Abstract
Mice lacking the suppressor of cytokine signalling-1 (SOCS1) die within weeks of birth with extensive fatty degeneration of the liver, consistent with acute hepatic toxicity to interferon-γ (IFN-γ), and inflammation of multiple organs. We show here that treatment for 1 week from birth with neutralizing antibody to IFN-γ rescues SOCS1−/− mice from lethal liver disease but the mice subsequently succumb to chronic inflammatory lesions characterized by T-lymphocyte infiltration of skeletal muscle, pancreas, lung, liver and skin. Elevated blood levels of eosinophils, neutrophils and platelets were also observed and the thymic lymphocyte population was depleted of CD4+ CD8+ T cells and showed a reduced CD4 : CD8 ratio. All T-cell populations in thymus, spleen and lymph node exhibited an increased proportion of cells bearing the activation marker CD44. These data suggest an important role for SOCS1 in T-lymphocyte regulation.
Introduction
The suppressor of cytokine signalling-1 (SOCS1) gene was simultaneously cloned by three groups,1–3 alternatively on the basis of the ability of SOCS1 to down-regulate interleukin-6 (IL-6) signalling,1 to inhibit signalling by signal transducers and activators of transcription (STAT),2 and to associate with janus kinases (JAK kinases).3 SOCS1 inhibits JAK kinase activity2–4 and also associates with elongins B and C with possible subsequent proteasomal targeting of the JAK–STAT–receptor complex via this association.5
Mice lacking SOCS1 appear normal at birth, but within 10 days exhibit growth retardation, and die by 3 weeks of age with fatty degeneration of the liver, a severely atrophic thymus and inflammatory infiltration in multiple organs.6 The liver disease observed in the SOCS1-deficient mice strongly resembles that described in neonates treated with interferon-γ (IFN-γ)7,8 and it was hypothesized that the disease observed in the SOCS1−/− mice might be IFN-γ-dependent. In a previous study, to test this hypothesis, mice were treated with IFN-γ neutralizing antibody for 3 weeks after birth and then analysed. The treated mice were normal at that time-point except for lymphoid cuffing of the lung vessels and persistence of erythropoiesis in the spleen.9 The unequivocal dependence on IFN-γ of neonatal disease development in SOCS1−/− mice was proven by the generation of mice which had functional inactivation of the genes for SOCS1 and for IFN-γ. These doubly deficient mice were healthy at weaning, were normal haematologically and exhibited only minor histological anomalies.9
In the process of generating the SOCS1−/− IFN-γ−/− mice a population of SOCS1−/− IFN-γ+/− mice was also produced. The majority of these mice became ill during early adult life with a disease distinct from that previously observed in SOCS1−/− IFN-γ+/+ neonates.6,10 Fatty degeneration of the liver was not a feature of disease in adult SOCS1−/− IFN-γ+/− mice, which instead exhibited polymyositis, myocarditis and corneal infiltration.10
The initial study on the effects of IFN-γ on neonatal mice identified this period as one of particular sensitivity to the toxic effects of this cytokine. Lethality was only observed when IFN-γ treatment was commenced within the first 6 days of birth, while administration of similar doses of the cytokine after this period appeared to be without toxicity.7 The altered disease in SOCS1−/− mice having only a single functional IFN-γ allele may therefore reflect the attenuated effects of a lower IFN-γ concentration, but might also represent a distinct disease process which develops in adult mice. To explore these alternatives the clinical manifestations of disease were monitored in SOCS1−/− mice which had been treated with IFN-γ neutralizing antibodies only briefly during the neonatal period. Although mice treated with anti-IFN-γ antibodies for the first 7 days of life were rescued from the neonatal fatal disease seen in untreated SOCS1−/− mice, they became moribund between 4 and 10 weeks of age and died with a complex inflammatory disease similar to that observed in SOCS1−/− IFN-γ+/− mice.10
Materials and methods
Generation and maintenance of mice and injection of antibody
SOCS1−/− mice on a mixed C57BL/6 × 129/Sv genetic background were generated as described.6 IFN-γ−/− mice were obtained from The Jackson Laboratory, Bar Harbor, ME. The SOCS1 and IFN-γ genotype of progeny of the intercross mice was determined by Southern blot analysis of tail tip genomic DNA as described previously.6,9 All mice were housed in conventional clean animal rooms.
Progeny of SOCS1+/− × SOCS1+/− matings (giving SOCS1+/+, SOCS1+/− and SOCS1−/− littermates) were injected intraperitoneally with IFN-γ neutralizing antibody (36 µg R4-6A2, American Type Culture Collection, Manasses, VA) within 3–4 hr of birth and then once daily for a total of seven injections. Pups were monitored continuously for external symptoms of disease and analysed either at weaning or when moribund. As each SOCS1−/− mouse was killed, one or more SOCS1+/+ or SOCS1+/− littermates were killed and subjected to a parallel analysis.
Haematological and histological analysis
Blood was collected from the orbital plexus or from the heart under anaesthesia, or, for the determination of blood glucose, from the tail. The peripheral blood haematocrit, differential white cell and platelet counts were determined using automated counting techniques (ADVIA 120, Bayer, Tarrytown, NY). Blood glucose was determined using the Advantage II Glucose Strips (Roche Diagnostics Group, Indianapolis, IN). Mice were killed by anaesthesia and their organs were fixed in 10% buffered formalin, sectioned and stained routinely with haematoxylin and eosin.
β-Galactosidase staining
Organs were fixed on ice for 3–4 hr in 4% paraformaldehyde containing 2 mm magnesium chloride and 5 mm ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), then given three 30 min washes in phosphate-buffered saline (PBS) pH 7·3, 2 mm magnesium chloride, 0·01% v/v sodium deoxycholate, 0·02% Nonidet P-40, 5 mm EGTA. Organs were stained overnight at 37° in PBS pH 7·3, 2 mm magnesium chloride, 0·01% v/v sodium deoxycholate, 0·02% Nonidet P-40, 5 mm potassium ferricyanide, 5 mm potassium ferrocyanide, 0·5 mg/ml 5-bromo-4-chloro-3-indolyl beta-d-galactopyranoside (X-gal). Stained organs were washed twice in PBS, 3% dimethyl sulphoxide, then post-fixed overnight at 4° in 4% paraformaldehyde, sectioned and counterstained with Nuclear Fast Red.
Flow cytometry
Dispersed cell suspensions were prepared from bone marrow, spleen, thymus and lymph node and mature red cells were lysed by incubation with buffered 156 mm ammonium chloride. Cells were incubated variously with specific monoclonal antibodies to the following murine cell surface antigens: CD4, CD8, Thy-1, CD44, B220 and immunoglobulin M (IgM). The antibodies were directly coupled to fluorescein isothiocyanate (FITC) or biotin, the latter being visualized with R–phycoerythrin–streptavidin. Analyses were performed on a FACScan cell sorter (Becton Dickinson, San Jose, CA) with dead cells and erythrocytes excluded after propidium iodide staining (1 µg/ml) and gating of forward angle and side scatter of light.
Interferon-γ assays
Serum IFN-γ was measured using a standard cytokine sandwich enzyme-linked immunosorbent assay (ELISA). IFN-γ specific antibodies (R4-6A2 as the capture antibody, XMG1.2 for detection) were obtained from Pharmingen (San Diego, CA) and the assay was performed as described by the manufacturer.
Results
Prevention of liver disease in SOCS1−/− weanlings
Antibody-treated mice
We have previously shown that treatment of SOCS1−/− mice for 3 weeks with IFN-γ neutralizing antibody prevents the neonatal lethality caused by SOCS1 deficiency.9 Consistent with this observation, in the current study of 58 SOCS1−/− mice treated during the first week of life with IFN-γ neutralizing antibody, all were apparently healthy at weaning, with the exception of one mouse that died at 21 days of age.
At weaning there was no difference between SOCS1−/− and SOCS1+/+ or SOCS1+/− in body weight (SOCS1−/−, 12·2 ± 1·8 g; SOCS1+/+ and SOCS1+/−, 12·1 ± 1·4 g). The reduced size of these mice when untreated, as originally observed,6 is therefore likely to be due to their illness rather than any SOCS1-dependent growth deficiency.
The antibody-treated SOCS1−/− weanlings displayed no significant haematological abnormalities (Table 1). No difference in blood glucose concentration was observed between healthy antibody-treated SOCS1−/− and SOCS1+/+ or SOCS1+/− weanlings (6·6 ± 1·4 mmol, n = 4, 5·6 ± 0·8 mmol, n = 20, respectively). Serum IFN-γ was above the limits of detection in only three of nine antibody-treated SOCS1−/− weanlings tested (33%), compared to six out of 27 antibody-treated SOCS1+/+ and SOCS1+/− littermates (22%). In contrast, IFN-γ was detectable in the sera of all four untreated 14-day-old SOCS1−/− pups tested while no IFN-γ was detectable in the sera of littermate SOCS1+/+ pups (data not shown).
Table 1.
The effects of anti-IFN-γ treatment and gene dosage on haematopoiesis in SOCS1−/− mice
| SOCS1genotype | SOCS1+/+/+/− | SOCS1−/− | SOCS1+/+/+/− | SOCS1−/− | SOCS1+/− | SOCS1−/− |
|---|---|---|---|---|---|---|
| IFN-γ genotype | IFN-γ+/+ | IFN-γ+/+ | IFN-γ+/+ | IFN-γ+/+ | IFN-γ+/− | IFN-γ+/− |
| State of health | healthy | sick | healthy | healthy | healthy | healthy |
| Treatment | Anti-IFN-γ Ab | Anti-IFN-γ Ab | Anti-IFN-γ Ab | Anti-IFN-γ Ab | Untreated | Untreated |
| Age (days) | 24–61 | 24–61 | 21 | 21 | 21 | 21 |
| No. of mice analysed | 21 | 14 | 20 | 7 | 9 | 9 |
| Total cells/μl | 11 100 ± 2700 | 14 700 ± 6500 | 7500 ± 2090 | 10 030 ± 7760 | 14 070 ± 8100 | 10 900 ± 3800 |
| Neutrophils/μl | 1070 ± 400** | 3160 ± 2400** | 750 ± 300 | 830 ± 660 | 590 ± 160 | 780 ± 300 |
| Lymphocytes/μl | 9360 ± 2400 | 9650 ± 4300 | 6030 ± 1800 | 7870 ± 6010 | 12 370 ± 7560 | 8330 ± 3170 |
| Monocytes/μl | 119 ± 90 | 110 ± 140 | 80 ± 47 | 136 ± 239 | 111 ± 60 | 96 ± 93 |
| Eosinophils/μl | 230 ± 100 | 420 ± 470 | 170 ± 107 | 206 ± 101 | 210 ± 85 | 200 ± 86 |
| Haematocrit % | 46 ± 4 | 44 ± 9 | 35 ± 7 | 21 ± 6 | 30 ± 6 | 27 ± 7 |
| Platelets/nl | 1145 ± 199* | 1617 ± 871* | 933 ± 200 | 962 ± 329 | 1004 ± 129 | 1149 ± 152 |
Figures are mean values ± SD. The data should be read pairwise by comparison of column 1 versus column 2, column 3 versus column 4, and column 5 versus column 6.
Significantly different, P < 0·02, Mann–Whitney test for non-parametric data.
Significantly different, P < 0·001, Mann–Whitney test for non-parametric data.
At the age of 3 weeks, changes in the lymphocyte profiles of antibody-treated SOCS1−/− mice were already evident. Though the weanling thymus was not visibly reduced in size compared to antibody-treated SOCS1+/+ or SOCS1+/− littermates there was a significant reduction in the proportion of CD4+ CD8+ T cells (82% of thymocytes in SOCS1+/+ and SOCS+/−, versus 57% in SOCS−/−, n = 7 and n = 3, respectively). There was also a shift in the ratio of CD4 : CD8 T cells, such that CD8+ T cells were present in nearly equal or greater numbers than CD4+ T cells. This change in CD4 : CD8 ratio was likewise observed in the spleen and the lymph nodes. Furthermore, there was a trend towards increased expression of the activation marker CD44 in all T-cell populations analysed (thymic, splenic and lymph node, data not shown).
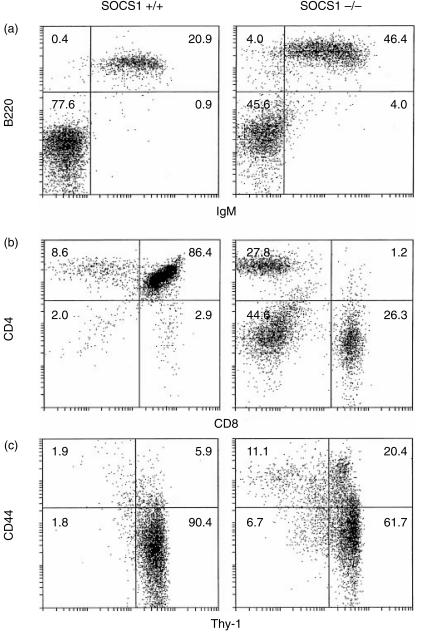
Intriguingly, the B-lymphocyte populations showed an organ-specific variation. The percentage of cells expressing the B-cell markers B220 and surface IgM was unchanged in the SOCS1−/− spleen and bone marrow. In contrast, there was a marked increase in the percentage of B220+ IgM+ B cells in the lymph nodes of antibody-treated SOCS1−/− weanlings (Fig. 1a) compared to antibody-treated SOCS1+/+ weanlings.
Figure 1.
Changes in the lymphocyte populations of antibody-treated SOCS1−/− mice compared to age-matched antibody-treated SOCS1+/+ mice. (a) Increased proportion of B220+ IgM+ cells in the lymph node of weanlings. (b) Loss of CD4+ CD8+ T cells in the thymus of adults and increased percentage of CD8+ T cells. Increased expression of the activation marker CD44 on thymocytes.
Despite the outwardly healthy appearance of these mice, histological analyses at weaning revealed the presence of several significant abnormalities, including lymphoid foci in the lung and salivary gland, and inflammatory infiltration of the skeletal muscle, pancreas, skin and tail marrow (Table 2). This infiltrate was predominantly lymphoid but also included monocytes, neutrophils and eosinophils in some tissues. In contrast to untreated SOCS1−/− pups6 no fatty degeneration or necrosis was detected in the liver of any of the weanlings examined (n = 6).
Table 2.
The effects of anti-IFN-γ treatment and IFN-γ heterozygosity on organ histology of SOCS1−/− mice
| SOCS1genotype | SOCS1+/+/+/− | SOCS1−/− | SOCS1+/+/+/− | SOCS1−/− | SOCS1+/− | SOCS1−/− |
|---|---|---|---|---|---|---|
| IFN-γ genotype | IFN-γ+/+ | IFN-γ+/+ | IFN-γ+/+ | IFN-γ+/+ | IFN-γ+/− | IFN-γ+/− |
| State of health | healthy | sick | healthy | healthy | healthy | healthy |
| Treatment | Anti-IFN-γ Ab | Anti-IFN-γ Ab | Anti-IFN-γ Ab | Anti-IFN-γ Ab | Untreated | Untreated |
| Age (days) | 35–70 | 27–70 | 21 | 21 | 21 | 21 |
| No. of mice analysed | 17–19 | 18–20 | 9 | 6 | 8–9 | 7–9 |
| Lymphoid foci | ||||||
| liver | 5 | 90 | 0 | 0 | 0 | 33 |
| lung | 16 | 75 | 0 | 88 | 0 | 67 |
| salivary gland | 0 | 53 | 0 | 25 | 0 | 78 |
| Liver fatty degeneration | 0 | 0 | 0 | 0 | 0 | 0 |
| Thymus cortex atrophy | 0 | 67 | 0 | 33 | 0 | 57 |
| Muscle infiltration | ||||||
| major | 0 | 70 | 0 | 50 | 0 | 56 |
| minor | 11 | 15 | 0 | 25 | 11 | 44 |
| Heart infiltration | ||||||
| major | 0 | 40 | 0 | 0 | 0 | 33 |
| minor | 5 | 35 | 0 | 29 | 0 | 44 |
| Corneal infiltration | 0 | 26 | 0 | 0 | 0 | 0 |
| ulcer | 0 | 26 | 0 | 0 | 0 | 0 |
| Pancreas infiltration | 5 | 100 | 0 | 88 | 13 | 100 |
| Kidney infiltration | 11 | 37 | 0 | 25 | 0 | 89 |
The values shown represent the percentage of mice displaying a particular lesion.
Interferon-γ heterozygous mice
In addition to the antibody-treated weanlings described above, a population of mice possessing only one functional allele of IFN-γ was also analysed at weaning (these mice were not treated at all).
At 3 weeks of age the SOCS1−/− IFN-γ+/− mice were externally indistinguishable from their SOCS1+/− IFN-γ+/− littermates. The SOCS1−/− mice showed no significant deviation in their haematological profile from that of their SOCS1+/− littermates (Table 1).
Despite their generally healthy appearance, at weaning perturbations in the lymphocyte populations of these SOCS1−/− IFN-γ+/− mice were already present. These changes were similar to those described above in the antibody-treated SOCS1−/− weanlings: reduced thymic CD4+ CD8+ T-cell populations, altered CD4 : CD8 T-cell ratio in the spleen, thymus and lymph node, and a general increase in the expression of the T-cell activation marker CD44. Likewise, as observed in the antibody-treated mice above, in the IFN-γ+/− mice there was an increase in the percentage of B220+ IgM+ cells in the lymph node, but not in the spleen or bone marrow (data not shown).
The histological profile of the SOCS1−/− IFN-γ+/− mice at weaning was also very similar to that seen in antibody-treated SOCS1−/− weanlings (Table 2). The major histological abnormalities were lymphoid foci in the lung, salivary gland and liver, atrophy of the thymic cortex, and inflammatory infiltration of skeletal and heart muscle, tail marrow, pancreas, kidney and skin. In contrast to untreated SOCS1−/− IFN-γ+/+pups no liver fatty degeneration was observed in any of the mice examined (n = 9).
Development of inflammatory disease in antibody-treated SOCS1 null mice
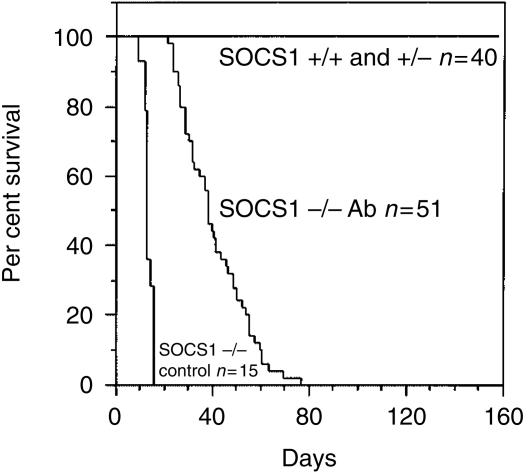
Although rescued from the lethal neonatal effects of the absence of SOCS1 and in apparent good health at weaning, SOCS1−/− mice treated with IFN-γ antibody did not remain disease-free. Of the 50 antibody-treated SOCS1−/− mice monitored after weaning, five developed scaliness of the skin on the tail at, or after, 3 weeks of age and the majority of mice began to show clinical signs of illness at between 4 and 9 weeks of age. All had become moribund by 11 weeks (Fig. 2). As expected, all untreated SOCS1−/− mice succumbed to disease within 2 to 3 weeks of birth. None of 40 antibody-treated control SOCS1+/+ or SOCS1+/− mice (littermates of the SOCS1−/− mice above) became ill over the 5-month period of observation (Fig. 2).
Figure 2.
Prolonged lifespan of antibody-treated SOCS1−/− mice compared to untreated SOCS1−/− mice but premature death compared with antibody-treated SOCS1+/+ and SOCS1+/− mice.
The cellular composition of the blood of sick antibody-treated SOCS1−/− mice was highly variable, compared with the antibody-treated littermates of the same age (Table 1). The most consistent differences noted were increases in the numbers of neutrophils and platelets (P < 0·001, n = 14, and P = 0·018, n = 14, respectively). The total number of white blood cells and the number of eosinophils also appeared higher, but was not significant due to the high degree of variability of these parameters, especially in the SOCS1−/− mice (Table 1).
In the thymus of sick antibody-treated SOCS1−/− mice there was a pronounced reduction in the percentage of CD4+ CD8+ T cells compared to that of treated wild-type littermates and this was greater than that noted above in these mice at weaning. There was also an increase in the ratio of CD8+ T cells relative to CD4+ T cells (Fig. 1b). Again, as observed in the antibody-treated weanlings described above, there was an increase in the expression of the T-cell activation marker CD44 in the thymus (Fig. 1b) and also in the spleen and lymph nodes.
The results from an analysis of B-lymphocyte populations in these mice revealed no significant differences in any of the B-cell populations analysed (spleen, lymph node, bone marrow, data not shown).
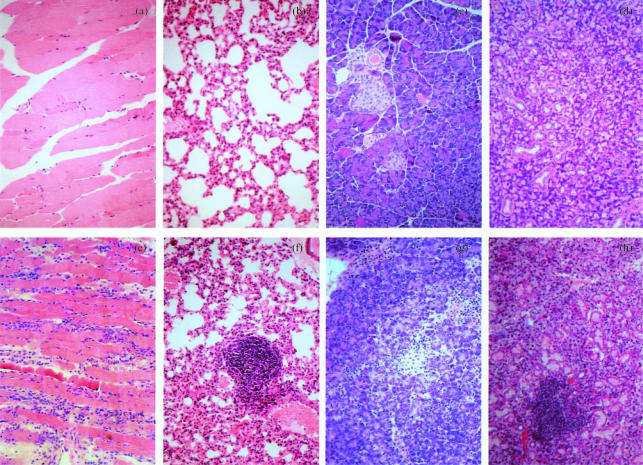
The pathology of disease in antibody-treated SOCS1−/− mice was characterized by inflammatory infiltration, predominantly lymphocytes (with monocytes, neutrophils and eosinophils also present in some tissues), of the skeletal muscle (Fig. 3a, e), lung (Fig. 3b, f), pancreas (Fig. 3c, g), salivary gland (Fig. 3d, h), heart muscle, and kidney (Table 2). Peri-islet infiltration was present in all pancreata analysed, however, no intra-islet infiltration was observed (Fig. 3c, g). To verify this several sections of pancreata were stained immunohistochemically for insulin and no abnormalities were detected (data not shown). Blood glucose was also monitored, in sick and healthy animals. There was no change in blood glucose in healthy animals and as hypoglycaemia was only observed in the last stages of illness it is unlikely that the inflammatory infiltrate had had any significant effect on insulin regulation.
Figure 3.
Inflammatory infiltration of organs from antibody-treated SOCS1−/− mice. Sections stained with haematoxylin and eosin. (a–d) Tissues from antibody-treated SOCS1+/+ or SOCS1+/− mice: (a) muscle, (b) lung, (c) pancreas, (d) salivary gland. (e–h) Tissues from antibody-treated SOCS1−/− mice: (e) muscle, (f) lung, (g) pancreas, (h) salivary gland.
Expression patterns from the SOCS1 locus in antibody-treated mice
Staining with X-gal was used to determine the pattern of SOCS1 expression in SOCS1+/− and SOCS1−/− mice, making use of the β-galactosidase substitution for SOCS1, incorporated during the disruption of the SOCS1 locus.6 Tissues were collected from sick antibody-treated SOCS1−/− mice and their littermates (both SOCS1+/+ and SOCS1+/−).
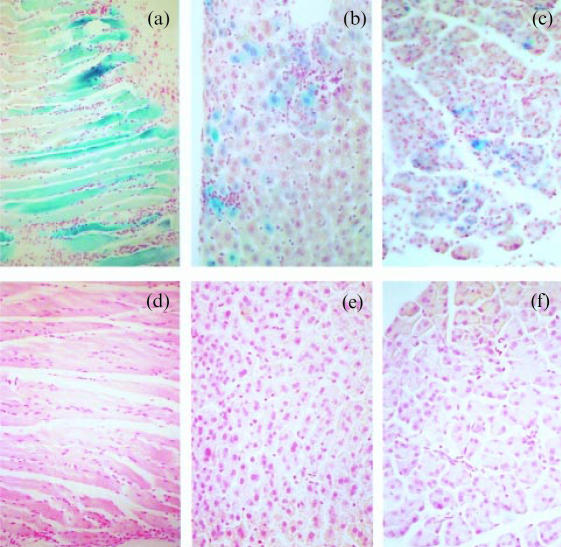
Tissues from SOCS1−/− and SOCS1+/− mice were stained for the presence of β-galactosidase activity. Skeletal muscle stained the most strongly of any tissue examined (Fig. 4a, d). Staining was present in the SOCS1−/− liver (Fig. 4b) and pancreas (Fig. 4c) though not in the SOCS1+/− liver or pancreas (Fig. 4e, f). These data seem to indicate a constitutive expressive of SOCS1 in skeletal muscle, and an up-regulation of SOCS1 promoter activity in the pancreas and liver of sick antibody-treated SOCS1−/− mice.
Figure 4.
β-galactosidase activity in organs from antibody-treated SOCS1−/−, SOCS1+/− and SOCS1+/+ mice: (a) SOCS1−/− muscle; (b) SOCS1−/− liver; (c) SOCS1−/− pancreas; (d) SOCS1+/+ muscle; (e) SOCS1+/− liver; (f) SOCS1+/− pancreas.
It was observed with interest that the regions of blue staining in the liver and pancreas were usually associated with areas of inflammatory infiltration. Up-regulation of SOCS1 expression in these tissues may be provoked by cytokines produced by the inflammatory infiltrate.
Discussion
The IFN-γ neutralizing treatment described here was sufficient to prevent the neonatally lethal liver fatty degeneration seen in untreated SOCS1−/− mice. Furthermore, there was no liver fatty degeneration observed in any of the 26 animals examined (adults and weanlings). However, this treatment was insufficient to prevent the development of an extensive inflammatory disease, characterized primarily by lymphocyte infiltration of multiple organs and tissues.
The increase in numbers of total cells, platelets, neutrophils and eosinophils in antibody-treated SOCS1−/− adults is interesting because a similar trend has been noted in SOCS1−/− IFN-γ−/− adults (W. Alexander, D. Metcalf, unpublished data). This is an important observation because it suggests that SOCS1 may be involved in the regulation of haematopoiesis.
The thymic T-lymphocyte populations of these SOCS1−/− mice were significantly altered compared with SOCS1+/+ and SOCS1+/− littermates, particularly with respect to CD4 and CD8 profile and the expression of the activation marker CD44. These alterations were evident in milder form in the antibody-treated SOCS1−/− mice prior to the onset of clinical disease, suggesting that some changes in the thymic populations might be a direct consequence of the absence of SOCS1, although involution induced by progressive disease is likely to be a major influence.
This model closely resembles the disease phenotype reported in SOCS1−/− IFN-γ+/− mice.10 The major differences between these models were the absence in the antibody-treated adults of major heart muscle infiltration and the lower frequency of corneal infiltration and ulceration. The absence of these lesions may simply reflect the lower age of the antibody-treated SOCS1−/− mice analysed (due to their reduced lifespan compared with SOCS1−/− IFN-γ+/− mice).
The increase of B220+ IgM+ cells in the lymph nodes of weanling antibody-treated SOCS1−/− and IFN-γ+/− SOCS1−/− mice and not in the spleen or bone marrow of either suggests that there may be some specific localization of B cells to nodes. The means by which such a localization might be mediated is presently unclear. The absence of this increase in antibody-treated SOCS1−/− adults may reflect the depletion of such populations as a result of the progressive illness of the mice.
The disease observed in weanling SOCS1−/− IFN-γ+/− mice supports previous findings in adult SOCS1−/− IFN-γ+/− mice,10 that these mice develop a distinctive inflammatory phenotype (Table 2) and suggests that disease is not acute in the mice, but begins to develop as early as weaning. Inflammatory infiltration and/or lymphoid foci were present in many organs and tissues, though to a lesser degree than that reported in adult mice.10 In contrast with previous observations,10 there was no inflammatory infiltration in the cornea, nor any corneal ulceration.
The near identical liver phenotypes of SOCS1−/− mice6 and mice treated with IFN-γ at birth7 suggest that lethality in SOCS1 mice results from an acute sensitivity of the neonatal liver to excess IFN-γ signalling. Given the lack of adverse effect of IFN-γ treatment on mice older than 6 days7 our data, derived from SOCS1−/− mice in which IFN-γ was neutralized for this initial period, establishes that SOCS1 is not only required to protect the neonatal liver from IFN-γ cytotoxicity, but also plays a key role beyond this early neonatal period.
The presence of IFN-γ in SOCS1−/− mice beyond the early neonatal period is sufficient to cause a lethal inflammatory disease characterized by dysregulated T-cell numbers, distribution and activation status, accompanied by widespread chronic inflammation. These studies suggest an important role for SOCS1 in T-cell development, selection or activation, the precise nature of which requires further investigation.
Acknowledgments
We thank S. Mihajlovic, E. Tsui, M. Chong, N. Sprigg, L. Viney, J. Mighall, L. Bryan, F. Rodda, J. Curtis, T. Baldwin and D. Advani for skilful technical assistance throughout this study. This work was supported by the National Health and Medical Research Council, Canberra, Australia; The National Institutes of Health, Bethesda, MD (Grant CA-22556), and the Australian Federal Government Cooperative Research Centres Program.
Abbreviations
- IFN-γ
interferon-γ
- SOCS1
suppressor of cytokine signalling-1
References
- 1.Starr R, Willson TA, Viney EM, et al. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–21. doi: 10.1038/43206. 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 2.Naka T, Narazaki M, Hirata M, et al. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–9. doi: 10.1038/43219. 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 3.Endo TA, Masuhara M, Yokouchi M, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–4. doi: 10.1038/43213. 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 4.Ohya K, Kajigaya S, Yamashita Y, et al. SOCS-1/JAB/SSI-1 can bind to and suppress Tec protein-tyrosine kinase. J Biol Chem. 1997;272:27178–82. doi: 10.1074/jbc.272.43.27178. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J-G, Farley A, Nicholson SE, et al. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci USA. 1999;96:2071–6. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starr R, Metcalf D, Elefanty AG, et al. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998;95:14395–9. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gresser I, Tovey MG, Maury C, Chouroulinkov I. Lethality of interferon preparations for newborn mice. Nature. 1975;258:76–8. doi: 10.1038/258076a0. [DOI] [PubMed] [Google Scholar]
- 8.Gresser I, Maury C, Tovey M, Morel-Maroger L, Pontillon F. Progressive glomerulonephritis in mice treated with interferon preparations at birth. Nature. 1976;263:420–2. doi: 10.1038/263420a0. [DOI] [PubMed] [Google Scholar]
- 9.Alexander WS, Starr R, Fenner JE, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf D, Di Rago L, Mifsud S, Hartley L, Alexander WS. The development of fatal myocarditis and polymyositis in mice heterozygous for IFN-γ and lacking the SOCS-1 gene. Proc Natl Acad Sci USA. 2000;97:9174–9. doi: 10.1073/pnas.160255197. [DOI] [PMC free article] [PubMed] [Google Scholar]