Abstract
Systemic and topical administration routes of tacrolimus and cyclosporin A (CsA) were compared in effects on early and late phases of elicited T-cell-mediated contact sensitivity (CS), and effects on early and late phases of cutaneous immunoglobulin E (IgE) antibody-mediated hypersensitivity responses in mice. Thus, both CS and IgE responses in the skin have an early mast-cell-dependent phase, and also a late inflammatory phase. We measured the effects of both immunosuppressants on both phases of the respective T cell versus IgE responses. Systemic administration of both agents completely suppressed CS and IgE late-phase responses, but failed to affect either early phase. In contrast, when topical CsA was used, low doses abolished the early phase of IgE responses, but even high doses did not inhibit the early phase of CS. Conversely, topical tacrolimus inhibited the early phase of CS more potently than the early phase of cutaneous IgE hypersensitivity responses. Thus, topical treatment was needed to inhibit the early phases and the two agents acted differentially, suggesting differing susceptibility of the early phases, that are probably due to different signalling mechanisms. These studies underscore the potential value of topical administration of these powerful immunosuppressive agents in the treatment of allergic diseases that exhibit features of early-phase mast-cell-dependent inflammation, and late inflammation due to mast cells or to T cells, such as atopic dermatitis or asthma, since the early phase is predominantly susceptible to topical application, while the last phase of both IgE and T-cell inflammation responds to systemic treatment with both agents.
Introduction
The effector phase of contact sensitivity (CS) is an important in vivo manifestations of T-cell allergy mediated by sensitized CD4+ T cells that recruit various non-specific, bone marrow-derived effector leucocytes into the extravascular tissue, via release of cytokines.1 We showed previously in mice that elicitation of CS responses requires an early, immediate hypersensitivity-like initiating phase that is due to release of the vasoactive amine serotonin from local mast cells2,3 and platelets,4 and tumour necrosis factor-α (TNF-α) from mast cells.5,6 This CS-initiation is due to antigen (Ag)-specific factors that sensitize the tissues for mast cell release of serotonin and cytokines like TNF-α,5,6 following local challenge with Ag.7,8 Although similar to immediate hypersensitivity, the early phase of CS responses is not due to immunoglobulin E (IgE),8,9 but is due to complement-fixing IgM antibodies that are produced very early after sensitization by the B-1 subset of B cells,10 that bind Ag, leading to activation of complement (C) to locally generate the C-fragment C5a, that in turn activates mast cells and platelets via C5a receptors.6,10–12
Cyclosporin A (CsA) and tacrolimus are potent immunosuppressants, used to prevent rejection of transplants and to treat a variety of immune-mediated disorders.13–15 The main action of CsA and tacrolimus appears to affect transcription of genes encoding cytokines in recently activated T cells.16,17 There are several other possible sites of action, including: inhibition of T-cell-receptor-mediated exocytosis of cytotoxins from killer T cells;18 inhibition in mast cells,19 and basophil20,21 IgE Fcε receptor-mediated exocytosis and cytokine transcription; and effects on antigen-presenting cells.22–24
Prior studies showed that CsA and tacrolimus inhibit the late, effector phase CS-related responses, suggesting an effect on production of cytokines by sensitized effector T cells.25 The mechanism of this effect is still not fully understood. CsA and tacrolimus bind to different intracellular proteins; termed cyclophillin and FK-506-binding protein, respectively.26 Although structurally distinct, both proteins possess isomerase activity, thought to be of importance in protein folding. However, blockade of isomerase activity and protein folding may not be sufficient to explain immunosuppression.27 Binding of the drug–immunophilin complex to calcineurin, a serine/threonine, calcium/calmodulin-dependent protein phosphatase,28 is now thought to mediate immunosuppression by affecting transcription of lymphokine genes that require phosphorylation for activation.29,30
Several differences in activity indicate that a mechanism of action involving calcineurin may not fully explain the effects of these drugs. For example, tacrolimus is 50–100 times more potent than CsA in inhibiting interleukin-2 (IL-2) secretion in vitro, even though its ability to bind calcineurin is similar; albeit at different sites.29,30 Also, these drugs mediate differential effects on T-cell activation in vivo,31 and differences have been noted when employed in organ transplantation.32 These data suggest that in vivo, CsA and tacrolimus may act on different targets producing alternative mechanisms of immunoregulation.
Although there is evidence that these drugs inhibit the in vitro function of mast cells and basophils,19–21 there has been no confirmation in vivo. We postulated that these agents might affect in vivo mast cell function differentially. Therefore, we examined the effects of either systemic or topical CsA versus tacrolimus on the early versus late effector phases of CS in mice, which involve early-phase mast-cell-dependent T-cell-mediated immune responses in the skin. We compared these effects on CS to the effects of the immunosuppressive agents on IgE-mediated mast-cell-dependent cutaneous responses, generated in mice after passive transfer of antigen-specific IgE, which also feature early- and late-phase components. These latter IgE responses therefore depend on mast cells, but in this model are independent of T-cell immunization. Therefore, these procedures allowed us to examine potential differential effects of these agents by using these contrasting in vivo models of cutaneous allergy, that are relevant to human allergic diseases.
Materials and methods
Mice
Male CBA/J and female BALB/cJ mice (5–6-week-old) were obtained from the Jackson Laboratory, Bar Harbor, ME. Mice were maintained in filter-topped microisolators in a locked bioclean room and fed autoclaved food and water. Cages were changed in a laminar flow hood and mice were handled by personnel wearing sterile gloves, garments and masks. Mice were rested for at least 1 week before use.
Reagents
Picryl chloride (PCl) [trinitrophenyl (TNP) chloride (Chemotronix, Swannonoa, NC)], recrystallized from ethanol/H2O before use, and oxazolone (OX) (Gallard-Schlesinger, Carle Place, NY), were protected from light in a desiccator during storage at room temperature. Ascites fluid containing 2 mg/ml of mouse anti-DNP/TNP IgE monoclonal antibody (H1 DNP-C-26),33 was a gift from Dr F. T. Liu (Scripps Clinic, La Jolla, CA) and was stored at −70°. For each experiment, a freshly thawed sample of IgE-rich ascites was centrifuged for 5 min at 16 000 g in a microfuge to remove aggregates, and diluted in sterile pyrogen-free phosphate-buffered saline (PBS, pH 7·2) containing 1% fetal bovine serum (Sigma, St Louis, MO). Different doses of IgE were injected intravenously (i.v.) via the retro-orbital plexus in a volume of 0·5 ml, 24 hr prior to skin challenge. CsA (Sandimmune, Novartis, Summit, NJ) for oral use, 100 mg/ml in olive oil and 12·5% ethanol, was diluted 1 : 10 in olive oil and 0·2 ml (2 mg) was injected subcutaneously. CsA for i.v. use at 50 mg/ml in a sterile ampoule was diluted 1 : 10 in PBS (pH 7·2) and 1 mg was injected i.v. per mouse. For topical use, CsA powder (kind gift of Prof. Robert E. Handschumacher, Yale University) was dissolved initially in absolute ethanol to which was added olive oil 1 : 2, and 20 µl of a 10% w/v solution was applied to the ears following local challenge with PCl. Tacrolimus (FK506) in powder was a kind gift of Dr S. Puppala (Fujisawa, Deerfield, IL) and was reconstituted in absolute ethanol to a concentration of 10 mg/ml and stored until use at −70°. Dilutions were made in sterile pyrogen-free PBS (pH 7·2) for intravenous administration, or in olive oil for topical treatment, and used as described above for CsA.
Immunization and elicitation of cutaneous hypersensitivity responses
CBA mice were contact sensitized by painting with 0·15 ml. of a 5% solution of PCl or 3% OX in an ethanol/acetone mixture (4 : 1 v/v) on all four paws, and the skin of the clipped abdomen. Mice were challenged by topical application of one drop (27-g needle) of 0·8% PCl or 0·8% OX, in olive oil to both sides of the ears. Duplicate measurements of ear thickness were made bilaterally with an engineer's micrometer (Mitotoyo, Paramus, NJ) before challenge, and at 2 and 24 hr after challenge. The increment in ear thickness was expressed as the mean±SE in units of 10−3 cm. In each experiment there were four or five mice per group, and the ears of a separate group of non-immunized controls were challenged and measured similarly, to provide non-specific background responses. IgE-mediated cutaneous sensitivity was induced by i.v. transfer of various amounts of anti-TNP IgE antibody. After 24 hr, mice were challenged on the ears with 0·8–1·0% PCl (TNP-chloride) in olive oil, and ear swelling responses were then measured at various time-points, with an engineer's micrometer.
Statistical analysis
Statistical analysis was performed using Student's t-test via Stat View 1.0 software, after tests confirmed normality of distribution of the data. We confirmed the significance by anova, using Sigma Stat for Windows 2.03. A P-value of less than or equal to 0.05 was considered significant. If indicated, adjustment was made to correct for multiplicity.
Results
Systemic CsA inhibits the late but not the early component of PCl CS
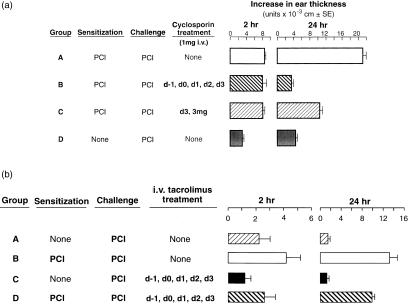
Systemic administration of CsA i.v. from 1 day prior to sensitization and daily thereafter, until 1 day prior to testing on day 4, abolished the late 24 hr component of PCl CS (Fig. 1a, Group B), compared to sensitized mice treated with vehicle alone (Group A), and unsensitized challenged controls (Group D). In fact, mice treated i.v. with CsA once, just 1 day prior to testing, had almost 75% inhibition of 24 hr CS (Group C), suggesting that CsA influenced the late effector phase of CS needed for elicitation of CS, more than acting at the T-cell sensitization phase. In contrast, CsA by both regimens had no effect on the elicited early 2 hr component of PCl CS (Groups B and C versus Group A). We concluded that systemic CsA inhibited the late, but not early, effector phase of CS, probably by affecting cytokine production by late-acting, effector T cells, rather than affecting early initiating mechnisms.
Figure 1.
Effects of systemic treatment with cyclosporin (CsA) and tacrolimus on the early and late components of PCl CS. (a) Intravenous CsA inhibits the late component of PCl CS but not the early component. CBA mice were contact sensitized with 5% PCl on day 0. Four days later CS responses were elicited by challenging the ears with 0·8% PCl. Mice were treated daily with 1 mg CsA in PBS (pH 7·2) i.v. on day −1 through to day +3 (Group B), or with vehicle alone on day −1 to day +3 (Group A), or with 3 mg i.v. just on day +3 (Group C). Ear thickness was quantified prior to challenge, and at 2 and 24 hr following challenge. Controls were not immunized nor treated with CsA, but were just challenged on the ears with PCl (Group D). P < 0·001 for Group B versus A and P < 0·005 for Group C versus A, at 24 hr. (b) Intravenous treatment with tacrolimus suppresses both early and late PCl CS responses. CBA mice were sensitized with PCl and 4 days later their ears were challenged with 0·8% PCl. Groups B and D received tacrolimus at a dose of 0·02 mg/day intraperitoneal from 1 day prior to sensitization, until the day of challenge, and were compared to untreated Group C, and to Group A, that received vehicle alone. P < 0·05 Group C versus A at 2 hr, and Groups B versus D at 2 hr. P < 0·005 Group C versus A and Group B versus D, at 24 hr.
Systemic tacrolimus diminishes the late component of CS and modestly affects the early component
Tacrolimus is about 50–100 times more potent than CsA using in vitro assays.21 As comparison, similar experiments were performed to determine the effects of tacrolimus in vivo. We used tacrolimus at a dose of 0·02 mg/day i.v., which is approximately equivalent to 1·0–2 mg CsA per day used above, and is one-third of the dose used for clinical immunosuppression.15,32,34 Figure 1(b) shows that tacrolimus at 0·02 mg/day i.v. beginning 1 day prior to sensitization and continuing daily until testing, significantly reduced 24-hr CS responses, and had a small but significant inhibitory effect on 2-hr ear swelling (Group D). We concluded that systemic tacrolimus also inhibited, but did not abolish, the late effector phase of CS, but also had a small inhibitory effect on the early 2 hr component of CS.
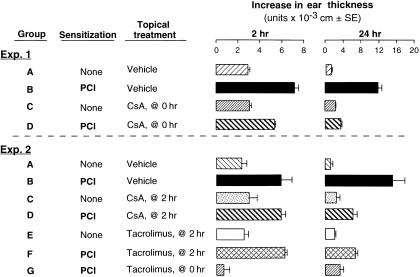
Topical tacrolimus, but not CsA, inhibits both early and late components of CS
CS reactions afford an opportunity to test the local topical effects of immunosuppressants. Therefore, effects of topical CsA and tacrolimus on CS responses were also examined. Topical CsA (Fig. 2, Exp. 1, Group B versus D, and Exp. 2, Group D versus B), and topical tacrolimus (Fig. 2, Exp. 2, Group G versus B) applied on the ears at the time of Ag challenge decreased both early (2-hr) and late (24-hr) CS ear swelling responses. Notably, late swelling was suppressed more, and topical tacrolimus was more potent.
Figure 2.
Differential effects of topical CsA and tacrolimus on early and late phases of PCl CS responses. CBA mice were contact sensitized with PCl on day 0 and ear challenged on day 4. Ear swelling was measured 2 and 24 hr after challenge. Mice were treated topically with either oil vehicle alone (Exp. 1 and 2, Group A), or 10% CsA (Exp.1, Groups C and D), or 0·1% tacrolimus (Exp. 2, Group G) at the time of Ag challenge (0 hr), or just after the 2-hr ear swelling was measured (Exp. 2). n = 4–5 per group. Exp. 1: P < 0·025 Group B versus D at 2 hr, P < 0·01 Group B versus D at 24 hr. Exp 2: for 2 hr data: P < 0·001 Group G versus B, P = NS, Group D and F versus B; for 24 hr data: P < 0·01 Group D and F versus B, P < 0·05 Group F versus G.
Since the early 2 hr component of CS is required for elicitation of the late 24 hr component, i.e. the early component is CS-initiating, and thus allows subsequent elicitation of the classical late 24-hr component of CS,35–38 we treated with CsA and tacrolimus just after the 2-hr CS-initiating component. Since this treatment was after the early CS-initiating component occurred, it allowed examination of effects of the topical immunosuppressants on the late-isolated, late-phase, T-cell-dependent component, separated from the early CS-initiating phase. Interesting differential effects of CsA versus tacrolimus were obtained. The inhibitory effect of topical tacrolimus on the late effector phase (24-hr CS ear swelling) was significantly less when the drug was administered after the early 2-hr component was allowed to occur (Fig. 2, Exp. 2, Group F). This was compared to another group of mice in which tacrolimus was applied at the time of initial antigen challenge prior to the early component, and thus at a time when tacrolimus clearly was able to influence the early effector phase (Fig. 2, Exp. 2, Group G).
In contrast, CsA inhibition of late CS was equivalent whether given either at the time of Ag challenge, or after the 2-hr ear swelling was measured (Fig. 2, Exp. 1, Group D versus Exp. 2, Group D). This striking difference suggested that a prominent component of the ability of tacrolimus to inhibit CS, was due to an effect on the early occurring, CS-initiating, and probably mast cell-dependent, events of the CS-initiating phase, that occur soon after Ag challenge, and upon which the subsequent late phase depends. This contrasted with CsA, which seemed to have far less of an effect on the early component of CS, and acted primarily on the late component (Fig. 1a versus Figure 1b), probably due to predominant effects on T-cell production of cytokines.
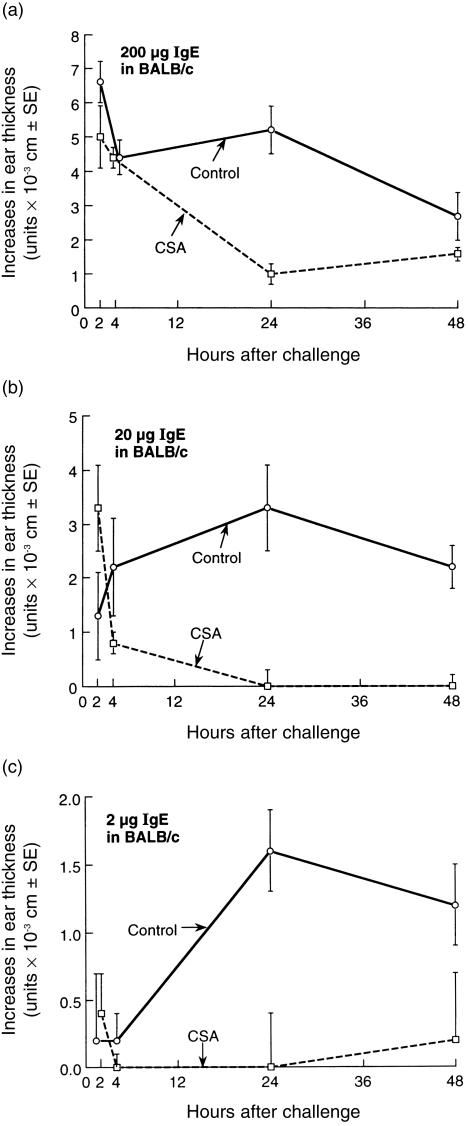
IgE-mediated hypersensitivity is inhibited in its late phase, but not the early phase, by systemic CsA
Although, CsA generally is known to inhibit T cells, CsA can also inhibit mast cell and basophil in vitro release of mediators, including vasoactive amines such as serotonin and histamine.19–21 However, the above in vivo experiments showed that the early component of CS in mice, which depend on release of serotonin from local skin mast cells,3,4 was not affected by systemic CsA, but was inhibited by topical CsA, and that tacrolimus inhibited both components, but the early component more potently (Figs 1 and 2). We therefore also tested the effects of these agents on passively transferred IgE-dependent in vivo cutaneous hypersensitivity, that depends on mast-cell-release of mediators, and does not involve T cells in the activation process.
Since CBA mice have unpredictable IgE late phase responses, while IgE immediate responses in BALB/c mice are regulatory followed by 24-hr late-phase ear swelling responses, we used BALB/c. Figure 3 shows IgE-mediated early and late biphasic ear swelling responses in BALB/c, and that systemic CsA had no effect on the immediate responses, but significantly inhibited the anti-TNP IgE-dependent late phase in mice topically challenged with PCl (TNP-Cl) (Fig. 3a,b). This was true at both 24 and 48 hr, and was true of strong responses mediated by passive transfer of 200 µg IgE per mouse (Fig. 3a), moderate responses mediated by 20 µg IgE per mouse (Fig. 3b), and weak late-phase responses, accompanied by barely detectable early responses, mediated by 2 µg IgE per mouse (Fig. 3c). We concluded that systemic treatment with CsA inhibited the IgE-mediated late-phase responses, that probably depended on mast cell production of cytokines, but did not affect the preceding IgE mast cell-mediated immediate responses, that probably depend on early-phase mast cell exocytosis and release of vasoactive mediators like serotonin and TNF-α. This was similar to findings in CS, where CsA inhibited late, but not early effector phases of these T-cell-dependent responses (Figs 1 and 2).
Figure 3.
Intravenous CsA inhibits the late phase but not the early phase of IgE-mediated hypersensitivity. BALB/c mice were passively sensitized on day 0 by i.v. injection of monoclonal anti-TNP IgE antibody in varying doses (a) 200 µg, (b) 20 µg, and (c) 2 µg. Then, 24 hr later, mice were challenged on the ears with 0·8% PCl and ear thickness was measured at various times later. A separate group of mice that received identical doses of IgE were treated i.v daily with 1 mg CsA in saline on day −1 to day +1. A negative control group was not immunized, nor treated with CsA, and received saline vehicle alone, and then were identically ear challenged with PCl to provide background swelling responses, that were then subtracted from the responses of the experimental groups, to derive the net swelling responses shown (note different scale of y-axis in each part of the figure).
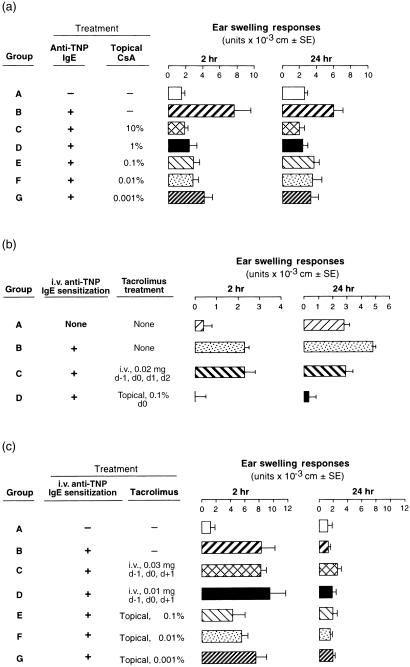
Topical CsA and tacrolimus suppress IgE-mediated early responses
Employing the same passive sensitization of anti-TNP IgE and then subsequent PCl ear challenge, CBA mice could also sometimes elicit macroscopic late-phase IgE ear swelling responses, that were also inhibited by systemic CsA, with no effect on IgE-dependent immediate responses (data not shown). In contrast, when CsA was administered topically to the ears in increasing doses, and just after Ag challenge at time 0, there was complete inhibition of strong early responses and also inhibition of the late effector phase, even at doses as small as 0·001% CsA (Fig. 4a, Groups C–G versus Group B).
Figure 4.
Effects of topical CSA or tacrolimus on both early- and late-phase, IgE-mediated reactions in CBA and BALB/c mice. Mice were given 20 µg of TNP-specific IgE i.v. on day 0, and ear challenged with 1% PCl 1 day later. Immediately after challenge, CSA (a) or tacrolimus (b,c), were topically applied to the pinna of both ears at various concentrations, and ear swelling measurements were made 2 and 24 hr later; n = 4–5 mice/group. (a) 2 hr data: P < 0·0005 for Group B versus C and D; P < 0·01 for Group B versus G. 24 hr data: P < 0·01 for Group B versus C, F and G. (b) 2 hr data: P < 0·01 for Group D versus B. 24 hr data: P < 0·01 for Group D versus C. (c) 2 hr data: P < 0·01 for Group E versus B; P < 0·05 Group F versus B; P = NS Group G versus B.
Thus, a very small dose of CsA (0·001% solution, or a 10 000-fold dilution of stock solution), given topically significantly suppressed strong IgE-mediated early phase responses in CBA mice. Similarly, in BALB/c mice, systemically administered tacrolimus modestly inhibited the late but not the early phase of IgE responses (Fig. 4b, Group C), whereas topical application of tacrolimus powerfully inhibited both phases (Fig. 4b, Group D). Furthermore, in CBA mice with isolated IgE early responses and no late-phase responses, systemic tacrolimus did not inhibit the early response (Fig. 4c, Groups C and D), while topical administration of tacrolimus resulted in dose-dependent inhibition of IgE immediate hypersensitivity (Fig. 4c, Groups E–G). However, topical CsA seemed to act more powerfully than topical tacrolimus in this instance (Fig. 4a, Groups E–G versus Figure 4c, Groups E–G).
Taken together, CsA and tacrolimus are two immunosuppressive agents that produced powerful, route-dependent, differential inhibitory effects on early versus late phases of cutaneous allergy due to IgE and to T-cell-dependent mechanisms in CS. Importantly, inhibition was more powerful when these drugs were given topically, perhaps because of greater effects on the early and required mast cell-dependent aspects that are characteristic of both of these responses.
Discussion
It is now clear that classical T-cell-dependent CS responses have an important early, immediate hypersensitivity-like component, and that conventional IgE-dependent immediate hypersensitivity has a prominent late-phase component. Importantly, in both cases the early component is required for elicitation of the late component.2–4,11,35–38 Furthermore, the late phase of IgE-dependent responses is now recognized to be perhaps more clinically important than the early phase in some allergic diseases, such as asthma.39 However, in actual allergic diseases, such as asthma or atopic dermatitis, both IgE- and T-cell-mediated early and late phases are involved and present a complex mixture; the overlapping effects due to IgE versus T cell are difficult to sort out. Thus, we have chosen to study a model system in mice, in which the effects of CsA versus tacrolimus given systemically or topically, can be studied in relatively pure models of either IgE-dependent or T-cell-dependent hypersensitivity.
The principal findings of this study are that systemically administered CsA and tacrolimus inhibited the late phase of both IgE- and T-cell-mediated cutaneous allergic responses, but not the early phase of either response. In contrast, when given topically, both inhibited the early and the late phases and inhibition was differential. Topical CsA and tacrolimus exerted the expected suppressive effects on the late phases of CS and IgE cutaneous hypersensitivity responses. However, tacrolimus more powerfully inhibited the early phase of CS, while CsA more powerfully inhibited the early phase of IgE responses. Thus topical tacrolimus, but not CsA, completely inhibited the early and the late components of CS, while topical CsA, but not tacrolimus, completely inhibited both phases of IgE responses (Table 1). These findings again suggested differential effects of the two immunosuppressants on the two different hypersensitivities. It is possible that tacrolimus inhibition of both early and late CS responses (Fig. 2, Group G) was due to a barrier of effect preventing Ag penetration in the skin, but the failure of tacrolimus applied similarly to block the early 2-hr response to IgE (Fig. 3, Group E) is against this.
Table 1.
Inhibitory effect of systemic versus topically administered CsA and tacrolimus on CS/DTH and IgE-mediated responses
| CS/DTH | IgE-mediated | |||
|---|---|---|---|---|
| Treatment | Early | Late | Early | Late |
| CsA | ||||
| Systemic | 0 | 4+ | 0 | 4+ |
| Topical | 0 | 3+ | 4+ | 4+ |
| Tacrolimus | ||||
| Systemic | 1+ | 2+ | 0 | 4+ |
| Topical | 4+ | 4+ | 2+ | 4+ |
Grading system: 0, less than 10% inhibition; 1+, 10–25% inhibition; 2+, 26–50% inhibition; 3+, 51–75% inhibition; 4+, greater than 75% inhibition.
Both CsA and tacrolimus have strong effects on cytokine production by T cells and by mast cells, that probably largely account for their effects on the late effector phase of both responses. In addition, we hypothesize that when administered topically each agent can better affect the early mast cell participation that is required for elicitation of the late component in both responses. However, there appear to be differential effects of the two agents on mast cell activation in the two cutaneous allergic responses. This may be due to different drug actions on intracellular signalling events that could be associated with different mechanisms of mast cell activation that are involved in these two types of responses. CsA had more powerful inhibitory effects on early IgE mast cell mechanisms, while tacrolimus was more inhibitory of early mast-cell-dependent aspects of CS. These findings support the idea that the early components of delayed-type hypersensitivity (DTH)/CS responses, and of IgE-mediated reactions, that both involve mast cells, and have a similar early time-course, but are due to different mechanisms of mast cell activation, as suggested previously.2,3,8,40 It is known that IgE activates mast cells via FcεRI signalling pathways of mediator release, while recent findings established that early mast cell activation in CS is due instead to B-1 cell-derived IgM antibody activating C to generate C5a to act on the mast cell C5a receptor signalling pathway of mediator release.10,12,13 Thus, we have concluded that IgE activation of mast cells via FcεRI, and the early component of CS, which also involves mast cell activation, but via G-coupled C5a receptors,10–13 therefore act via different signalling pathways, and thus may have been affected differentially by tacrolimus compared to CsA.
Differences in skin absorption, of these agents could contribute to the differences we found. However, we do not think that differences in skin absorption explain the differential effects of these drugs on the effector phase of these two models of cutaneous allergic responses. Absorption of tacrolimus and CsA are similar in skin, at least in humans.41 Although partition coefficient experiments showed CsA to be more lipophilic than tacrolimus, both drugs were present in the epidermis and dermis in comparable percentages of total applied dose. The greater topical efficacy of tacrolimus in clinical dermatitis could not clearly be explained by the authors on the basis of differences in skin absorption.42 In our study, we adjusted the dose to compensate for potency differences of CsA and tacrolimus. Further, absorption differences cannot explain the reciprocal power of the two agents on the different early versus late components of IgE versus CS responses.
Therefore, our findings extend understanding of the effects of CsA and tacrolimus on mast cells. It was shown previously that in vitro treatment of mast cells or basophils with CsA or tacrolimus inhibited activated release of histamine and serotonin.20,21,25,26 These prior in vitro findings may be akin to our findings with topical application of these agents. Our results suggest that inhibition of basophils or mast cells in the skin is probably not achievable by systemic administration, employing even relatively high doses of either CsA or tacrolimus, since these drugs did not affect the early eliciting phase IgE or CS ear swelling responses when administered intravenously. However, they probably affected skin mast cells when applied topically, and did so differentially.
Tacrolimus and CsA offer a newly expanding approach to the treatment of allergic skin conditions. For example, systemically administered CsA has been employed successfully to treat atopic dermatitis in adults.43 Our results in CS and IgE model systems of mice, suggest that this may be due to an effect of systemic CsA on late components of either or both of the IgE and T-cell aspects of this disease. In another study, a topical formulation of CsA showed significant efficacy in patients with allergic contact dematitis.44 Finally, a comprehensive study of topical tacrolimus in paediatric atopic dermatitis showed significant suppression of disease when used in concentrations similar to those used in this study.44 Our results support the use of topical therapy with these agents44,45 and further suggest that topical tacrolimus may be more effective in the topical treatment of immune allergic responses in the skin, such as atopic dermatitis, compared to topical CsA. This may not necessarily be due to differences in skin absorption, but due in part to a distinct locus of action on the IgE/mast cell and T-cell signalling mechanisms, that are both likely to underly this complex and sometimes serious allergic skin disease.
In summary, we found that CsA and tacrolimus both strongly inhibit the late phases of IgE and CS responses when given systemically, and when applied topically to the skin. However, these drugs are differentially active on the early, mast-cell-dependent, effector phases of these responses. Tacrolimus more potently inhibits the early component of CS, while CsA more powerfully inhibits the early immediate hypersensitivity phase of IgE responses. Our findings demonstrate powerful topical effects of these agents on T-cell- and IgE-mediated allergic skin models. Topical treatment is less likely to be limited by significant side-effects compared to systemic administration, thus probably resulting in an improved therapeutic index. These findings support the use of these agents in the treatment of combined T-cell- and IgE-mediated allergic diseases with early mast cell-dependent components, that may be amenable to topical treatment, such as atopic dermatitis in the skin, and possibly allergic asthma in the airways.
Acknowledgments
1This work was supported by grants from the National Institutes of Health (AI-43371, AR-41942 and HL-56389); and the Maria Sklodowska Curie Fund and Committee for Scientific Research.
Abbreviations
- Ag
antigen
- CsA
cyclosporin A
- CS
contact sensitivity
- i.v.
intravenous
- OX
oxazolone
- PCl
picryl chloride
- TNP
trinitrophenyl.
References
- 1.Askenase PW. Effector and regulatory molecules and mechanisms in delayed-type hypersensitivity (DTH) In: Middleton E, Reed CE Jr, Ellis EF, Atkinson NF, Yunginger JW, Busse WW, editors. Allergy: Principles and Practice. 5. St. Louis: C.V Mosby Co.; 1998. pp. 323–41. [Google Scholar]
- 2.Van Loveren H, Meade R, Askenase PW. An early component of delayed-type hypersensitivity mediated by T cells and mast cells. J Exp Med. 1983;157:1604–7. doi: 10.1084/jem.157.5.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Askenase PW, Herzog W, Millet I, Paliwal V, Ramabhadran R, Rochester C, Geba GP, Ptak W. Serotonin initiation of delayed-type hypersensitivity: mediation by a primitive Thy-1+ antigen-specific clone or by monoclonal IgE antibody. Skin Pharmacol. 1991;4:25–42. doi: 10.1159/000210981. [DOI] [PubMed] [Google Scholar]
- 4.Geba GP, Ptak W, Anderson GM, Paliwal V, Ratzlaff RE, Levin J, Askenase PW. Delayed-type hypersensitivity in mast cell-deficient mice. Dependence on platelets for expression of contact sensitivity. J Immunol. 1996;157:557–65. [PubMed] [Google Scholar]
- 5.McHale J, Harari OA, Marshall D, Haskard DO. Vascular endothelial cell expression of ICAM-1 and VCAM-1 at the onset of eliciting contact hypersensitivity in mice: Evidence for a dominant role of TNF-α. J Immunol. 1999;162:1648–55. [PubMed] [Google Scholar]
- 6.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–98. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 7.Askenase PW, Rosenstein RW, Ptak W. T cells produce an antigen-binding factor with an in vivo activity analogous to IgE antibody. J Exp Med. 1983;157:862–73. doi: 10.1084/jem.157.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kops SK, Van Loveren H, Rosenstein RW, Ptak W, Askenase PW. Mast cell activation and vascular alterations in immediate hypersensitivity-like reactions induced by T cell-derived antigen-binding factor. Lab Invest. 1984;50:421–34. [PubMed] [Google Scholar]
- 9.Askenase PW. Delayed-type hypersensitivity (DTH) recruitment of T cell subsets via antigen-specific non IgE factors, and IgE antibodies: relevance to asthma, autoimmunity and immune resistance to tumors and parasites. In: Coffman R, editor. The Regulation and Functional Significance of T Cell Subsets; Progress in Chemical Immunology. Vol. 54. Basel: S. Karger; 1992. pp. 166–211. [PubMed] [Google Scholar]
- 10.Askenase PW. B-1 B cell IgM antibody initiates T cell elicitation of contact sensitivity. Curr Top Microbiol Immunol. 2000;252:171–9. doi: 10.1007/978-3-642-57284-5_18. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji RF, Geba GP, Wang Y, Kawamoto K, Matis LA, Askenase PW. Required early complement activation in contact sensitivity with generation of local C5-dependent chemotactic activity and late T cell IFN-γ: a possible initiating role of B cells. J Exp Med. 1997;186:1015–26. doi: 10.1084/jem.186.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askenase PW, Kawikowa I, Paliwal V, Akahira-Azuma M, Gerard C, Hugli T, Tsuji R. A new paradigm of T cell allergy: Requirement for the B-1 cell subset. Int Arch Allergy Immunol. 1999;118:145–9. doi: 10.1159/000024052. [DOI] [PubMed] [Google Scholar]
- 13.Shevach EM. The effects of Cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- 14.Cohen DJ, Loertscher R, Tilney NL, Carpenter CC, Strom TR. Cyclosporine: a new immunosupressive agent for organ transplantation. Ann Int Med. 1984;101:667–82. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- 15.Parsons WH, Sigal NH, Wyvratt MJ. FK-506-a novel immunosupressant. NY Acad Sci. 1993;685:22–36. doi: 10.1111/j.1749-6632.1993.tb35847.x. [DOI] [PubMed] [Google Scholar]
- 16.DeFranco AL. Immunosuppressants at work. Nature. 1991;352:754–5. doi: 10.1038/352754a0. [DOI] [PubMed] [Google Scholar]
- 17.Sigal NH, Dumont FJ. Cyclosporin A, FK-506 and rapamycin: pharmacologic probes of lymphocyte activation. Annu Rev Immunol. 1992;10:519–60. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 18.Trenn G, Taffs R, Hohman R, Kincaid EM, Shevach M Sitkovsky. Biochemical characterization of the inhibitory effect of cyclosporin A on cytolytic T lymphocyte effector functions. J Immunol. 1989;142:3796–802. [PubMed] [Google Scholar]
- 19.Draberova L. Cyclosporin A inhibits rat mast cell activation. Eur J Immunol. 1990;20:1469–73. doi: 10.1002/eji.1830200710. [DOI] [PubMed] [Google Scholar]
- 20.Hultsch T, Rodriguez JL, Kaliner MA, Hohman R. Cyclosporin A inhibits degranulation of rat basophilic leukemia cells and human basophils. Inhibition of mediator release without affecting PI hydrolysis or Ca 2+ fluxes. J Immunol. 1990;144:2659–64. [PubMed] [Google Scholar]
- 21.Cirillo R, Triggiani M, Siri L, Ciccarelli A, Pettit GR, Condorelli M, Marone G. Cyclosporin A rapidly inhibits mediator release from human basophils presumably by interacting with cyclophilin. J Immunol. 1990;144:3891–7. [PubMed] [Google Scholar]
- 22.Peguet-Navarro J. Does cyclosporin A directly affect human Langerhans cell function? J Invest Dermatol. 1991;96:953–5. doi: 10.1111/1523-1747.ep12491847. [DOI] [PubMed] [Google Scholar]
- 23.Dupuy P, Bagot M, Michel M, Descourt B, Dubetret L. Cyclosporin A inhibits the antigen-presenting function of freshly isolated human Langerhans cells in vitro. J Invest Dermatol. 1991;96:408–13. doi: 10.1111/1523-1747.ep12469772. [DOI] [PubMed] [Google Scholar]
- 24.Furue M, Katz SL. The effect of cyclosporin on epidermal cells. I. Cyclosporin inhibits accessory cell functions of epidermal Langerhans cells in vitro. J Immunol. 1988;140:4139–43. [PubMed] [Google Scholar]
- 25.Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentimno D, Nourse J, Crabtree GR. The mechanism of action of cyclosporin A and FK-506. Clin Immunol Immunopathol. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK-506. Immunol Today. 1992;13:136–42. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 27.Fruman DA, Burakoff SJ, Bierer BE. Immunophilins in protein folding and immunosupression. FASEB J. 1994;8:391–400. doi: 10.1096/fasebj.8.6.7513288. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKB-FK-506 complexes. Cell. 1991;66:807–15. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 29.O'Keefe SJ, Tamara J, Kincaid RL, Tocci MJ, O'Neill EA. FK-506 and CsA-sensitive activation of the IL-2 promoter by calcineurin. Nature. 1992;357:692–4. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu J. FK-506 and cyclosporin, molecular probes for studying intracellular signal transduction. Immunol Today. 1993;14:290–5. doi: 10.1016/0167-5699(93)90048-P. [DOI] [PubMed] [Google Scholar]
- 31.Bishop DK, Li W. Cyclosporin A and FK-506 mediate differential effects on T cell activation in vivo. J Immunol. 1992;148:1049–54. [PubMed] [Google Scholar]
- 32.Jiang H, Kobayashi M. Differences between cyclosporin A and tacrolimus in organ transplantation. Transplant Proceed. 1999;5:1978–80. doi: 10.1016/s0041-1345(99)00235-3. [DOI] [PubMed] [Google Scholar]
- 33.Liu FT, Bohn JW, Ferry EL, Yamamoto H, Molinaro CA, Sherman LA, Klinman NR, Katz DH. Monoclonal dinitrophenyl-specific murine IgE antibody. Preparation, isolation characterization. J Immunol. 1980;124:2728–37. [PubMed] [Google Scholar]
- 34.Busuttil RW, Holt CD. Tacrolimus is superior to cyclopsorine in liver transplantation. Transplant Proc. 1994;30:2174–8. doi: 10.1016/s0041-1345(98)00579-x. 10.1016/s0041-1345(98)00579-x. [DOI] [PubMed] [Google Scholar]
- 35.Van Loveren H, Askenase PW. Delayed-type hypersensitivity is mediated by a sequence of two different T cell activities. J Immunol. 1984;133:2397–401. [PubMed] [Google Scholar]
- 36.Van Loveren H, Kato K, Meade R, Green DR, Horowitz M, Ptak W, Askenase PW. Characterization of two different Ly1+ T cell populations that mediate delayed-type hypersensitivity. J Immunol. 1984;133:2402–11. [PubMed] [Google Scholar]
- 37.Herzog WR, Ferreri NR, Ptak W, Askenase PW. The antigen-specific DTH-initiating Thy-1+ cell is double negative (CD4−, CD8−) and CD3 negative; and expresses IL-3 receptors, but no IL-2 receptors. J Immunol. 1989;143:3125–33. [PubMed] [Google Scholar]
- 38.Ptak W, Herzog WR, Askenase PW. Delayed-type hypersensitivity initiation by early-acting cells that are antigen mismatched or MHC incompatible with late-acting, delayed-type hypersensitivity effector T cells. J Immunol. 1991;146:469–75. [PubMed] [Google Scholar]
- 39.Bentley AM, Kay AB, Durham SR. Human late asthmatic responses. In: Kay AB, editor. Allergy and Allergic Diseases. Vol. 2. Cambridge: Blackwell Science; 1997. pp. 1113–330. [Google Scholar]
- 40.Van Loveren H, Kraeuter-Kops S, Askenase PW. Different mechanisms of release of vasoactive amines by mast cells occur in T cell-dependent compared to IgE-dependent cutaneous hypersensitivity responses. Eur J Immunol. 1984;14:40–7. doi: 10.1002/eji.1830140108. [DOI] [PubMed] [Google Scholar]
- 41.Lauerma AI, Surber C, Maibach HI. Absorption of topical tacrolimus (FK-506) in vitro through human skin: comparison with Cyclosporin A. Skin Pharmacol. 1997;10:230–4. doi: 10.1159/000211510. [DOI] [PubMed] [Google Scholar]
- 42.Sowden JM, Berth-Jones J, Ross JS, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991;338(8760):137–40. doi: 10.1016/0140-6736(91)90134-b. [DOI] [PubMed] [Google Scholar]
- 43.Surber C, Itin P, Buehner S, Maibach HI. Effect of a new topical cyclosporin formulation on human allergic contact dermatitis. Contact Dermatitis. 1992;26:116–19. doi: 10.1111/j.1600-0536.1992.tb00895.x. [DOI] [PubMed] [Google Scholar]
- 44.Boguniewicz M, Fiedler VC, Raimer S, Lawrence VD, Leung DJ, Hanifin JM. A randomized, vehicle controlled trial of tacrolimus ointment for the treatment of atopic dermatitis in children. J Allergy Clin Immunol. 1998;102:637–44. doi: 10.1016/s0091-6749(98)70281-7. [DOI] [PubMed] [Google Scholar]
- 45.Bieber T. Topical tacrolimus (FK-506): a new milestone in the treament of atopic dermatitis. J Allergy Clin Immunol. 1998;102:555–7. doi: 10.1016/s0091-6749(98)70270-2. [DOI] [PubMed] [Google Scholar]