 In brief, weighed liver samples (≈150 mg each) were homogenized in 5 ml of 4 m guanidinium–isothiocyanate buffer, diluted to obtain equalized amounts of g of liver/ml of buffer, and after acid phenol extraction of total RNA, cDNA was obtained using murine moloney leukemia virus (MMLV) reverse transcriptase (Gibco-BRL, Eggenstein, Germany) and oligo-dT (12–18mer; Sigma) as a primer. PCR was performed on a Light Cycler (Roche Diagnostics Corporation, Indianapolis, IN) using the proprietary Light-Cycler-DNA Master SYBR Green I kit (Roche) and the following primer sets: β2-microglobulin: sense 5′-TGACCGGCTTGTATGCTATC-3′, antisense 5′-CAGTGTGAGCCAGGATATAG-3′; interferon-γ (IFN-γ): sense 5′-GCTCTGAGACAATGAACGCT-3′, antisense 5′-AAAGAGATAATCTGGCTCTGC-3′; TNF: sense 5′-GATCTCAAAGACAACCAACTAGTG-3′, antisense 5′-CTCCAGCTGGAAGACTCCTCCCAG-3′. After amplification (denaturation at 94° for 1 second, annealing at 60° for 5 seconds and extension at 72° for 5 seconds) melting curve analysis was performed to exclude the presence of confounding primer dimers during quantification. Semiquantitative comparisons of amplified products were made based on the crossing points obtained for each sample compared to a serially diluted, arbitrarily selected standard cDNA run in parallel. In this manner, arbitrary units could be assigned to mRNA levels present in each sample. This format allows for semiquantitative comparisons to be made within individual cycler runs only, but precludes comparison of mRNA levels between different cytokines. For purposes of statistical comparison, units were normalized by calculating the cDNA ratios of cytokine/β2-microglobulin for each sample.
Statistics
CFU data are expressed as the means of the log10 counts of four mice per group ±SD, and cDNA ratios are expressed as the means of four samples ±SD. Statistical analysis was performed using the Student's t-test.
Results
Cytokine production in response to stimulation with M. avium in bone marrow-derived Mφ from CD14+/+ and CD14−/− mice
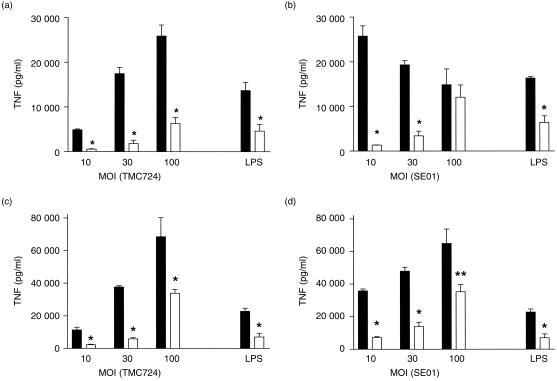
Bone marrow-derived Mφ, prepared from CD14+/+ and CD14−/− mice, were incubated with two strains of viable M. avium at different multiplicities of infection (MOI). In CD14+/+ Mφ, the highly virulent strain TMC724 always induced less TNF secretion than SE01, the strain of intermediate virulence, regardless of the dose used for stimulation, a finding previously reported for other M. avium isolates differing in virulence.32 Of note, the dose required to elicit in CD14−/− bone marrow-derived Mφ a response equivalent to that in CD14+/+ Mφ was at least 10-fold higher for both strains tested 6 hr after infection (Fig. 1a, 1b). At 24 hr postinfection, the difference between the capacities of CD14−/− and wild-type bone marrow-derived Mφ to produce TNF upon stimulation with M. avium was still evident, but less pronounced, in that only three- to 10-fold higher inocula were needed to achieve, in CD14−/− Mφ, TNF secretion equivalent to that obtained in CD14+/+ Mφ. Thus, prolonged stimulation, particularly with higher doses of M. avium, could also induce TNF secretion in a CD14-independent manner (Figs 1c, 1d). In conclusion, relative to the kinetics and magnitude of TNF secretion, bone marrow-derived Mφ from CD14−/− mice were markedly less responsive to stimulation with viable M. avium.
Figure 1.
Mycobacterium avium-induced tumour necrosis factor (TNF) secretion in bone-marrow-derived macrophages (Mφ) of CD14+/+ (wild type) and CD14-deficient (CD14−/−) mice. Bone marrow-derived Mφ from CD14+/+ (black bars) and CD14−/− (open bars) mice were incubated with the indicated multiplicities of infection (MOI) of viable M. avium TMC724 (a,c) or M. avium SE01 (b,d). Supernatants were harvested 6 hr (a,b) or 24 hr (c,d) postinfection, and TNF concentrations were measured by enzyme-linked immunosorbent assay (ELISA). Each point indicates the means +SD (error bars) of triplicate values. As a control, 10 ng/ml of lipopolysaccharide (LPS) was used. *P < 0·001; **P < 0·01.
Cytokine production in response to stimulation with M. avium in thioglycollate-elicited Mφ from CD14+/+ and CD14−/− mice
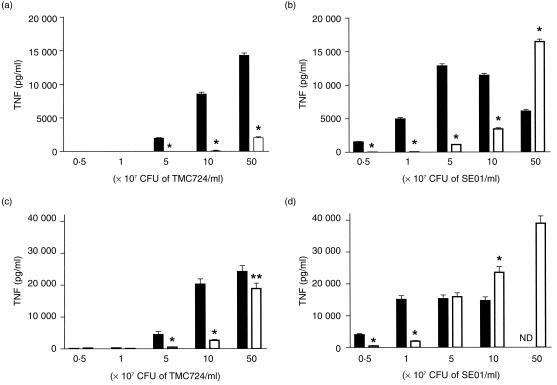
It could be argued that mycobacteria, following infection in vivo, might also encounter prestimulated, inflammatory Mφ whose CD14 expression levels might differ from those of resident Mφ. Therefore, in vitro infections analogous to those described above for bone marrow-derived Mφ were also performed with thioglycollate-elicited peritoneal Mφ from CD14−/− or wild-type mice. Again, the dose required to elicit in CD14−/− Mφ a response equivalent to that in CD14+/+ Mφ was at least 10-fold higher for both strains tested 4 hr after infection, although much higher doses of mycobacteria were generally required to induce TNF secretion in peritoneal Mφ (Fig. 2a, 2b). For instance, at stimulating doses lower than 5 × 107 CFU of SE01/ml (i.e. at a multiplicity of infection of 100:1), there was no detectable TNF secretion by CD14−/− Mφ, whereas CD14+/+ Mφ stimulated similarly produced up to 1300 pg/ml of TNF (P < 0·01). Even at higher inocula of M. avium, TNF secretion by CD14−/− Mφ was always five- to 10-fold lower than by CD14+/+ Mφ. This difference was less pronounced, but still evident, when Mφ supernatants were analysed after 24 hr of co-incubation (Fig. 2c, 2d). Particularly following stimulation with very high inocula, TNF levels in the supernatants of CD14−/− Mφ were similar to or higher than those induced in CD14+/+ Mφ. Therefore, prolonged stimulation with higher doses of M. avium induced TNF secretion, in a CD14-independent manner, also in this type of Mφ population.
Figure 2.
Mycobacterium avium-induced tumour necrosis factor (TNF) secretion in peritoneal macrophages (Mφ) of CD14+/+ (wild type) and CD14-deficient (CD14−/−) mice. Peritoneal Mφ from CD14+/+ (black bars) and CD14−/− (open bars) mice were incubated with the indicated concentrations of viable M. avium TMC724 (a,c) or M. avium SE01 (b,d). Supernatants were harvested 4 hr (a,b) or 24 hr (c,d) postinfection, and TNF concentrations were measured by enzyme-linked immunosorbent assay (ELISA). Each point indicates the means±SD (error bars) of triplicate values. ND, not determined. *P < 0·001; **P < 0·05.
Analogous results were obtained when IL-6 levels were measured in the supernatants of peritoneal Mφ stimulated with M. avium for 4 hr. For instance, when CD14+/+ Mφs were incubated with 5 × 108 CFU/ml of TMC724, 151 ± 48 pg/ml of IL-6 was secreted into the supernatants, whereas CD14−/− Mφ stimulated with this dose produced undetectable levels of IL-6. Similarly, CD14+/+ Mφ stimulated with 5 × 107 CFU/ml of SE01 secreted 157 ± 49 pg/ml of IL-6, whereas the supernatants of CD14−/− Mφ stimulated identically contained only 26 ± 4 pg/ml of IL-6 (P < 0·01).
Therefore, in terms of TNF and IL-6 secretion, CD14−/− peritoneal Mφ were markedly less responsive to M. avium stimulation.
Course of M. avium infection in CD14+/+ and CD14−/− mice
Both CD14−/− and control mice were infected intravenously with either 1 × 105 CFU of M. avium strain SE01 or 1 × 105 CFU of M. avium strain TMC724, and the course of bacterial replication was determined in infected organs (Fig. 3). Bacterial loads in the liver, spleen and lungs of mice infected with either strain were almost identical in CD14−/− mice to those found in CD14+/+ mice at any of the time-points investigated (Fig. 3). In order to establish whether dissemination of M. avium into other organs might be different in CD14−/− mice by comparison with CD14+/+ mice, CFU counts were also determined in the kidneys and in the blood of infected mice. No significant differences between the two groups of infected mice were observed with either strain of M. avium (Fig. 4). Thus, deficiency for CD14 did not appreciably influence the emergence of antibacterial protective mechanisms during M. avium infection and did not have any effect on the organ distribution of the infecting inoculum, regardless of the virulence of the M. avium strain used for infection.
Figure 3.
Course of Mycobacterium avium infection in CD14+/+ (wild type) and CD14-deficient (−/−) mice. CD14+/+ (closed symbols) and CD14−/− (open symbols) mice were infected intravenously with either 1 × 105 colony-forming units (CFU) of M. avium TMC724 (a,c,e) (circles), or 1 × 105 CFU of M. avium SE01 (b,d,f) (squares). Bacterial CFU counts were determined in the liver (a,b), spleen (c,d), and lung (e,f) at the indicated time-points postinfection. Each point reflects the means±SD (error bars) of four mice per group. There was no significant difference (P > 0·05) when data from CD14+/+ mice were compared with data from CD14−/− mice at the same time-points.
Figure 4.
Dissemination of Mycobacterium avium in CD14+/+ (wild type) and CD14-deficient (−/−) mice. CD14+/+ (black bars) and CD14−/− (open bars) mice were infected intravenously with either 1 × 105 colony-forming units (CFU) of M. avium TMC724 or 1 × 105 CFU M. avium SE01and killed 5 weeks postinfection. Bacterial CFU counts were determined in the blood (a) and the kidney (b). Data reflect the means ±SD (error bars) of four mice per group. There was no significant difference (P > 0·05) when data from CD14+/+ mice were compared with data from CD14−/− mice.
Even when higher levels of inocula were used (i.e. 106 CFU of TMC724 or 5 × 106 CFU of SE01 administered via the intravenous route), no significant differences in bacterial loads in the lungs, livers or spleens were apparent between CD14+/+ and CD14−/− mice at 3 and 8 weeks postinfection (data not shown).
Cytokine mRNA levels in the livers of CD14+/+ and CD14−/− mice in response to infection with M. avium
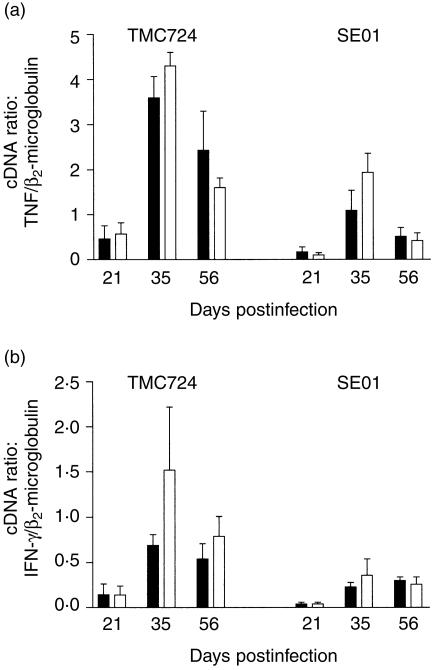
In order to determine whether the differences in cytokine secretion observed when stimulating CD14+/+ and CD14−/− Mφ in vitro would also be evident in vivo, we semiquantitatively determined the mRNA levels for TNF and IFN-γ in the livers of M. avium-infected mice at 3, 5 and 8 weeks postinfection. As shown in Fig. 5(a), TNF mRNA expression was similar in CD14−/− and CD14+/+ mice during the entire time frame of infection with either strain. IFN-γ mRNA expression appeared to be slightly enhanced in CD14−/− mice infected with TMC724 when compared to CD14+/+ mice, although this difference did not reach statistical significance (Fig. 5b). When a higher infective dose of TMC724 was chosen (106 CFU/mouse), IFN-γ mRNA expression was found to be enhanced almost eightfold in CD14−/− mice when directly compared to similarly infected BALB/c mice at 3 weeks postinfection (data not shown). Infection with SE01 induced very similar levels of IFN-γ mRNA expression in both mouse strains, and the results were the same when a higher dose of infection was used (data not shown). Thus, although there was a tendency for enhanced IFN-γ mRNA expression in CD14−/− mice when compared with CD14+/+ mice at early time-points of infection, these correlated more with the inoculum dose and the virulence of the M. avium isolate, and thus probably reflected different dynamics of bacterial growth in vivo.
Figure 5.
Cytokine mRNA levels in the livers of Mycobacterium avium-infected CD14+/+ (wild type) and CD14-deficient (−/−) mice. CD14+/+ (black bars) and CD14−/− (open bars) mice were infected intravenously with either 1 × 105 colony-forming units (CFU) of M. avium TMC724 or 1 × 105 CFU M. avium SE01, and killed on day 21, 35 or 56 postinfection. Reverse transcription–polymerase chain reaction (RT–PCR) was performed on mRNA purified from liver biopsies with primers specific for β2-microglobulin, tumour necrosis factor (TNF) and interferon-γ (IFN-γ), and serial dilutions of a standard cDNA were included in each run on a Light Cycler. The ratio of cytokine cDNA units/β2-microglobulin cDNA units calculated from four individual samples ±SD (error bars) is shown, data representing one experiment out of two performed. There was no significant difference (P > 0·05) when data from CD14+/+ mice were compared with data from CD14−/− mice at the same time-points.
Granuloma formation in CD14+/+ and CD14−/− mice in response to infection with M. avium
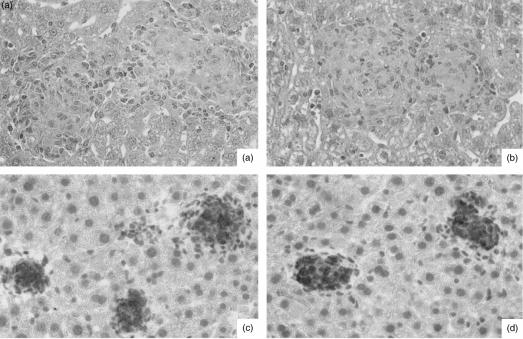
Although CFUs were not affected in CD14−/− mice, it appeared possible that the slight differences observed in IFN-γ mRNA expression might have resulted in alterations of either the quantity or quality of granuloma formation in CD14−/− mice when compared to CD14+/+ mice. Careful histological analysis, however, revealed that both the cellular composition and the kinetics of granuloma induction were similar in both groups of mice, regardless of the M. avium isolate and inoculum size used for infection. Therefore, the number and size of granulomas, as well as overall granuloma morphology, was identical in CD14−/− and CD14+/+ mice, both early during infection (3 and 5 weeks) when granuloma formation was incipient (Fig. 6a, 6b) and late during infection (8 weeks), when full-blown epithelioid differentiation of granulomas had occurred (Fig. 6c, 6d). Likewise, induction of iNOS proceeded in a similar manner in CD14−/− and control, infected mice (Fig. 6c, 6d), showing that Mφ activation occurred to a comparable degree in both groups of mice.
Figure 6.
Comparative liver histopathology in Mycobacterium avium-infected CD14+/+ (wild type) and CD14-deficient (−/−) mice. CD14+/+ (a) and (c) and CD14−/− (b) and (d) mice were infected intravenously with either 1 × 105 colony-forming units (CFU) of M. avium TMC724 (a) and (b), or 1 × 105 CFU of M. avium SE01 (c) and (d), and killed 5 weeks (a) and (b) or 8 weeks (c) and (d) postinfection for histological analysis of the liver. (a) and (b) Well-structured granulomas with epithelioid macrophages (haematoxylin & eosin staining; magnification ×128). (c) and (d) Circumscript inducible nitric oxide synthase (iNOS)-positive granulomas (immunoperoxidase staining; magnification × 128).
Discussion
In these studies, we used gene-targeted mice to elucidate the role of CD14 in response to infection with whole M. avium organisms in vitro and in vivo. A deficiency in CD14 resulted in significantly reduced and delayed TNF production by either bone marrow-derived or thioglycollate-elicited peritoneal Mφ stimulated with viable M. avium in vitro. In a murine model of disseminated infection with M. avium, however, CD14 was not critically involved in either the antibacterially protective or the chronic inflammatory response against M. avium, regardless of the virulence of the strain used for infection.
A number of studies, using human or mouse primary cells or cell lines, have conclusively demonstrated a requirement for CD14 in TNF production following stimulation with LPS, peptidoglycan, lipoproteins or lipoarabinomannan.4–8,14,15,18 In addition, a few studies have confirmed that CD14 is necessary for TNF secretion induced by intact, viable or heat-killed micro-organisms.14 Recently, we obtained evidence that TNF induction by viable M. avium is also abrogated in human monocyte-derived Mφ by co-incubation with a mAb against CD14 (N. Reiling et al., unpublished). The results presented here concerning the in vitro stimulation (with viable M. avium) of peritoneal Mφ from CD14−/− mice are in good agreement with these data and demonstrate that interaction of whole mycobacteria with CD14 facilitates TNF secretion from Mφ. It has previously been noted that M. avium strains of differing virulence induce secretion of different amounts of TNF from Mφ in vivo.32 Our data extend these findings and show that strains of both high and intermediate virulence induce TNF in a CD14-dependent manner.
It is, however, clear from our in vitro dose–response experiments that the requirement for CD14 is far from absolute. When higher doses of M. avium were used and incubation times were extended, TNF was induced also in CD14−/− Mφ, reaching levels at least equivalent to those induced in CD14+/+ Mφ. This is in agreement with the fact that CD14−/− Mφ stimulated with Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides were also capable of producing TNF.13 Thus, in contrast to the majority of results obtained with LPS and Gram-negative bacteria, Gram-positive micro-organisms and mycobacteria can induce Mφ activation in a CD14-independent manner.
In vivo experiments using CD14−/− mice have established that the role of CD14 differs depending on the challenge inoculum. Thus, CD14−/− mice were completely protected not only from LPS-mediated shock, but also from a lethal challenge with whole Escherichia coli organisms, whereas shock induced by S. aureus was unmitigated in these mice.12,16 When CD14−/− mice were infected with viable E. coli organisms, a delayed, but significant, rise in serum concentrations of both TNF and IL-6 was noted, attesting to the fact that in this situation CD14-independent cytokine induction was also possible.12 Remarkably, when CD14−/− mice were infected with either heat-killed or viable S. aureus, there was a threefold increase in TNF levels in their sera.16
Our data were obtained in a model of chronic mycobacteria-induced infection and thus add another dimension to our understanding of the in vivo role of CD14. In contrast to its dramatic effect in the acute model of LPS-induced shock, CD14 deficiency did not preclude an intact antimycobacterial defence or the ordered recruitment of monocytes into granulomatous lesions. In contrast to infection in vitro, where CD14−/− Mφ were markedly impaired in terms of TNF secretion, there were no significant differences between infected CD14−/− and wild-type, CD14+/+ mice regarding TNF and IFN-γ mRNA expression in the liver at 3, 5 and 8 weeks postinfection. Although we failed to detect levels of TNF and IFN-γ mRNA above background levels at 1 week postinfection in either mouse strain (data not shown), it remains possible that small, but significant, differences between CD14−/− and CD14+/+ mice, particularly in terms of TNF expression, may have occurred at very early time-points of infection. Assuming that such early decreases in TNF production would entail counter-regulatory effects, the tendency towards enhanced IFN-γ mRNA levels in CD14−/− mice, particularly when higher concentrations of infecting inocula were used, might thus be explained. This could potentially also lead to the recruitment and activation of additional types of cells into inflammatory foci, e.g. granulocytes, which have limited capacities of directly and indirectly reducing mycobacterial growth.33,34 Regardless, our data underscore the fact that it is almost impossible to predict, from in vitro Mφ stimulation and cytokine secretion data, the protective and inflammatory response in vivo when mice genetically deficient for a single, non-essential molecule are examined in vivo.
CD14−/− mice, owing to their inability to activate monocytes through CD14, were previously reported to have a greatly reduced bacteraemia and bacterial load in organs, such as the lung, when infected with whole organisms of E. coli or Bacteroides fragilis.12,35 We did not observe such a redistribution of micro-organisms in our experiments with M. avium. This probably relates to the fact that, in contrast to many other micro-organisms, mycobacteria are capable of using a large variety of different receptors on the surface of Mφ for adherence and invasion, e.g. the mannose receptor, the complement receptors CR1, CR3 and CR4, the fibronectin receptor, an αvβ3integrin, the CD11/CD18 integrin and the scavenger receptor.36 It is therefore probable that, in the absence of CD14, phagocytosis proceeded unaltered via these mechanisms, leading to similar organ distributions of the bacterial load.
Recently, a major signalling pathway, leading to Mφ activation by mycobacterial products, was identified.10,37 Following recruitment to the phagosome, the toll-like receptor 2 (TLR2) is capable of detecting lipoproteins, lipopeptides and glycolipids from Gram-positive bacteria and mycobacteria, and signals TNF secretion via an associated adaptor protein, MyD88.9,10,37–39 In line with this concept, blocking M. avium interaction of Mφ with an antibody against TLR2 was found to completely abrogate TNF induction in human mononuclear cells.9 TLR2 is therefore now thought to be the essential component mediating Mφ activation in response to microbial structures derived from Gram-positive bacteria and mycobacteria, and the role of CD14 has been relegated to a molecule facilitating the interaction of microbial products with TLR2.9,38
Our data imply that it may be premature to extrapolate from in vitro findings to the overall relevance of a given molecule in an in vivo infection. Particularly during chronic infections, such as those caused by mycobacteria, a plethora of compensatory mechanisms may dwarf the role of any single molecule. As demonstrated here during chronic infection with M. avium, the role of CD14 in antibacterial protection and granuloma formation is certainly minor. It may be prudent to refrain from speculating on the potentially pivotal role of other cell surface receptors in signalling Mφ activation until their true role is defined in vivo.
Acknowledgments
We would like to thank Johanna Suwinski, Svenja Kröger-Albrecht and Stefanie Kutsch for expert technical assistance, and Ernst Th. Rietschel for critically reading the manuscript. This work was funded in part by DFG grant Re 1228/3-1 to N.R. and S.E. and NIH grant AI23859 to S.G.
References
- 1.Bazil V, Baudys M, Hilgert I, Stefanova I, Low MG, Zbrozek J, Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein, CD14. Mol Immunol. 1989;26:657–62. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 2.Goyert SM, Ferrero E, Rettig WJ, Yenamandra AI, Obata F, Le Beau MM. The CD14 monocyte differentiation antigen maps to a region encoding growth factors and receptors. Science. 1988;239:497–500. doi: 10.1126/science.2448876. [DOI] [PubMed] [Google Scholar]
- 3.Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–52. [PubMed] [Google Scholar]
- 4.Dziarski R, Ulmer AJ, Gupta D. Interactions of CD14 with components of Gram-positive bacteria. Chem Immunol. 2000;74:83–107. doi: 10.1159/000058761. [DOI] [PubMed] [Google Scholar]
- 5.Gupta D, Kirkland TN, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–6. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 6.Ulmer AJ, Dziarski R, El-Samalouti V, Rietschel ET, Flad HD. CD14, an innate immune receptor for various bacterial cell wall components. In: Brade H, Opal SM, Vogel SN, Morrison DC, editors. Endotoxin in Health and Disease. New York: Marcel Dekker; 1999. pp. 463–75. [Google Scholar]
- 7.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of LPS and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 8.Pugin JI, Heumann D, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 9.Lien E, Sellati TJ, Yoshimura A, et al. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–25. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 10.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–7. [PubMed] [Google Scholar]
- 11.Ferrero E, Jiao D, Tsuberi BZ, Tesio L, Rong GW, Haziot A, Goyert SM. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci USA. 1993;90:2380–4. doi: 10.1073/pnas.90.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haziot A, Ferrero E, Köntgen F, Hijiy N, Yamamoto S, Silver J, Stewart CL, Goyert SM. Resistance to endotoxin shock and reduced dissemination of Gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–14. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 13.Sellati T, Bouis DA, Kitchens RL, et al. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–64. [PubMed] [Google Scholar]
- 14.van Furth AM, Verhard-Seijmonsbergen EM, Langermans JA, van Dissel JT, van Furth R. Anti-CD14 monoclonal antibodies inhibit the production of tumor necrosis factor alpha and interleukin-10 by human monocytes stimulated with killed and live Haemophilus influenzae or Streptococcus pneumoniae organisms. Infect Immun. 1999;67:3714–8. doi: 10.1128/iai.67.8.3714-3718.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weidemann B, Brade H, Rietschel ET, Dziarski R, Bazil V, Kusumoto S, Flad HD, Ulmer AJ. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–15. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haziot A, Hijiya N, Schultz K, Zhang F, Gangloff SC, Goyert SM. CD14 plays no major role in shock induced by Staphylococcus aureus but down-regulates TNF-alpha production. J Immunol. 1999;162:4801–5. [PubMed] [Google Scholar]
- 17.Bernardo J, Billinglea AM, Blumenthal RL, Seetoo KF, Simons ER, Fenton MJ. Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannan: role of CD14 and the mannose receptor. Infect Immun. 1998;66:28–35. doi: 10.1128/iai.66.1.28-35.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Doerfler M, Lee TC, Guillemin B, Rom WN. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993;91:2076–83. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inderlied CB, Kemper CA, Bermudez LE. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hänsch HCR, Smith DA, Mielke MEA, Hahn H, Bancroft GJ, Ehlers S. Mechanisms of granuloma formation in murine Mycobacterium avium infection: the contribution of CD4+ T cells. Int Immunol. 1996;8:1299–310. doi: 10.1093/intimm/8.8.1299. [DOI] [PubMed] [Google Scholar]
- 21.Pedrosa J, Florido M, Kunze ZM, Castro AG, Portaels F, McFadden J, Silva MT, Appelberg R. Characterization of the virulence of Mycobacterium avium complex (MAC) isolates in mice. Clin Exp Immunol. 1994;98:210–6. doi: 10.1111/j.1365-2249.1994.tb06127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benini J, Ehlers EM, Ehlers S. Different types of pulmonary granuloma necrosis in immunocompetent vs. TNFRp55-gene-deficient mice aerogenically infected with highly virulent Mycobacterium avium. J Pathol. 1999;188:127–37. doi: 10.1002/(SICI)1096-9896(199909)189:1<127::AID-PATH398>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.Flynn JL, Goldstein MM, Chan J, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–72. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 24.Kindler V, Sappino AP, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–40. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 25.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–70. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DA, Hänsch HCR, Bancroft GJ, Ehlers S. T-cell-independent granuloma formation in response to Mycobacterium avium – role of tumour necrosis factor alpha and interferon gamma. Immunology. 1997;92:413–21. doi: 10.1046/j.1365-2567.1997.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gangloff SC, Hijiya N, Haziot A, Goyert SM. Lipopolysaccharide structure influences the macrophage response via CD14-independent and CD14-dependent pathways. Clin Infect Dis. 1999;28:491–6. doi: 10.1086/515176. [DOI] [PubMed] [Google Scholar]
- 28.Medina E, Rogerson BJ, North RJ. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology. 1996;88:479–81. doi: 10.1046/j.1365-2567.1996.d01-700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel ET, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–9. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cattoretti G, Piteri S, Parraricini C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993;171:83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- 31.Ehlers S, Seitzer U. Measuring immune responses in vivo. In: Kaufmann S, Kabelitz D, editors. Methods in Microbiology. Vol. 25. London, UK: Academic Press; 1998. pp. 365–87. Immunology of Infection. [Google Scholar]
- 32.Sarmento AM, Appelberg R. Relationship between virulence of Mycobacterium avium strains and induction of tumor necrosis factor alpha production in infected mice and in in vitro-cultured mouse macrophages. Infect Immun. 1995;63:3759–64. doi: 10.1128/iai.63.10.3759-3764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geertsma MF, Nibbering PH, Pos O, van Furth R. Interferon-gamma-activated human granulocytes kill ingested Mycobacterium fortuitum more efficiently than normal granulocytes. Eur J Immunol. 1990;20:869–73. doi: 10.1002/eji.1830200423. [DOI] [PubMed] [Google Scholar]
- 34.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection in mice. Infect Immun. 2000;68:577–83. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woltmann A, Gangloff SC, Bruch HP, Rietschel ET, Solbach W, Silver J, Goyert SM. Reduced bacterial dissemination and liver injury in CD14-deficient mice following a chronic abscess-forming peritonitis induced by Bacteroides fragilis. Med Microbiol Immunol. 1998;187:149–56. doi: 10.1007/s004300050087. 10.1007/s004300050087. [DOI] [PubMed] [Google Scholar]
- 36.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–81. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brightbill HD, Libraty DH, Krutzik SR, et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–6. doi: 10.1126/science.285.5428.732. 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 38.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–5. doi: 10.1038/44605. 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 39.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]