Abstract
Mast cell chymase plays important roles in inflammation and tissue remodeling. Here we show that mast cell chymase also functions as an enhancer of immunoglobulin production. In the culture of murine spleen cells stimulated with lipopolysaccharide and interleukin-4, purified rat chymase (rat mast cell protease-I; RMCP-I), at physiological concentrations, enhanced immunoglobulin E (IgE) and IgG1 syntheses but not IgG3 synthesis. The enhancement was also evident when spleen cells depleted of T cells and macrophages were employed as responding cells. Enzymatic activity of RMCP-I was required to enhance IgE and IgG1, because two inhibitors for chymotryptic enzymes, chymostatin and Y-40613, a novel chymase inhibitor, suppressed the enhanced immunoglobulin production, and phenylmethylsulphonyl fluoride, an irreversible inhibitor for serine proteases, totally abolished the enhancing effect. Furthermore, a specific inhibitor for Zn2+-dependent metalloproteases, GI 129471, could also completely inhibit the production of IgE and IgG1 that was enhanced by RMCP-I, suggesting that a metalloprotease also played an essential role in the immunoglobulin production. Our results together with others show that proteases from mast cell granules have important function not only in the efferent phase but also in the afferent phase of immune responses.
Introduction
Mast cells contain two types of serine proteases in their granules, and secrete them upon degranulation induced by various stimuli.1 Mast cell chymase [EC 3.4.21.39] is a chymotrypsin-like enzyme, and participates in inflammation and subsequent tissue remodelling through various actions, including conversion of angiotensin I to angiotensin II, activation of pro-interleukin (IL)-1β and various metalloproteases (MMPs), and degradation of various neuropeptides and extracellular matrices.2,3 Chymase released by degranulation of mast cells can also induce activation of other mast cells in the vicinity, and degranulation of airway serous cells.4,5 Although the cellular activation requires enzymatic activity of chymase, the underlying mechanism has not been determined in detail.
We are interested in the role of mast cell proteases in various immunological and cardiovascular diseases, and have developed synthetic chymase inhibitors as novel therapeutics. In the series of pharmacological evaluations of the inhibitors in animal models of inflammation driven by the T helper type 2 lymphocyte (Th2)-associated immune response, we observed that the inhibitors not only suppressed indices of the inflammation, but also lowered serum immunoglobulin E (IgE) level in the immunized animals. We hypothesized that mast cell chymase might be directly involved in IgE production, because various serine proteases have been reported to modulate IgE response.
Ishizaka and colleagues described a glycosylation enhancing factor (GEF) that was produced by T cells stimulated with antigens, which in turn directed the generation of IgE-potentiating factor by another type of T cell.6 The resulting IgE-potentiating factor augmented IgE production by stimulated B cells. Iwata et al. reported that GEF was a kallikrein-like serine protease, and the GEF activity itself was dependent on its enzymatic activity.7 Matsushita et al. described a T-cell derived protein, ε receptor modulating protein (εRMP), that modulated the binding avidity between IgE and CD23.8 The protein was a chymotrypsin-like serine protease, as it had amidolytic activity against a synthetic substrate, N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide, and its enzymatic activity was required for the modulation of CD23.9 εRMP by itself could induce IgE production in the presence of IL-4 in mouse B lymphocytes.10 They also showed that bovine chymotrypsin as well as kallikrein enhanced IgE and IgG1 syntheses of murine B cells elicited by lipopolysaccharide (LPS) plus IL-4 in vitro at relatively low concentrations, and that these enzymes inhibited the immunoglobulin production at higher concentrations.11
In this study, we have tested the possibility that mast cell chymase modulates IgE production, employing a purified enzyme and its specific inhibitors.
Materials and methods
Enzymes and inhibitors
Rat mast cell protease-I (RMCP-I) was purified from rat skin according to the purification procedure for human chymase by Urata et al.12 with some modifications. Briefly, shaved skin of Wistar rats was minced, then homogenized with polytron homogenizer in 20 mm sodium phosphate, pH 7·4, centrifuged at 8000 r.p.m. for 60 min, and the supernatant was discarded. High-salt buffer (10 mm sodium phosphate, pH 7·4, 2 m KCl, 0·1% Triton-X-100) was added to the precipitate, further homogenized, and the resulting supernatant was collected after centrifugation. The supernatant was dialysed against 20 mm Tris, pH 8·0, 0·3 m KCl, and mixed with Hitrap Heparin Sepharose (Pharmacia Biotech, Uppsaala, Sweden). After extensively washing the gel with 20 mm Tris, pH 8·0, 0·3 m KCl, RMCP-I was eluted with a linear gradient of 20 mm Tris, pH 8·0, 0·3 m KCl and 20 mm Tris, 1·8 m KCl, 0·1% Triton-X-100. Purified RMCP-I was dialysed against 20 mm Tris, pH 8·0, 1 m NaCl, 0·1% Triton-X-100 and stored at a concentration of 1·4 mg/ml at −80°. Protein concentration was determined with a BCA kit (Pierce Chemical Co., Rockford, IL). The RMCP-I was homogeneous according to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis using a Laemmli gel under both reducing and non-reducing conditions (TEFCO, Matsumoto, Japan), and hydrolysed a synthetic substrate, succinyl-l-Ala-l-Ala-l-Pro-l-Phe-p-nitroanilide (Sigma Chemical Co., St. Louis, MO). To inactivate RMCP-I, phenylmethylsulphonyl fluoride (PMSF; Sigma Chemical Co.) was dissolved in isopropanol at the concentration of 50 mm, and added to RMCP-I at the final concentration of 1·5 mm in three aliquots. The inactivated RMCP-I was extensively dialysed against 20 mm Tris, pH 8·0, 1 m NaCl, 0·1% Triton-X-100. No hydrolysing activity against the synthetic substrate was detected after inactivation. When either RMCP-I or PMSF-treated RMCP-I were added to the culture, the control culture included the same dilution of the buffer, 20 mm Tris, pH 8·0, 1 m NaCl, 0·1% Triton-X-100. Bovine chymotrypsin, diisopropyl fluorophosphate (DFP)-treated chymotrypsin and chymostatin were purchased from Sigma Chemical Co. A specific chymase inhibitor, Y-40613 (2-[5-amino-2-(4-fluorophenyl)-1,6-dihydro-6-oxo-1-pyrimidinyl]-N-{1-[(5-methoxycarbonyl-2-benzoxazolyl) carbonyl]-2-phenylethyl} acetamide), was designed and synthesized in our Pharmaceutical Research Division.13 A specific MMP inhibitor, GI129471, was also synthesized in our Research Division. All enzyme inhibitors were first dissolved in dimethylsulphoxide (DMSO) at concentrations between 0·1 and 100 mm, and diluted with culture medium. The final concentration of DMSO did not exceed 0·1%.
Cell culture
Spleen cells were prepared from female BALB/c mice (Japan SLC, Hamamatsu, Japan) aged more than 7 weeks. B cells were purified from the spleen cells using magnetic-activated cell sorting (MACSTM) according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Briefly, the spleen cells were incubated with anti-Thy1.2 beads, anti-CD4 beads, anti-CD8α beads and anti-CD11b beads, applied to a CS column (Miltenyi Biotec), and the unbound cells were collected. Adherent cells were removed by plating cells to plastic dishes (F3003; Falcon Becton Dickinson, Franklin Lakes, NJ) overnight in RPMI-1640 (Life Technologies, Rockville, MD) supplemented with 5 × 10−5 m 2-mercaptoethanol (2-ME) and 10% fetal bovine serum (FBS; Hyclone, Logan, UT). In order to assess the purity of B cells, cells were stained with a fluorescein isothiocyanate (FITC)-labelled anti-CD3 antibody and a FITC-labeled anti-CD11b antibody (PharMingen, San Diego, CA), and analysed by fluorescence-activated cell sorting (FACSCaliburTM; Becton Dickinson, San Jose, CA). Depending on the preparation, the enriched B-cell preparation contained less than 1·2% of CD3+ cells as well as 0·4% CD11b+ cells.
Spleen cells or purified B cells (3 × 105) were cultured in 200 µl of RPMI-1640 supplemented with 5 × 10−5 m 2-ME and 10% FBS in the presence of 20 µg/ml Salmonella typhimurium LPS (Sigma Chemical Co.) and 500 ng/ml recombinant murine IL-4 (WAKO Pure Chemical Industries, Osaka, Japan) for 7 days at 37°. IgE and IgG1 in the supernatant were quantitated by sandwich enzyme-linked immunosorbent assay (ELISA). In the case of IgG3 production, IL-4 was omitted from the culture.
ELISA
ELISA plates (Corning Costar Japan, Tokyo, Japan) were coated with 250 ng of a monoclonal anti-mouse IgE antibody (clone R35-72; Pharmingen) in 50 µl phosphate-buffered saline (PBS). After blocking with 50 mm Tris, pH 7·6, 150 mm NaCl containing 5% skimmed milk, culture supernatant or standard IgE was added to the plate for 1 hr at room temperature. After washing with PBS containing 0·05% Tween-20, the plates were sequentially incubated with a biotinylated anti-mouse IgE antibody (clone LO-ME-2, 1 µg/ml; Serotec, Oxford, UK) and horseradish peroxidase-conjugated streptavidin (1 µg/ml; Vector Laboratories, Burlingame, CA) for 1 hr each. The bound peroxidase was quantitated with o-phenylenediamine and hydrogen peroxide as substrate. Mouse monoclonal IgE purified from IGELb4 hybridoma (TIB141, American Type Culture Collection, Rockville, MD) supernatant was employed as a standard.14 IgG1 and IgG3 were quantitated in the same manner, except that an anti-mouse IgG1 antibody (clone A85–1; PharMingen) and an antimouse IgG3 antibody (clone R40–82; PharMingen) were used as capture antibodies, and that a biotinylated anti-mouse IgG1 antibody (clone LO-MG1–2; Cymbus Biotechnology, Chandlers Ford, UK) and a biotinylated anti-mouse κ antibody (clone R8-140; PharMingen) were used as detecting antibodies, respectively. Mouse myeloma IgG1 (clone MOPC-21; PharMingen) and IgG3 (clone J606; PharMingen) were employed as standards.
Reverse transcription–polymerase chain reaction (RT–PCR)
RNA was prepared from spleen cells stimulated for 4 days as described above, except that six-well plates were used instead of 96-well plates. Trizol reagent (Life Technologies) was used to isolate RNA according to the manufacturer's instructions. The RNA was reverse-transcribed and amplified by PCR using TaKaRa RNA PCR Kit (Takara, Tokyo, Japan) according to the manufacturer's instructions. As reported by Roper et al.,15 primers to amplify mature Cε complementary DNA (cDNA)were 5′-TCAAGGAACCTCAGTCACCGTC-3′ (sense primer) corresponding to JH4 gene, and 5′-CTAGGATAGTCTGTCAGGT-3′ (antisense primer) from the sequence of exon 1 of Cε and the size of the PCR product from mature Cε was approximately 300 bp. Primers to amplify β-actin cDNA were 5′-ATGGATGACGATATCGCT-3′ (sense primer) and 5′-ATGAGGTAGTCTGTCAGGT-3′ (antisense primer). Cycle conditions were 94° for 1 min for denaturation, 57° for 1 min for annealing, and 72° for 1·5 min for extension. After 25, 28, 31 or 34 cycles, the PCR product was resolved in agarose gel electrophoresis, and stained with ethidium bromide.
Results
Bovine chymotrypsin enhances the production of IgE and IgG1 by mouse spleen cells
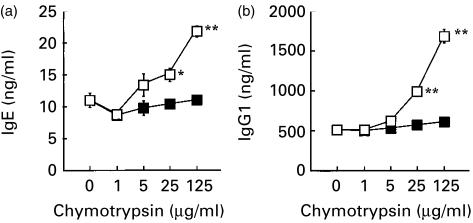
In the first series of experiments, we estimated the potency of bovine chymotrypsin to modulate IgE response in our culture system. When added to the culture of mouse spleen cells, chymotrypsin enhanced the production of both IgE and IgG1 stimulated with LPS and IL-4 (Fig. 1a, b, respectively). Its action was dependent on the concentration, and evident above 25 µg/ml in the experiment shown in Fig. 1. Enzymatic activity was essential for its enhancing activity, because DFP-treated chymotrypsin was totally inactive (Fig. 1).
Figure 1.
Bovine chymotrypsin enhances the production of IgE and IgG1 by murine spleen cells stimulated with LPS plus IL-4. Spleen cells from BALB/c mice were cultured with or without indicated concentration of bovine chymotrypsin (open squares) or DFP-treated chymotrypsin (closed squares) in the presence of LPS plus IL-4 for 7 days. IgE (a) and IgG1 (b) in the supernatant were quantitated by ELISA. Each symbol and bar represent mean and standard error of mean (SEM; n = 6). Values obtained from the culture containing chymotrypsin were compared with values from the culture without chymotrypsin by Dunnett's method (*, P < 0·05; **, P < 0·01).
Thus, though a higher concentration was required than that reported by Matsushita and Katz,11 chymotrypsin clearly enhanced the IgE and IgG1 responses in our culture system, and these results prompted us to test the effects of mast cell chymase on the IgE production.
RMCP-I enhances the production of IgE and IgG1
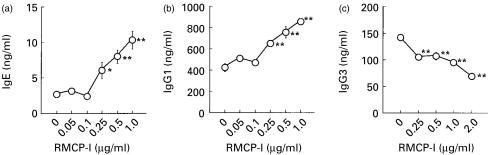
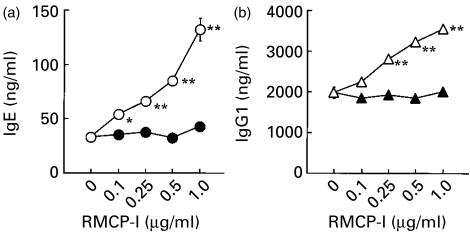
Mast cells are distributed throughout the body, and a subset of mast cells secretes a chymotryptic enzyme, mast cell chymase, when activated. In order to explore the possibility that mast cell chymase may also modulate the IgE production, RMCP-I was purified from rat skin, and added to the culture. We employed rat chymase instead of mouse enzyme, because RMCP-I could be obtained in large enough quantities for our experiments, and it is closely homologous to mouse chymase, mouse mast cell protease-4 (about 90% identical in primary sequence).16 The purified RMCP-I migrated as a single band with a molecular weight of about 30 000 MW in SDS–PAGE under both reducing and non-reducing conditions (data not shown). As shown in Fig. 2(a), up to 1·0 µg/ml RMCP-I enhanced the IgE synthesis stimulated with LPS plus IL-4 in a concentration-dependent manner. The same concentration of RMCP-I also enhanced the IgG1 production (Fig. 2b), and the minimum concentration of RMCP-I effective to enhance the production of IgE and IgG1 was as low as 250 ng/ml. In contrast to IgE and IgG1, RMCP-I suppressed the IgG3 production stimulated with LPS (Fig. 2c), suggesting that RMCP-I did not exert its enhancing effect by improving culture conditions or cell viability.
Figure 2.
RMCP-I enhances the production of IgE and IgG1, but not IgG3. Spleen cells from BALB/c mice were cultured with or without indicated concentration of RMCP-I in the presence of either LPS plus IL-4 (a and b) or LPS alone (c) for 7 days. IgE (a), IgG1 (b) and IgG3 (c) in the supernatant were quantitated by ELISA. Each symbol and bar represents mean and SEM (n = 6). Values obtained from the culture containing chymase were compared with values from the culture without chymase by Dunnett's method (*, P < 0·05; **, P < 0·01).
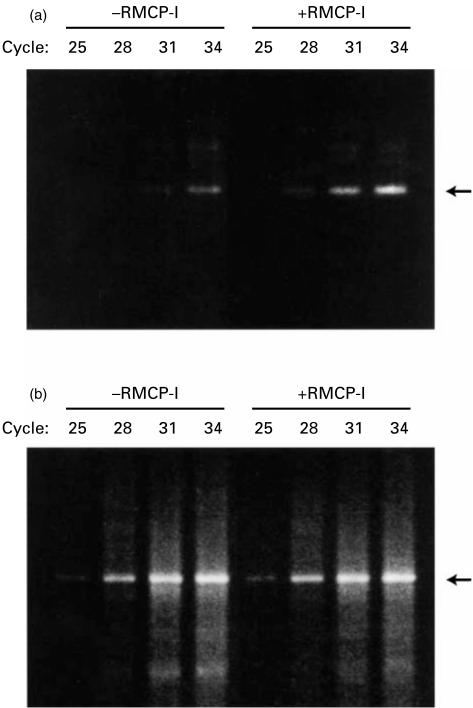
In order to confirm the enhancement of the IgE production by RMCP-I, the amount of mature Cε mRNA was estimated with RT–PCR (Fig. 3). RNA was extracted from the spleen cells that had been cultured with or without RMCP-I (1·0 µg/ml) in the presence of LPS plus IL-4 for 4 days, and analysed by RT–PCR using the primers for mature Cε described by Roper et al.15 The amplified band from mature Cε cDNA was detected after the 28th cycle in the spleen cells cultured with RMCP-I, whereas the band could be seen after the 31st cycle in the cells cultured without RMCP-I (Fig. 3a). The intensity of the bands derived from β-actin did not differ significantly between the two groups (Fig. 3b). These results indicated that mRNA for mature Cε was more abundant in the B cells cultured with RMCP-I.
Figure 3.
RT–PCR analysis of mature Cε mRNA.BALB/c spleen cells were cultured with or without 1 µg/ml RMCP-I in the presence of LPS plus IL-4 for 4 days. cDNA was reverse transcribed from mRNA, and amplified with the primer pairs for mature Cε (a) and β-actin (b) for indicated cycles. Bands in agarose gel were visualized with ethidium bromide.
Proteolytic activity of RMCP-I is required for enhancing immunoglobulin production
To test whether enzymatic activity of RMCP-I was required, two inhibitors for chymase were used: chymostatin and Y-40613. Chymostatin inhibits chymotryptic enzymes, such as chymotrypsin and chymase, as well as cathepsins B and D, and papain. The production of both IgE and IgG1 enhanced by RMCP-I was suppressed in the presence of chymostatin, although its inhibitory effect was only partial (Fig. 4). The inhibition of the immunoglobulin production was dependent on the concentration of chymostatin; the IgG1 production was significantly inhibited above the inhibitor concentration of 0·1 µm in the experiment of Fig. 4(b), whereas the inhibition of the IgE production was statistically significant at the inhibitor concentration of 1·0 µm (Fig. 4a). The inhibitory effect of chymostatin was not caused by possible toxicity of this compound, because the concentration used in our experiments was well below the toxic concentration. Chymostatin up to 100 µg/ml (170 µm) has been used in lymphocyte culture without any effects on cell growth or viability.17
Figure 4.
Enzymatic activity of RMCP-I is required for enhancing IgE and IgG1. BALB/c spleen cells were cultured with (grey column) or without (open column) 1 µg/ml RMCP-I in the presence of LPS plus IL-4 for 7 days. Indicated concentration of either chymostatin (closed symbols) or Y-40613 (open symbols) was added to some culture with RMCP-I. Concentrations of IgE (a) and IgG1 (b) were determined by ELISA. Each symbol and bar represents mean and SEM (n = 6). The data obtained in the presence of the inhibitors were compared to the data obtained in the absence of the inhibitors by Williams' Multiple Comparison Test (*, P < 0·05; **, P < 0·01).
Y-40613 was a novel inhibitor specific for chymotryptic enzymes including chymase.13 The structure dictates its fine specificity against endopeptidases that cleave peptide bonds carboxyl-terminal to aromatic residues, and Y-40613 did not affect the activity of tryptic enzymes, such as mast cell tryptase, thrombin, plasmin and kallikrein. Y-40613 inhibits RMCP-I with a Ki value of 103 nm. The production of both IgE and IgG1 was suppressed by Y-40613 in a dose-dependent manner, though the inhibition was again partial (Fig. 4). The suppressive effect of Y-40613 as well as chymostatin indicated that chymotryptic activity was required for the enhancement of IgE and IgG1, and other materials possibly contaminating in our RMCP-I preparation, especially other proteases, were not responsible for the enhancement. In other experiments mast cell tryptase purified from rat skin could not enhance the IgE production in our system (data not shown). The only partial inhibition of the enhancement by two inhibitors could be caused by their limited potency, because RMCP-I, inactivated irreversibly with PMSF, was totally inactive in enhancing immunoglobulin production (see Fig. 5). These results indicated that proteolytic activity of RMCP-I was essential for enhancing both IgE and IgG1.
Figure 5.
RMCP-I enhances the production of IgE and IgG1 by enriched B cells. B cells enriched from BALB/c spleen cells were cultured with or without indicated concentration of either RMCP-I (open symbols) or PMSF-treated RMCP-I (closed symbols) in the presence of LPS plus IL-4 for 7 days. Concentrations of IgE (a) and IgG1 (b) in the supernatant were determined by ELISA. Each symbol and bar represents mean and SEM (n = 6). Values obtained from the culture containing chymase were compared with values from the culture without chymase by Dunnett's method (*, P < 0·05; **, P < 0·01).
B cells are the target cells of RMCP-I
In order to examine the possibility that RMCP-I might act on B cells directly, B cells were enriched by MACSTM, and cultured with or without graded concentration of RMCP-I in the presence of LPS plus IL-4. As shown in Fig. 5, the production of both IgE and IgG1 by the enriched B cells was enhanced by RMCP-I in a concentration-dependent manner: the enhancement was evident above 100–250 ng/ml of RMCP- I, the same concentration required for the whole spleen cells, and the immunoglobulin production was maximal at 1·0 µg/ml as shown in the whole spleen cells (see Fig. 2). Thus, T cells and macrophages were not required for enhancement of the immunoglobulin production, suggesting that B cells might be the direct target cells of RMCP-I. We could frequently observe higher immunoglobulin production in the enriched B cells (compare the vertical axes of Fig. 2a,b and Fig. 5a,b). This might be a result of the suppressive effects of macrophages, because removal of CD11b+ cells caused enhancement of immunoglobulin production (data not shown). RMCP-I pretreated with PMSF neither enhanced nor suppressed immunoglobulin production, demonstrating that enzymatic activity was essential for modulating the production of both IgE and IgG1 (Fig. 5).
An essential role of an MMP in enhancing immunoglobulin syntheses
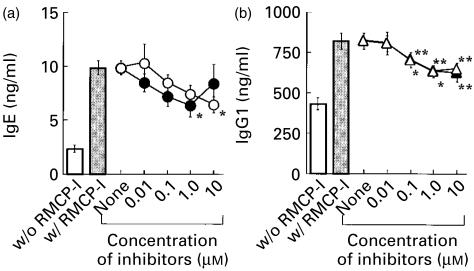
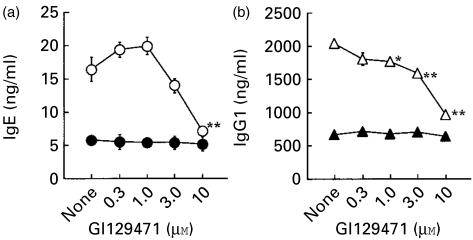
Mast cell chymase could activate various MMPs,18–21 and some MMPs were shown to be involved in IgE production in vitro.22,23 In order to examine the possibility that RMCP-I exerted its effects through MMP(s), a specific inhibitor for MMPs, GI 129471, was added to the culture with RMCP-I. GI 129471 was synthesized as a collagenase inhibitor and shown to inhibit various MMPs including tumour necrosis factor-α (TNF-α)-converting enzyme.24 GI 129471 inhibited the production of both IgE and IgG1 by the enriched B cells that was enhanced by RMCP-I, in a dose-dependent fashion, and the inhibition was nearly complete at the highest concentration of the inhibitor (10 µm; Fig. 6). GI 129471, however, did not affect the immunoglobulin production in the absence of RMCP-I (Fig. 6), indicating that the inhibitory effect was not caused by any toxicity of the compound on B cells. Given that GI 129471 did not affect the proteolytic activity of RMCP-I, these results indicated that MMPs played a role when RMCP-I enhanced the IgE and IgG1 responses.
Figure 6.
A metalloprotease inhibitor, GI129471, blocks the production of IgE and IgG1 enhanced by RMCP-I. B cells enriched from BALB/c spleen cells were cultured with (open symbols) or without (closed symbols) 1 µg/ml RMCP-I in the presence of LPS plus IL-4 for 7 days. Indicated concentration of GI129471 was added to some culture. Concentrations of IgE (a) and IgG1 (b) in the supernatant were determined by ELISA. Each symbol and bar represents mean and SEM (n = 6). Values obtained from the culture containing GI129471 were compared with values from the culture without the inhibitor by Dunnett's method (*, P < 0·05; **, P < 0·01).
Discussion
We have been interested in the function of mast cell proteases, and this report shows that rat mast cell chymase (RMCP-I) as well as bovine chymotrypsin could enhance the production of both IgE and IgG1 by mouse B cells stimulated with LPS plus IL-4. The enhancement of IgE and IgG1 responses by bovine chymotrypsin is in agreement with the report by Matsushita and Katz,11 although we could not observe inhibition of the IgE and IgG1 responses at relatively high concentrations of chymotrypsin. It seemed unlikely that chymotrypsin would be present in the milieu of B lymphocytes enough to enhance their immunoglobulin production, because a high concentration of the enzyme (25 µg/ml) was required (Fig. 1). Our data support the idea that mast cell chymase acts as a ‘physiological’ chymotryptic enzyme to modulate the IgE and IgG1 responses. Mast cells are largely classified into two types according to their serine proteases: T type contains tryptase alone, while TC type contains both tryptase and chymase, and both types are distributed throughout the body, especially along surfaces exposed to the environment. Mast cells of TC type are abundant in, for example, skin, conjunctiva and submucosa of small intestine.25 Mast cell chymase could be released in tissues at the concentration enough to affect the immunoglobulin production. For instance, local concentration of chymase would reach 1 µm (30 µg/ml) after degranulation of skin mast cells, considering that 5 × 106 mast cells exist per 1 cm3 skin.26
RMCP-I enhanced the production of both IgE and IgG1 stimulated with LPS plus IL-4, while it slightly inhibited the IgG3 production stimulated with LPS (Fig. 2), indicating that mast cell chymase selectively augmented the immunoglobulin isotypes that dominated in the Th2 immune response. Given that RMCP-I acts on B lymphocytes (Fig. 5), signals delivered by mast cell chymase may exert its enhancing effects on the immunoglobulin production in B cells in concert with the signals from a Th2 cytokine, IL-4. The mechanism however, remains to be determined; it is possible that mast cell chymase could enlarge the population of IgE-producing B cells as well as IgG1-producing B cells, either by promoting class switch or cell proliferation, or that chymase could augment IgE and IgG1 syntheses by individual B cells.
The action of RMCP-I is dependent on its proteolytic activity, because two inhibitors, chymostatin and Y-40613, suppressed the enhancing effect of RMCP-I on the production of IgE and IgG1 (Fig. 4), and an irreversible inhibitor, PMSF, totally abolished the enhancing effect (Fig. 5). Requirement for the enzymatic activity is similar to bovine chymotrypsin (Fig. 1), and it is likely that both chymase and chymotrypsin exert their effects through the same target molecule, though mast cell chymase appeared even more effective than chymotrypsin.
Our data employing an MMP inhibitor, GI 129471, indicate that a Zn2+-dependent MMP is somehow involved in the enhancement of the IgE and IgG1 responses (Fig. 6). A precursor of some MMP that putatively modulates immunoglobulin syntheses could be a direct target of mast cell chymase, because chymase has been shown to activate precursors of various MMPs, such as MMP-1 (collagenase), MMP-3 (stromelysin) and MMP-9 (gelatinase) by proteolytic cleavage of the specific peptide bonds.18–21 MMP inhibitors have been shown to inhibit IgE production in human B cells stimulated with anti-CD40 antibody and IL-4 through inhibition of proteolysis of CD23,22,23 and one of them also reported that an MMP inhibitor, batimastat, suppressed IgE production of mouse B cells stimulated with LPS plus IL-4.22 GI 129471, however, only suppressed the response enhanced by RMCP-I, but not the immunoglobulin production in the absence of RMCP-I in this report (Fig. 6). The reason for the discrepancy between Christie et al.22 and ours is largely unknown, though there are some differences in the experimental procedure, such as the methods for enrichment of B cells: they used spleen cells depleted of T cells by complement-dependent cytolysis, in contrast to our use of MACSTM and adherence to remove both T cells and macrophages.
Modulation of IgE response by serine proteases was first described by Ishizaka and his colleagues to our knowledge. GEF was a T-cell derived soluble factor that converted IgE-binding factor produced by primed Lyt-1+ FcR+ T cells to IgE-potentiating factor by enhancing its N-linked glycosylation, which in turn augmented IgE production by primed B lymphocytes.6 They showed that GEF was a kallikrein-like serine protease, and that its enzymatic activity was essential for the GEF activity, because treatment of GEF with inhibitors for serine proteases such as PMSF and DFP abrogated its activity on the IgE response.7 Other serine proteases, trypsin, kallikrein and plasmin, also had GEF activity, and they postulated that at least a part of the GEF activity was a result of bradykinin generated from high molecular weight kininogen by its proteolytic activity, as bradykinin in concentrations as low as 10 ng/ml enhanced glycosylation of IgE-binding factor produced by a T-cell hybridoma.7 Mast cells of both TC type and T type also contain a trypsin-like enzyme, tryptase, in their granules, and it is most likely that mast cell tryptase could have the GEF activity, as human tryptase could not only activate plasma prekallikrein but directly generate bradykinin from high- and low-molecular-weight kininogens.27 There is, however, no data available about the GEF activity of mast cell tryptase.
In inflammation driven by the Th2-type immune response the number of mast cells would increase in the inflamed tissue through the action of chemokines such as eotaxin and RANTES (regulated on activation of normal T-cell expressed and secreted), because human mast cells of TC type and their progenitors express CCR3.28,29 Th2 cytokines produced locally, such as IL-10 and IL-4, may further promote the preferential development of TC type over the T type of mast cells from the progenitors.28,30,31 The mast cells express the elevated level of the high affinity receptor for IgE under the influence of IL-4 and IgE.30,32–35 When mast cells thus primed efficiently with specific IgE are activated with the cognate antigen, these activated mast cells may not only initiate an allergic reaction but also induce IgE synthesis, because the activated mast cells could express CD40L and secrete IL-4 and IL-13.36–39 Most importantly, Pawankar et al. showed that nasal mast cells from perennial allergic patients could express these molecules abundantly and elicit IgE production directly in naive B cells, when triggered with a specific allergen.40 Thus, B cells would be stimulated with the activated mast cells as well as Th2 cells in the inflamed tissues and form germinal centres producing IgE in situ, as shown in humans and in mice.41,42 Both our findings and others have indicated that granular contents of mast cells other than cytokines further support local IgE production: chymase augments IgE production by naive B cells elicited by IL-4, as shown in this report; tryptase generates bradykinin which in turn potentiates IgE production from primed B lymphocytes through IgE-binding factor;6,7,27 and histamine enhances IgE synthesis, as shown in human naive B cells stimulated with anti-CD58 antibody plus IL-4 or IL-13.43 Small vesicles called ‘exosomes’ were identified in the granules in murine mast cells, and the exosomes could also stimulate murine B cells.44 Another protease derived from the activated mast cells may also facilitate this process: cathepsin G produced by TC type mast cells can stimulate CD4+ T cells and is a chemokinetic factor for T cells.45,46 It is now obvious that mast cell products involved in efferent phase of immune responses may have quite a large impact on the afferent phase. A positive regulatory loop may exist in local immune responses: activation of mast cells is dependent on specific IgE, and the granular contents from the activated mast cells further potentiate the local IgE production. The local IgE production was suggested to be critical in some diseases such as intrinsic asthma.47
Genetic analysis also gives us a clue that mast cell chymase has an important role in atopic inflammation. Human mast cell chymase gene resides on chromosome 14q11, and Mao et al. reported an association of BstXI restriction fragment length polymorphism of the chymase locus with atopic eczema.48 The association is significant both in patients with pure atopic eczema with a lower IgE level (< 500 IU/ml) and in patients with a high IgE level (> 2000 IU/ml) and with a history of asthma.49,50 Although mast cell chymase is likely involved in proteolytic modulation of skin architecture in eczema, our data suggest that chymase may also be associated with atopic disorders at the stage of pathogenic IgE production.
The above scenario for Th2-driven inflammation may also be relevant in cardiovascular diseases. Mast cells of TC type are abundant in human heart tissue and in adventitia and intima of coronary arteries, and their function has been largely elusive.51 Recent research discovered a role of mast cell chymase in renin–angiotensin system: human chymase is a major enzyme that generates angiotensin II from angiotensin I, and some studies suggested that chymase may be much more potent than the angiotensin-converting enzyme.52 The induction and the amplification of IgE response by chymase may be involved in the pathogenesis of cardiovascular diseases. Marone et al. reviewed the increment of serum IgE level in the patients of coronary artery diseases, and suggested the role of IgE in the activation of cardiac mast cells in the pathogenesis.53
In order to demonstrate that mast cell chymase could act as an enhancer of IgE production in vivo, we took advantage of the fact that Y-40613 was orally active and had a long half life in vivo. After subcutaneous injection of mercuric chloride, Brown Norway rats mounted a high IgE response and multiple disorders including polyarthritis and nephritis.54 Oral administration of Y-40613 suppressed the IgE response in a dose-dependent fashion, and reduced the severity of arthritis and nephritis, suggesting the involvement of chymase in the IgE response in vivo (Kobayashi, F. et al. submitted for publication).
It is also important to know whether our finding is relevant to humans. Our preliminary data showed that recombinant human chymase expressed in Pichia pastoris enhanced the IgE production by normal human peripheral blood lymphocytes stimulated with IL-4 at a similar concentration as RMCP-I enhanced murine IgE synthesis, although the mechanism in detail remains to be determined (data not shown). Thus, inhibitors for mast cell chymase could be novel therapeutics that inhibit IgE-dependent disorders at both the induction and effector phases.
Acknowledgments
We thank Prof. Mizuo Miyazaki, Drs Naotaka Shiota, and Shinji Takai of Department of Pharmacology, Osaka Medical College for continuous support and valuable advice. We are also indebted to Drs Shigeki Kuwahara, Fujio Kobayashi and Naruyasu Komorita of Pharmaceutical Research Division, Mitsubishi Pharma Corporation for providing the enzyme inhibitors and for helpful discussions.
Abbreviations
- GEF
glycosylation enhancing factor
- MMP
metalloprotease
- RMCP
rat mast cell protease.
References
- 1.Lagunoff D. Mast cell proteases: a historical perspective. In: Caughey GH, editor. Mast Cell Proteases in Immunology and Biology. New York: Marcel Dekker, Inc.; 1995. pp. 1–8. [Google Scholar]
- 2.Redington AE, Polosa R, Walls AF, Howarth PH, Holgate ST. Role of mast cells and basophils in asthma. Chem Immunol. 1995;62:22–59. [PubMed] [Google Scholar]
- 3.Fukami H, Okunishi H, Miyazaki M. Chymase: its pathophysiological roles and inhibitors. Curr Pharm Des. 1998;4:439–53. [PubMed] [Google Scholar]
- 4.Schick B, Austen KF, Schwartz LB. Activation of rat serosal mast cells by chymase, an endogenous secretory granule protease. J Biol Chem. 1984;132:2571–7. [PubMed] [Google Scholar]
- 5.Sommerhoff CP, Caughey GH, Finkbeiner WE, Lazarus SC, Basbaum CB, Nadel JA. Mast cell chymase: a potent secretagogue for airway gland serous cells. J Immunol. 1989;142:2450–6. [PubMed] [Google Scholar]
- 6.Ishizaka K. Regulation of IgE synthesis. Ann Rev Immunol. 1984;2:159–82. doi: 10.1146/annurev.iy.02.040184.001111. [DOI] [PubMed] [Google Scholar]
- 7.Iwata M, Munoz JJ, Ishizaka K. Modulation of the biologic activities of IgE-binding factor. IV. Identification of glycosylation-enhancing factor as a kallikrein-like enzyme. J Immunol. 1983;131:1954–60. [PubMed] [Google Scholar]
- 8.Matsushita S, Marcelletti JF, Katz LR, Katz DH. Purification of murine suppressive factor of allergy into distinct CD23-modulating and IgE-suppressive proteins. Proc Natl Acad Sci USA. 1991;88:4718–22. doi: 10.1073/pnas.88.11.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsushita S, Katz DH. The murine ε receptor modulating protein: a novel serine protease which modulates CD23 binding to IgE. Cell Immunol. 1991;137:252–9. doi: 10.1016/0008-8749(91)90075-m. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita S, Katz DH. εRMP: a novel serine protease expressed on activated T-cell membranes enhanced IL-4-induced IgE synthesis by B cells. FASEB J. 1992;6:A1986. [Google Scholar]
- 11.Matsushita S, Katz DH. Biphasic effect of kallikrein on IgE and IgG1 syntheses by LPS/IL-4-stimulated B cells. Cell Immunol. 1993;146:210–4. doi: 10.1006/cimm.1993.1018. 10.1006/cimm.1993.1018. [DOI] [PubMed] [Google Scholar]
- 12.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–57. [PubMed] [Google Scholar]
- 13.Akahoshi F, Ashimori A, Sakashita H, et al. Synthesis, structure–activity relationships and pharmacokinetic profiles of non-peptidic α-keto heterocycles as novel inhibitors of human chymase. J Med Chem. 2001;44:1286–96. doi: 10.1021/jm000496v. [DOI] [PubMed] [Google Scholar]
- 14.Naito K, Hirama M, Okumura K, Ra C. Recombinant soluble form of the human high-affinity receptor for IgE prevents anaphylactic shock in mice. J Allergy Clin Immunol. 1996;97:773–80. doi: 10.1016/s0091-6749(96)80155-2. [DOI] [PubMed] [Google Scholar]
- 15.Roper RL, Brown DM, Phipps RP. Prostaglandin E2 promotes B lymphocytes Ig isotype switching to IgE. J Immunol. 1995;154:162–70. [PubMed] [Google Scholar]
- 16.Lutzelschwab C, Peiler G, Aveskogh M, Hellman L. Secretory granule proteases in rat mast cells. Cloning of 10 different serine proteases and a carboxypeptidase A from various rat mast cell populations. J Exp Med. 1997;185:13–29. doi: 10.1084/jem.185.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puri J, Factorovich Y. Selective inhibition of antigen presentation to cloned T cells by protease inhibitors. J Immunol. 1988;141:3313–7. [PubMed] [Google Scholar]
- 18.Saarinen J, Kalkkinenn N, Welgus HG, Kovanen P. Activation of human interstitial procollagenase through direct cleavage of the Leu83–Thr84 bond by mast cell chymase. J Biol Chem. 1994;269:18134–40. [PubMed] [Google Scholar]
- 19.Lees M, Taylor DJ, Woolley DE. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur J Biochem. 1994;223:171–7. doi: 10.1111/j.1432-1033.1994.tb18980.x. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki K, Lees M, Newlands GF, Nagase H, Woolley DE. Activation of precursors for matrix metalloproteinases 1 (interstitial collagenase) and 3 (stromelysin) by rat mast-cell proteinases I and II. Biochem J. 1995;305:301–6. doi: 10.1042/bj3050301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang KC, Raymond WW, Blount JL, Caughey GH. Dog mast cell alpha-chymase activates progelatinase B by cleaving the Phe88–Gln89 and Phe91–Glu92 bonds of the catalytic domain. J Biol Chem. 1997;272:25628–35. doi: 10.1074/jbc.272.41.25628. [DOI] [PubMed] [Google Scholar]
- 22.Christie G, Barton A, Bolognese B, et al. IgE secretion is attenuated by an inhibitor of proteolytic processing of CD23 (Fc epsilon RII) Eur J Immunol. 1997;27:3228–35. doi: 10.1002/eji.1830271221. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler DJ, Parveen S, Pollack K, Williams RJ. Inhibition of sCD23 and immunoglobulin E release from human B cells by a metalloproteinase inhibitor, GI 129471. Immunology. 1998;95:105–10. doi: 10.1046/j.1365-2567.1998.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGeehan GM, Becherer JD, Bast RC, Jr, et al. Regulation of tumor necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature (London) 1994;370:558–61. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 25.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–8. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schechter NM. Chymotrypsin-like proteinases of human skin mast cells. In: Caughey GH, editor. Mast Cell Proteases in Immunology and Biology. New York: Marcel Dekker, Inc.; 1995. pp. 47–69. [Google Scholar]
- 27.Imamura T, Dubin A, Moore W, Tanaka R, Travis J. Induction of vascular permeability enhancement by human tryptase: dependence on activation of prekallikrein and direct release of bradykinin from kininogens. Lab Invest. 1996;74:861–70. [PubMed] [Google Scholar]
- 28.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnani P, de Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, Romagnani S, Marone G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195–204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia HZ, Du Z, Craig S, et al. Effect of recombinant human IL-4 on tryptase, chymase, and Fcε receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J Immunol. 1997;159:2911–21. [PubMed] [Google Scholar]
- 31.Toru H, Eguchi M, Matsumoto R, Yanagida M, Yata J, Nakahata T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91:187–95. [PubMed] [Google Scholar]
- 32.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Induction of the high-affinity IgE receptor (Fc epsilon RI) on human mast cells by IL-4. Int Immunol. 1996;8:1367–73. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi M, Lanzt CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, Kinet JP, Galli SJ. IgE enhanced mouse mast cell Fc (epsilon) RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185:663–72. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaikh N, Rivera J, Hewlett BR, Stead RH, Zhu FG, Marshall JS. Mast cell Fc epsilon RI expression in the rat intestinal mucosa and tongue is enhanced during Nippostrongylus brasiliensis infection and can be up-regulated by in vivo administration of IgE. J Immunol. 1997;158:3805–12. [PubMed] [Google Scholar]
- 35.Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, Costa JJ, Galli SJ. IgE enhanced Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J Immunol. 1999;162:5455–65. [PubMed] [Google Scholar]
- 36.Gauchet JF, Henchoz S, Mazzi G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature (London) 1993;365:340–3. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 37.Okayama Y, Semper A, Holgate ST, Church MK. Multiple cytokine mRNA expression in human mast cells stimulated via Fc epsilon RI. Int Arch Allergy Immunol. 1995;107:158–9. doi: 10.1159/000236963. [DOI] [PubMed] [Google Scholar]
- 38.Burd PR, Thompson WC, Max EE, Mills FC. Activated mast cells produce interleukin 13. J Exp Med. 1995;181:1373–80. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toru H, Pawankar R, Ra C, Yata J, Nakahata T. Human mast cells produce IL-13 by high-affinity IgE receptor cross-linking: enhanced IL-13 production by IL-4-primed human mast cells. J Allergy Clin Immunol. 1998;102:491–502. doi: 10.1016/s0091-6749(98)70140-x. [DOI] [PubMed] [Google Scholar]
- 40.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the FcεRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slavin RG, Gleich GJ, Hutcheson PS, Kephart GM, Knutsen AP, Tsai CC. Localization of IgE to lung germinal lymphoid follicles in a patient with allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol. 1992;90:1006–8. doi: 10.1016/0091-6749(92)90479-l. [DOI] [PubMed] [Google Scholar]
- 42.Kosco-Vibois MH, Bonnofoy JY, Chvatchko Y. The physiology of murine germinal center reactions. Immunol Rev. 1997;156:127–36. doi: 10.1111/j.1600-065x.1997.tb00964.x. [DOI] [PubMed] [Google Scholar]
- 43.Kimata H, Fijimoto M, Ishioka C, Yoshida A. Histamine selectively enhances human immunoglobulin E (IgE) and IgG4 production induced by anti-CD58 monoclonal antibody. J Exp Med. 1996;184:357–64. doi: 10.1084/jem.184.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skokos D, Le Panse S, Villa I, Rousselle JC, Peronet R, David B, Namane A, Mecheri S. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 45.Hase-Yamazaki T, Aoki Y. Stimulation of human lymphocytes by cathepsin G. Cell Immunol. 1995;160:24–32. doi: 10.1016/0008-8749(95)80005-4. [DOI] [PubMed] [Google Scholar]
- 46.Chertov O, Ueda H, Xu LL, et al. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186:739–47. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humbert M, Menz G, Ying S, Corrigan CJ, Robinson DS, Durham SR, Kay AB. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999;20:528–33. doi: 10.1016/s0167-5699(99)01535-2. 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 48.Mao XQ, Shirakawa T, Yoshikawa T, et al. Association between genetic variants of mast-cell chymase and eczema. Lancet. 1996;348:581–3. doi: 10.1016/s0140-6736(95)10244-2. [DOI] [PubMed] [Google Scholar]
- 49.Mao XQ, Shirakawa T, Enomoto T, Shimizu S, Dake Y, Kitano H, Hagihara A, Hopkin JM. Association between variants of mast cell chymase gene and serum IgE levels in eczema. Hum Hered. 1998;48:38–41. doi: 10.1159/000022782. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka K, Sugiura H, Uehara M, Sata H, Hashimoto-Tamaoki T, Furuyama J. Association between mast cell chymase genotype and atopic eczema: comparison between patients with atopic eczema alone and those with atopic eczema and atopic respiratory disease. Clin Exp Allergy. 1999;29:800–3. doi: 10.1046/j.1365-2222.1999.00388.x. [DOI] [PubMed] [Google Scholar]
- 51.Patella V, Genovese A, Marone G. What are human heart mast cells for? Chem Immunol. 1995;62:171–86. [PubMed] [Google Scholar]
- 52.Takai S, Jin D, Sakaguchi M, Miyazaki M. Chymase-dependent angiotensin II formation in human vascular tissue. Circulation. 1999;100:654–8. doi: 10.1161/01.cir.100.6.654. [DOI] [PubMed] [Google Scholar]
- 53.Marone G, Casolaro V, Patella V, Florio G, Triggiani M. Molecular and cellular biology of mast cells and basophils. Int Arch Allergy Immunol. 1997;114:207–17. doi: 10.1159/000237670. [DOI] [PubMed] [Google Scholar]
- 54.Mathieson PW, Thiru S, Oliveira DBG. Mercuric chloride-treated Brown Norway rats develop widespread tissue injury including necrotizing vasculitis. Lab Invest. 1992;67:121–9. [PubMed] [Google Scholar]