Abstract
Major histocompatibility complex (MHC) class I-restricted αβ T cells express the CD8αβ heterodimer, which acts as a MHC class I-specific co-receptor. Rats are so far the only species with frequent expression of the CD8αβ by MHC-unrestricted γδ T cells. This study compares CD8αβ expression by splenic rat αβ and γδ T cells and reveals a lineage-specific difference in the control of CD8β expression. After activation in vitro, many γδ T cells, but not αβ T cells, persistently down-modulate the expression of CD8β, but not CD8α, at the RNA level. Down-regulation occurred after stimulation with T-cell receptor (TCR)-specific monoclonal antibody (mAb) and interleukin-2 (IL-2) or CD28-mediated costimulation, and after activation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. Functional differences between modulating and non-modulating cells were not found with respect to interferon-γ (IFN-γ) production and cytolytic activity. The modulation could be indicative for a fundamental difference between αβ and γδ T cells and also limits the use of CD8β as a stable marker of γδ T-cell subsets. Possibly, CD8β modulation provides a mechanism to escape over-stimulation by (auto-)antigens by increasing the threshold of TCR-mediated activation in γδ T cells.
Introduction
CD8 exists either as CD8αβ heterodimer or as CD8αα homodimer. In principle, both isoforms are capable of acting as major histocompatibility complex (MHC) class I-specific co-receptors during recognition of MHC class I-restricted antigens,1 but there is accumulating evidence that CD8αβ is a more efficient co-receptor than CD8αα.2 On αβ T cells, CD8αβ expression is restricted to thymically selected MHC class I-restricted αβ T cells and immature thymocytes.3–5 The CD8αα isoform occurs also on some T cells where a function as an MHC class I-specific co-receptor appears unlikely. Examples are CD8αα+ αβ T-cell receptor (TCR) lymphocytes of the mouse gut epithelium which depend in their development on the presence of the MHC Ib molecule, Qa-2,6 but where a co-receptor function of CD8 is unlikely owing to a lack of binding of Qa-2 to CD8.6 Other cell types with an as yet undefined function of CD8αα are activated rat7 and human8 CD4 T cells, which also express CD8αα.
Expression of the CD8αβ co-receptors by αβ TCR-positive cells can be modulated under certain circumstances. The best defined example is the regulation of CD8 expression during transition of thymocytes from the double-positive (CD4+ CD8+) to the single-positive (CD4+ or CD8+) stage after encounter of the selecting MHC/peptide.9 Modulation of CD8 chains also provides a mechanism allowing thymocytes10 and peripheral T cells11,12 to escape negative selection or to limit stimulation by (self)antigens by increasing the threshold for TCR-mediated stimulation. Sometimes CD8 modulation precedes apoptotic cell death of strongly activated cells.12
γδ T cells recognize a variety of largely unknown ligands in an MHC unrestricted manner. They can be divided into several subsets with different tissue localization, ontogeny, co-receptor expression and TCR-V composition. Coinciding with a lack of MHC restriction, γδ T cells rarely express CD4 or CD8αβ co-receptors.13 A remarkable exception are rats where more than 70% of splenic γδ T cells carry a CD8αβ heterodimer,14 which is indistinguishable from that of MHC class I-restricted αβ T cells in terms of serology and lck binding.15 Comparison of the complementarity-determining region 3 (CDR3) length of the TCRδ chain of CD8+ and CD8− rat γδ T cells, and comparison between mouse and rat cells, revealed for all γδ T-cell populations a considerably longer CDR3 than for β chains of MHC-restricted TCR. Consequently, despite CD8αβ expression, antigen recognition by CD8αβ+ rat γδ T cells is expected to be principally different from that of MHC class I-restricted CD8αβ+ αβ T cells.15
Despite the lack of knowledge about the function of CD8αβ expression by γδ T cells, it has become a marker for an extremely potent population of regulatory rat and mouse γδ T cells.16–18
This study addresses the changes in CD8β expression by splenic rat αβ and γδ T cells and shows a γδ T-cell-specific modulation of the CD8β chain.
Materials and methods
Animals
LEW rats (female) were bred in the facilities of the Institute of Virology and Immunobiology, Würzburg, by sibling mating from founders provided by Charles River (Sulzfeld, Germany). The rats were used when 8–16 weeks of age.
Antibodies and immobilization of antibodies
Fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or biotin-conjugated monoclonal antibodies (mAbs) were purchased from Pharmingen (San Diego, CA) or Serotec (Oxford, UK); the unconjugated mAbs used are as follows: TCR αβ, R73;19 TCR γδ, V65;14 CD8α hinge region, OX8;20 CD8α immunoglobulin domain, G28;3 CD8αβ, 3.4.1;3 CD4, W3/25,21 OX38 and OX35;22 CD28, JJ319;23 and NKR-P1A, 10/78.24 For intracellular cytokine staining, anti-interferon-γ (IFN-γ) mAbs DB-125 and DB-1-FITC (Serotec), and anti-interleukin-4 (IL-4) mAbs OX8126 and OX81-PE (Pharmingen) were used. Culture dishes for panning were prepared by incubation with 100 µg/ml of polyclonal sheep anti-mouse immunoglobulin G (IgG) (a kind gift of Dr L. Hübner, Roche-Diagnostics, Penzberg, Germany) in 50 mm sodium carbonate buffer (pH 9·5), overnight at 4°. Subsequently, dishes were washed three times, incubated for 2 hr with culture supernatants of hybridoma lines of the respective specificity, and washed again several times.
Preparation and activation of T cells
Spleen cells were obtained by passage through stainless steel sieves, and erythrocytes were lysed by incubation in Tris–ammonium chloride solution (155 mm, pH 7·2) for 5 min. Cells were washed and T cells were enriched by passage through nylon wool (NEN Life Science, Köln, Germany) columns.27 The remaining B cells were removed by a 20-min incubation at 4° on plates coated with sheep anti-mouse IgG antibody (cross-reactive with rat immunoglobulin). Cells not adhering to the plates were subject to T-cell lineage-specific purification and activation using a panning technique modified from ref 14. In brief, 1 × 108 or 2 × 106 T cells were incubated for 45 min at 4° on 145/20 mm Petri dishes (Greiner, Nürtingen, Germany), coated as described above with γδ TCR (V65) or αβ TCR (R73) specific mAbs, respectively. After TCR-specific panning, the adherent cells were cultured in 40 ml of RPMI-1640 medium (Gibco Life Technologies, Eggenstein, Germany), supplemented19 with 100 U/ml of recombinant human interleukin-2 (IL-2) (Chiron, Emeryville, CA) or with the indicated amounts of soluble CD28-specific mAb, JJ319, as a co-stimulus. After 24 or 40 hr, cells were removed with a rubber policeman, washed and cultured at 5 × 104–1 × 105 cells/ml in RPMI-1640 with 100 U/ml of IL-2 for an additional 2 to 3 days. In some experiments nylon wool-enriched T cells were depleted from CD4 cells by 25 min of incubation at 4° on anti-CD4 mAb (OX35)-coated 145/20 mm Petri dishes (3 × 107 cells per dish). Subsequently, 2 × 107 non-adherent cells were activated in a 145/20 mm Petri dish in 40 ml of RPMI-1640 with 5 ng/ml of phorbol 12-myristate 13-acetate (PMA; Sigma, Deisenhofen, Germany) and 400 ng/ml of ionomycin (Sigma). After 24 hr, cells were washed and cultured in RPMI-1640 (containing 100 U/ml of IL-2) for an additional 2 days.
Flow cytometry and intracellular staining of cytokines
All antibodies were used at a saturating concentration. Analysis was performed by three-colour immunofluorescence and flow cytometry by labelling 2 × 105 nucleated cells in 100 µl of fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS]/0·2% bovine serum albumin [BSA]/0·2% NaN3). Cells were sequentially exposed to 10 µg/ml of normal mouse IgG (Sigma) for 10 min and the respective mAb for 15 min at 4°. Cells were washed between incubation with the following differently conjugated mAbs: PE-conjugated mAb; biotinylated mAb; FITC-conjugated mAb, together with streptavidin CyChrome conjugate (Pharmingen).
For intracellular FACS staining, cells were restimulated with 5 ng/ml of PMA (Sigma) and 400 ng/ml of ionomycin (Sigma), for 4 hr, with 2 µg/ml of Brefeldin A being added (Sigma) for the last 2 hr. Cells were stained for two surface markers as described above, washed with PBS, fixed by addition of 100 µl of PBS/4% formaldehyde and then incubated at room temperature for 20 min. Thereafter, cells were washed with PBS and permeabilized by addition of 1 ml of saponin buffer (0·5% saponin [Sigma] in PBS/0·1% BSA/0·01% NaN3). After incubation for 20 min at room temperature, cells were resuspended in 100 µl of saponin buffer, and normal mouse immunoglobulin or unconjugated cytokine-specific mAbs (blocking control) were added to a final concentration of 10 µg/ml. Following 20 min of incubation at room temperature, cells were incubated for 30 min with directly conjugated cytokine-specific mAb, and washed once with saponin buffer and once with FACS buffer.
Analysis was performed on a FACScan flow cytometer (Becton-Dickinson, Mountain View, CA). Light scatter gates were set to exclude dead cells. The cytometer was calibrated using negative controls and single and two-colour stainings of rat lymphocytes. For calculation of cell frequency, values of isotype-matched controls were subtracted appropriately. The data were analysed using CellQuest 2·0 software.
Purification of CD8αβ-expressing cells
Nylon wool-enriched T cells were depleted from CD4-expressing cells by panning, as described above. CD4-depleted cells were stained with biotinylated anti-CD8αβ mAb (3.4.1) and purified by magnetic-activated cell sorting (MACS) using MACS beads conjugated to polyclonal anti-mouse IgG antibody (Miltenyi, Bergisch Gladbach, Germany) as described in the manufacturer's protocol. Cell purity was analysed by three-colour flow cytometry using CyChrome-conjugated streptavidin, CD8α-specific OX8-FITC and PE-labelled mAb specific for TCR γδ (V65) or αβ (R73).
51Cr cytotoxicity assays of CD8 subpopulations
Effector cells were generated as described above (by 2 days of culture on anti-γδ TCR-coated plates with IL-2 and by 2–3 days of culture with IL-2 alone). CD8 subpopulations were stained with the FITC-conjugated mAb anti-CD8β (3.4.1) and biotinylated anti-CD8α (OX8), and then sorted using a FACS vantage sorter (Becton-Dickinson). Purity was controlled by three-colour flow cytometry of γδ or αβ TCR, CD8α and CD8β. All cells were γδ TCR+ and CD4−, with less than 10% contamination by the undesired CD8 subpopulation. The indicated numbers of effector cells were incubated in triplicate with 5 × 103 51Cr-labelled target cells, in the presence of 25 U/ml of IL-2, in 96-well round-bottom plates. Target cells (106) were labelled for 1 h at 37° in the presence of 370 kBq of Na251CrO4 (Amersham Buchler, Braunschweig, Germany). Target cells used were the anti-γδ TCR mAb-producing hybridoma, V65, for redirected lysis and the anti-αβ TCR-producing hybridoma, R73, as a negative control. After 4 hr of incubation with effector cells, supernatants were taken and the radioactivity was determined. The per cent specific lysis, in counts per minute (c.p.m.) was calculated as follows:
Reverse transcription–polymerase chain reaction (RT–PCR) for CD8α and β chains
RNA was extracted from 5 × 105 cells of FACS-sorted CD8αβ- or CD8αα-expressing γδ T cells using the RNeasy kit (Quiagen, Hilden, Germany), according to the manufacturer's instructions. One microgram of the RNA was reverse transcribed, in 40-µl batches in the manufacturer's buffer, using superscript reverse transcriptase (Gibco Life Technologies) together with 1 µm dNTPs (Roth, Karlsruhe, Germany), 2·5 µm dithiothreitol (DTT), 40 U of RNAsin (Gibco Life Technologies) and 0·2 ng of 15-mer oligo dT primer (Promega, Madison, WI). After incubation for 10 min at room temperature, the RT–PCR was performed for 1 hr at 37° and stopped by a 5-min incubation at 95°. The resulting cDNA was calibrated to the same concentration as β-actin message by competitive PCR using different concentrations of a plasmid described previously.28 PCR amplification with calibrated cDNAs was performed in parallel and was carried out using different numbers of cycles in order to estimate the quantity of the respective cDNA for the CD8α and CD8β chains. PCR products were analysed on 1% agarose gels stained with ethidium bromide.
PCR primers were designed according to the published rat sequences29,30 and bind to separated exons. CD8α sense: 5′-TCAGTGGAGGGAATGGGATTGG-3′; CD8α antisense: 5′-TGAAGGTCTGGGCTTGACAAGG-3′; CD8β sense: 5′-AGCACTTTGAGTTCCTGGCGTC-3′; CD8β antisense: 5′-GGGGTTGGACATTGTTTCTTCTTC-3′. The CD8α primers fit to the hinge domain and the C2 exon of the cytoplasmatic domain. Consequently, a CD8α′-like variant of CD8 would also be amplified.31 CD8β primers are located in the V-like and in the membrane proximal domain and would allow the identification of splicing variants.32
Results
Different distribution of co-receptors on αβ and γδ T cells before and after activation in vitro
Freshly isolated and in vitro-activated αβ and γδ T cells of LEW rats were compared for expression of the co-receptors CD8αβ, CD8αα and CD4 by three-colour flow cytometry. In nine animals, 31·3 ± 2·0% of the splenic αβ T cells expressed the CD8αβ heterodimer; 66·1 ± 2·6% were CD4+ cells. No more than 1% of the cells expressed either both or none of the co-receptors. A small proportion (2–3%) of nylon wool-purified splenic T cells expressed the γδ TCR (75–85% αβ T cells, ≈ 5% natural killer [NK] cells). These cells could be divided into a major population of CD8αβ heterodimer-bearing cells (76·5 ± 4·0%) and two minor populations of either CD8αα homodimer positive (12·5 ± 3·3%) or CD8− cells (9·9 ± 1·9%). Only 1·1 ± 0·4% were CD4+.
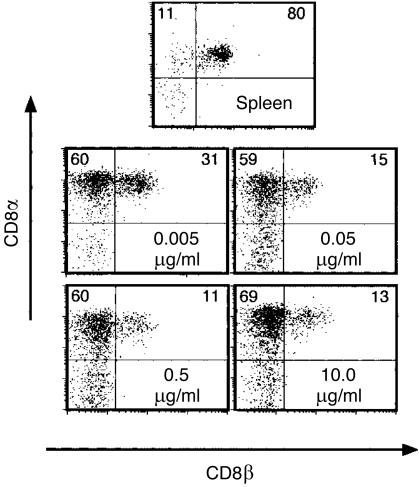
After activation in vitro with immobilized TCR-specific mAb and with IL-2 or CD28 mAb as a costimulus, αβ and γδ T cells showed a fundamental difference with regard to expression of CD4 and CD8. αβ T cells consistently expressed either CD4 or CD8αβ, which are the typical co-receptors of MHC-restricted and thymus-derived T cells. There was some variation in the ratio of CD4+ and CD8αβ+ cells, as well as in the proportion of cells co-expressing CD4 and CD8αα,7 but no enrichment of cells expressing neither CD4 nor CD8αβ (data not shown). Quite differently, the frequency of CD8αβ+ γδ T cells consistently decreased and at the same time the frequency of CD8αα+ cells often increased. This switch in the CD8 phenotype, as well as a rise in the total cell number, could be enforced by addition of an increasing concentration of costimulatory CD28 mAb (Fig. 1).
Figure 1.
Activation and culture of γδ T cells in the presence of CD28-specific monoclonal antibody (mAb) results in a reduction of the CD8αβ γδ T-cell frequency. Freshly isolated splenocytes and γδ T cells activated in the presence of the indicated concentration of CD28 mAb were analysed by three-colour flow cytometry for T-cell receptor (TCR), CD8αβ and CD8αα expression. Numbers in the quadrants represent the frequency of cells. Splenocytes were electronically gated for γδ TCR+ cells. Activated cells comprised 98% γδ T cells. Activation and purification was performed by panning on γδ TCR mAb-coated plates for 2 days, subsequent washing of the cells and then culture with interleukin-2 (IL-2) for a further 3 days, as described in the Materials and Methods. Cell numbers yielded at the end of culture were 5·7 × 106 (0·005 µg/ml), 8·3 × 106 (0·05 µg/ml), 19·8 × 106 (0·5 µg/ml) and 21·3 × 106 (10 µg/ml).
On γδ T cells, but again not on αβ T cells, a similar change in the composition of co-receptor was also seen using an activation protocol bypassing the TCR. CD8-enriched (CD4-depleted) splenocytes were stimulated with PMA and ionomycin (Table 1). Again, γδ T cells showed a reduction in the frequency of CD8αβ-bearing cells, which was paralleled by an increase in the number of CD8αα+ cells. In contrast, the αβ T-cell population showed an increased frequency of CD8αβ+ cells, which was paralleled by a decrease in the frequency of CD4 cells. This shows that the change in CD8 phenotype of the γδ T-cell population is not just a peculiarity of the activation by the γδ TCR-specific mAb V65.
Table 1.
Stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin leads to a reduction in the frequency of CD8αβ– γδT cells but not of CD8αβ– αβT cells
| % CD8αβ+ | % CD8αα+ | % CD8− | % CD4+ | |||||
|---|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | |
| αβ cells | ||||||||
| Resting | 78 | 71 | 2 | 8 | 20 | 21 | 18 | 19 |
| Stimulated | 88 | 81 | 7 | 9 | 6 | 10 | 4 | 11 |
| γδ T cells | ||||||||
| Resting | 80 | 78 | 12 | 13 | 9 | 9 | 0 | 0 |
| Stimulated | 60 | 56 | 36 | 40 | 4 | 5 | 0 | 0 |
CD4-depleted splenocytes (85–87% αβ T cells, 8–9% γδ T cells and 3–5% natural killer cells) were stimulated with 5 ng/ml of PMA and 400 ng/ml of ionomycin at 5 × 105 cells/ml for 1 day, washed, cultured for a further 2 days at 1 × 105 cells/ml in interleukin-2 (IL-2) medium and then analysed by three-colour flow cytometry.
Two mechanisms could be responsible for the shift in the CD8 phenotype of the γδ T cells: differences in the growth rate and/or death rate of CD8αβ-positive and -negative γδ T cells; or modulation of the CD8β chain.
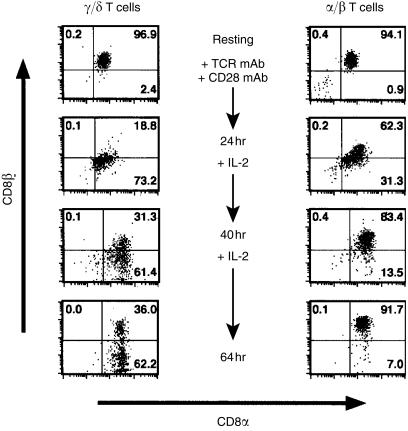
To test both possibilities a kinetic analysis was performed of cell-surface markers after activation of MACS-purified CD8αβ+ cells (Fig. 2). Purified CD8αβ+ αβ or γδ T cells were activated by panning on anti-TCR mAb-coated plates with soluble CD28 mAb (or with IL-2, data not shown) as a costimulus. Twenty-four hours later, the cells were harvested, washed, counted and propagated in medium with IL-2 for two more days. Analysis of expression of the TCR and co-receptors was performed directly after MACS sorting and after 24, 40 and 64 hr of culture.
Figure 2.
Purified CD8αβ+ αβ and γδ T cells differentially modulate CD8β after activation. CD4-depleted splenocytes were purified by magnetic-activated cell sorting (MACS) and activated by panning on T-cell receptor (TCR)-coated plates in the presence of 0·1 µg/ml of CD28 monoclonal antibody (mAb). After 24 hr, cells were washed and cultured in interleukin-2 (IL-2)-containing medium. Cells were analysed by three-colour flow cytometry for TCR, CD8αβ and CD8αα expression at the indicated time-points after initiation of the culture. The panels show cells electronically gated for expression of the respective TCR. Numbers in the quadrants represent the frequency of cells. The purity of γδ or αβ T cells was > 99%.
After 24 hr of activation, T cells of either lineage showed activation-dependent temporary internalization of the TCR, both chains of CD8 (Fig. 2) and other surface molecules such as CD2, CD5 and NKR-P1A (data not shown). After 40 hr, all cells had recovered nearly normal expression of these surface markers, with the exception of CD8β, which was no longer expressed by a major population of γδ T cells. After 64 hr, CD8αβ+ and CD8αβ− γδ T-cell populations could be clearly distinguished, but more than 60% of the γδ T cells remained CD8αβ−. Thus, the ratio of CD8αβ+ versus CD8αβ− cells was the same as the day before; this ratio did not change for two more days (data not shown).
The early separation of CD8αβ+ and CD8αβ− populations, as well as the stability of their ratio, favour modulation of CD8β and not outgrowth of CD8αα− cells to explain expansion of the CD8αβ− γδ T-cell population in our culture system. In the depicted experiment, outgrowth as an exclusive mechanism for generation of CD8αβ− cells would require an extreme and selective proliferation of CD8αα+ cells. Cell numbers were calculated by multiplying numbers of living cells with the proportion of the respective cell population determined by FACS. Based on these calculations, during 64 hr of incubation the number of γδ T cells and αβ T cells increased by 3·1-fold and 4·2-fold, respectively. If outgrowth had been the exclusive mechanism for the generation of CD8αβ− γδ T cells, this would require an 80-fold increase in the number of CD8αα+ cells versus a 1·1-fold increase of the CD8αβ+ population in 64 hr. This seems extremely unlikely.
The proportion of cells switching from CD8αβ to CD8αα expression was unaffected by the proportion of CD8αβ− cells in the starting population. In a parallel experiment, γδ T cells, partially depleted of CD8αβ (the eluate of the MACS column used for positive selection of cells depicted in Fig. 2), showed a very similar degree of modulation for both CD8 populations. The ratios of CD8αβ+ cells before versus after culture were: 1·66 for the CD8αβ-enriched cells (95% CD8αβ+ cells before stimulation and 57% CD8αβ+ cells after stimulation, see Fig. 2) and 1·74 for the CD8αβ-depleted cells (33% CD8αβ+ cells before stimulation and 19% CD8αβ+ cells after stimulation, results not shown).
For total γδ T cells, as well as for purified CD8αβ+ γδ T cells, the decrease of CD8αβ expression was more pronounced if the costimulus was CD28 mAb instead of IL-2. After costimulation of the starting population with 76·5% + 4·0% CD8αβ+ cells (eight experiments) using 0·1 µg/ml of CD28 mAb, the yield of CD8αβ+ cells was 44·1 + 12·1% CD8αβ+ cells (nine experiments, significantly different from the starting population; P < 0·001, unpaired Student's t-test). In addition, although not clearly statistically significant (P = 0·09, eight experiments), a reduction in the proportion of CD8αβ+ cells, from 76·8% + 4·0% to 69·1 + 10·3%, was found if IL-2 was used as a costimulus (P < 0·001 for CD28).
Modulation of CD8αβ cell-surface expression correlates with the loss of CD8β mRNA
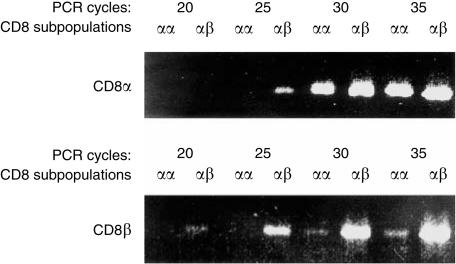
Expression of CD8 expression can be regulated at different levels ranging from accessibility of the chromatin33 at the post-transcriptional level, as described for CD8αα expression by human CD4 cells,34 to modulation at the cell surface after activation.35 To test whether the sustained modulation of CD8β by activated γδ T cells occurs prior to translation, CD8αβ+ and CD8αα+ γδ T cells were analysed by semiquantitative RT–PCR. The analysed cells were generated as shown in Fig. 2: purified CD8αβ γδ T cells were activated by incubation with γδ TCR-specific mAb and IL-2 or anti-CD28 for 2 days and, after two more days of propagation in IL-2-containing medium, CD8αα- and CD8αβ-bearing cells were separated by FACS. First, the amount of cDNA was calibrated by competitive PCR for β-actin28 and then CD8α and CD8β message was compared by semiquantitative PCR with different numbers of cycles (Fig. 3). The choice of primers allowed detection of CD8 variants previously found in other species, such as CD8α′31 or secreted CD8β splice variants,32 but the size of PCR products gave no indication for expression of such variants by peripheral rat γδ T cells.
Figure 3.
Quantification of CD8α and CD8β mRNA in CD8αα and CD8αβ γδ T cells. Cells were generated as described in the legend to Fig. 2 and in the Results. The cells were stained for γδ T-cell receptor (TCR), CD8αα and CD8αβ and sorted at the third day of culture for CD8αα+ and CD8αβ+ γδ T cells. cDNA was generated, calibrated for β-actin content, and polymerase chain reaction (PCR) amplification with CD8α- or CD8β-specific primers was performed. The number of amplification cycles and source of mRNA are indicated. The figure shows ethidium bromide staining of a 1·5% agarose gel.
In accordance with the expression on the cell surface (Fig. 2), CD8α message was expressed at nearly the same level in CD8αα+ and CD8αβ+ γδ T cells (Fig. 3a). CD8β mRNA was only found in cells with CD8αβ surface expression (Fig. 3b). The weak CD8β signal detected at high cycle numbers in the CD8αα population can be explained by a 3% contamination level with CD8αβ-expressing cells, which was detected by flow cytometry (data not shown). In conclusion, modulation of CD8β expression correlates with different CD8β mRNA levels, while CD8α appears to be unaffected at the levels of mRNA and surface expression.
CD8αα- and CD8αβ-expressing γδ T cells are indistinguishable regarding IFN-γ production and cytolytic capacity
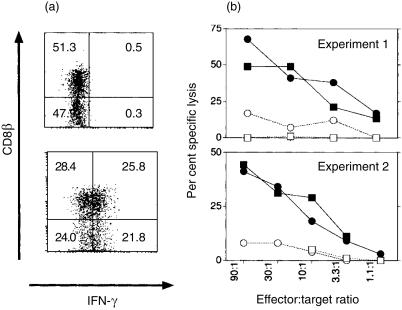
Loss of CD8 expression on CD8+ mouse αβ T cells has been found to be associated with peripheral inactivation of T cells12,36 or with loss of cytotoxicity and induction of T helper 2 (Th2) cytokine production.37 Therefore, the functional properties of the CD8αα- and CD8αβ-expressing γδ T cells were compared by intracellular staining for IFN-γ and IL-4 and by testing redirected TCR-mediated cytotoxicity.
Cells were activated with IL-2 or CD28 as costimuli. Both activation protocols led to IFN-γ production, and no differences were apparent between CD8αβ- and CD8αα-expressing γδ T cells (Fig. 4a). Intracellular staining for IL-4 was negative (data not shown). The lytic capacity of the activated subsets was compared by redirected lysis of a hybridoma cell line producing mAb against the γδ TCR. Cytotoxic CD8αα and CD8αβ γδ T cells were FACS sorted from cells generated with IL-2 as costimulus, and had identical cytolytic activity in 51Cr redirected lysis-release assays (Fig. 4b). Therefore, no functional differences between activated CD8αα- and CD8αβ-expressing γδ T cells were found with respect to cytolytic activity and lymphokine production.
Figure 4.
CD8αα and CD8αβ γδ T cells differ neither in interferon-γ (IFN-γ) production nor in redirected lysis. (a) IFN-γ production. Splenic γδ T cells were purified and activated with interleukin-2 (IL-2) as a costimulus and cultured for a further 2 days with IL-2. Cells were surface stained for CD8αα and CD8αβ and intracellularly for IFN-γ. The specificity of intracellular staining was controlled by a blocking control with unlabelled IFN-γ-specific monoclonal antibody (mAb). The majority (98%) of the cells were CD8α+. Numbers in the quadrants represent the percentage of cells. (b) Redirected lysis. Splenic γδ T cells were purified and activated with IL-2 as a costimulus for 3 days (exp. 1) or 2 days (exp. 2) and cultured for a further 2 days with IL-2. Finally, cells (more than 99% were γδ T cells) were sorted in a fluorescence-activated cell sorter for CD8αα (circles) and CD8αβ (squares) expressing populations. Contamination by the opposite CD8 subpopulation was less than 10%. Duration of the 51Cr-release assay was 4 hr. Targets were hybridoma lines producing mAb specific for the γδ T-cell receptor (TCR) (filled symbols) or the αβ TCR (open symbols).
Discussion
We demonstrated different types of modulation of CD8αβ expression by splenic αβ and γδ T cells. After activation in vitro, αβ T cells transiently modulate surface expression of CD8β as well as of many other surface molecules; γδ T cells show the same type of modulation but in addition they persistently down-regulate CD8β at the mRNA level. γδ T cells that had undergone CD8β modulation, and those remaining CD8β+, did not differ in their potential to produce IFN-γ after stimulation with PMA and ionomycin or to kill in a redirected lysis assay. Thus, CD8β modulation does not lead to a general functional impairment of the activated cells but it remains possible that modulating and non-modulating cells differ in functions not tested.
Antigenic ligands for rat γδ T cells have not yet been identified, which prevents a direct test of the consequences of CD8β modulation for antigen recognition. Hypothesizing that CD8 could act as co-receptor for antigen recognition, not only for αβ but also for γδ T cells, CD8β modulation could be a mechanism for increasing the threshold for generation of TCR-mediated signals. This could prevent persistent activation by (auto)antigens. Alternatively, CD8 on γδ T cells may accomplish a function independent of antigen recognition which also would be affected by the CD8β modulation. Especially in the rat, antigen receptor-independent functions of CD8 are not purely hypothetical, as certain macrophages and mast cells38 express a CD8αβ variant that mediates activation signals. Finally, it cannot be excluded that CD8αβ modulation, and consequently CD8β modulation, would be of little, if any, importance for γδ T-cell function. An analogous example may be the CD4 coreceptor expressed by CD1d-restricted NKT cells, which is also modulated after activation in vitro.39
Besides its use as a marker for MHC class I restriction, CD8 expression is also a fairly reliable surface marker for T cells with cytotoxic T-lymphocyte (CTL) commitment and for CTLs. It has been reported that mouse CTL clones lose CD8 after culture with PMA, ionomycin, IL-2 and IL-4,37 and that this loss coincides with loss of cytolytic capacity and a gain of Th2-like functions. Our experiments with γδ T cells did not show such a correlation, which was consistent with a report on in vivo-primed CD8αα- and CD8αβ-expressing γδ T cells40 that found no difference in the killing of a tumour line.
So far we have observed the CD8β modulation only in vitro, but it may also occur in vivo after massive γδ T-cell activation such as intraperitoneal infection with Listeria monocytogenes41 or Toxoplasma gondii,42 where a substantial proportion of the activated cells do not express CD8αβ.41 An interesting case is the distribution of CD8αβ versus CD8αα γδ and αβ T cells in the gut epithelium. In very young rats (8 days old) both populations consist mainly of CD8αβ cells. Until 24 days of age, their frequency is reduced by ≈ 50%, which is compensated by an increasing frequency of CD8αα cells. This switch in phenotype could reflect differential developmental stages of either population, but might also be a consequence of activation by antigens in the gut. It remains unclear as to whether the variation in CD8 phenotype results from differential death/expansion rates of both subsets during gut development or from activation.43 In addition, it cannot be excluded that lineage specificity of CD8αβ modulation applies only to splenic (see the Results), and not gut, lymphocytes.
Thus, in summary, rat γδ T cells persistently down-modulate CD8β after activation. This modulation may increase the threshold for TCR-mediated activation or fulfil other hitherto unknown TCR-independent functions. Whatever the reason, down-modulation limits the use of CD8β as a marker for γδ T-cell subpopulations and provides – at least for splenic T cells – one of the rare examples for a lineage-specific difference between αβ and γδ T cells.
Acknowledgments
We thank Anke Marxer and Sonja Rotzoll for help with the cell sorting and Drs T. Hünig and A. Schimpl for comments on the manuscript. The work was supported by a grant of the Deutsche Forschungsgemeinschaft through SFB 479.
Abbreviations
- IFN-γ
interferon-γ
- IL
interleukin
- mAb
monoclonal antibody
- TCR
T-cell receptor.
References
- 1.Dembic Z, Haas W, Zamoyska R, Parnes J, Steinmetz M, von Boehmer H. Transfection of the CD8 gene enhances T-cell recognition. Nature. 1987;326:510. doi: 10.1038/326510a0. [DOI] [PubMed] [Google Scholar]
- 2.Renard V, Romero P, Vivier E, Malissen B, Luescher IF. CD8 beta increases CD8 coreceptor function and participation in TCR–ligand binding. J Exp Med. 1996;184:2439. doi: 10.1084/jem.184.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres-Nagel N, Kraus E, Brown MH, Tiefenthaler G, Mitnacht R, Williams AF, Hünig T. Differential thymus dependence of rat CD8 isoform expression. Eur J Immunol. 1992;22:2841. doi: 10.1002/eji.1830221113. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama K, Negishi I, Kuida K, Louie MC, Kanagawa O, Nakauchi H, Loh DY. Requirement for CD8 beta chain in positive selection of CD8-lineage T cells. Science. 1994;263:1131. doi: 10.1126/science.8108731. [DOI] [PubMed] [Google Scholar]
- 5.Bosselut R, Kubo S, Guinter T, Kopacz J, Altman J, Singer A. Role of CD8beta domains in CD8 coreceptor function: importance of MHC I binding, signaling, and positive selection of CD8+ T cells in the thymus. Immunity. 2000;12:409. doi: 10.1016/s1074-7613(00)80193-4. [DOI] [PubMed] [Google Scholar]
- 6.Teitell M, Holcombe H, Cheroutre H, Aldrich CJ, Stroynowski I, Forman J, Kronenberg M. The alpha 3 domain of the Qa-2 molecule is defective for CD8 binding and cytotoxic T lymphocyte activation. J Exp Med. 1993;178:2139. doi: 10.1084/jem.178.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez F, McKnight AJ, Silva A, Mason D. Glucocorticoids induce the expression of CD8 alpha chains on concanavalin A-activated rat CD4+ T cells: induction is inhibited by rat recombinant interleukin 4. J Exp Med. 1992;176:1551. doi: 10.1084/jem.176.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spits H, Paliard X, Engelhard VH, de Vries JE. Cytotoxic activity and lymphokine production of T cell receptor (TCR)-alpha beta+ and TCR-gamma delta+ cytotoxic T lymphocyte (CTL) clones recognizing HLA-A2 and HLA-A2 mutants. Recognition of TCR-gamma delta+ CTL clones is affected by mutations at positions 152 and 156. J Immunol. 1990;144:4156. [PubMed] [Google Scholar]
- 9.Ellmeier W, Sawada S, Littman DR. The regulation of CD4 and CD8 coreceptor gene expression during T cell development. Annu Rev Immunol. 1999;17:523. doi: 10.1146/annurev.immunol.17.1.523. [DOI] [PubMed] [Google Scholar]
- 10.Chidgey A, Boyd R. Agonist peptide modulates T cell selection thresholds through qualitative and quantitative shifts in CD8 co-receptor expression. Int Immunol. 1997;9:1527. doi: 10.1093/intimm/9.10.1527. 10.1093/intimm/9.10.1527. [DOI] [PubMed] [Google Scholar]
- 11.Schonrich G, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hammerling GJ, Arnold B. Distinct mechanisms of extrathymic T cell tolerance due to differential expression of self antigen. Int Immunol. 1992;4:581. doi: 10.1093/intimm/4.5.581. [DOI] [PubMed] [Google Scholar]
- 12.Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161:1686. [PubMed] [Google Scholar]
- 13.Kaufmann SHE. γ/δ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2772. [Google Scholar]
- 14.Kühnlein P, Park JH, Herrmann T, Elbe A, Hünig T. Identification and characterization of rat gamma/delta T lymphocytes in peripheral lymphoid organs, small intestine, and skin with a monoclonal antibody to a constant determinant of the gamma/delta T cell receptor. J Immunol. 1994;153:979. [PubMed] [Google Scholar]
- 15.Straube F, Herrmann T. Expression of functional CD8alpha beta heterodimer on rat gamma delta T cells does not correlate with the CDR3 length of the TCR delta chain predicted for MHC class I-restricted antigen recognition. Eur J Immunol. 2000;30:3562. doi: 10.1002/1521-4141(200012)30:12<3562::AID-IMMU3562>3.0.CO;2-J. 10.1002/1521-4141(200012)30:12<3562::aid-immu3562>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.McMenamin C, McKersey M, Kühnlein P, Hünig T, Holt PG. Gamma delta T cells down-regulate primary IgE responses in rats to inhaled soluble protein antigens. J Immunol. 1995;154:4390. [PubMed] [Google Scholar]
- 17.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 18.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184:2167. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hünig T, Wallny H-J, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. J Exp Med. 1989;169:73. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brideau RJ, Carter PB, McMaster WR, Mason DW, Williams AF. Two subsets of T-lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 21.Mason DW, Arthur RP, Dallman MJ, Green JR, Spoickett GP, Thomas ML. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 22.Jefferies WA, Green JR, Williams AF. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985;162:117. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tacke M, Clark GJ, Dallman MJ, Hünig T. Cellular distribution and costimulatory function of rat CD28. Regulated expression during thymocyte maturation and induction of cyclosporin A sensitivity of costimulated T cell responses by phorbol ester. J Immunol. 1995;154:5121. [PubMed] [Google Scholar]
- 24.Kraus E, Lambracht D, Wonigeit K, Hunig T. Negative regulation of rat natural killer cell activity by major histocompatibility complex class I recognition. Eur J Immunol. 1996;26:2582. doi: 10.1002/eji.1830261107. [DOI] [PubMed] [Google Scholar]
- 25.van der Meide PH, de Borman TH, Labie MC, Wubben JA, Botman CA, Vijverberg K, Schellekens H. A sensitive two-site enzyme immunoassay for the detection of rat interferon-gamma in biological fluids. J Interferon Res. 1990;10:183. doi: 10.1089/jir.1990.10.183. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156:2406. [PubMed] [Google Scholar]
- 27.Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- 28.Siegling A, Lehmann M, Platzer C, Emmrich F, Volk HD. A novel multispecific competitor fragment for quantitative PCR analysis of cytokine gene expression in rats. J Immunol Methods. 1994;177:23. doi: 10.1016/0022-1759(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 29.Johnson P, Gagnon J, Barclay AN, Williams AF. Purification, chain separation and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985;4:2539. doi: 10.1002/j.1460-2075.1985.tb03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson P. A human homolog of the mouse CD8 molecule, Lyt-3: genomic sequence and expression. Immunogenetics. 1987;26:174. doi: 10.1007/BF00365908. [DOI] [PubMed] [Google Scholar]
- 31.Zamoyska R, Derham P, Gorman SD, von Hoegen P, Bolen JB, Veillette A, Parnes JR. Inability of CD8 alpha′ polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. Nature. 1989;342:278. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]
- 32.DiSanto JP, Smith D, de Bruin D, Lacy E, Flomenberg N. Transcriptional diversity at the duplicated human CD8 beta loci. Eur J Immunol. 1993;23:320. doi: 10.1002/eji.1830230203. [DOI] [PubMed] [Google Scholar]
- 33.Kieffer LJ, Yan L, Hanke JH, Kavathas PB. Appropriate developmental expression of human CD8 beta in transgenic mice. J Immunol. 1997;159:4907. [PubMed] [Google Scholar]
- 34.Gao MH, Walz M, Kavathas PB. Post-transcriptional regulation associated with control of human CD8A expression of CD4+ T cells. Immunogenetics. 1996;45:130. doi: 10.1007/s002510050180. [DOI] [PubMed] [Google Scholar]
- 35.Takada S, Engleman EG. Evidence for an association between CD8 molecules and the T cell receptor complex on cytotoxic T cells. J Immunol. 1987;139:3231. [PubMed] [Google Scholar]
- 36.Arnold B, Schönrich G, Hämmerling GJ. Multiple levels of peripheral tolerance. Immunol Today. 1993;14:12. doi: 10.1016/0167-5699(93)90317-E. [DOI] [PubMed] [Google Scholar]
- 37.Erard F, Wild MT, Garcia-Sanz JA, Le Gros G. Switch of CD8 T cells to noncytolytic CD8− CD4− cells that make TH2 cytokines and help B cells. Science. 1993;260:1802. doi: 10.1126/science.8511588. [DOI] [PubMed] [Google Scholar]
- 38.Hirji NS, Lin TJ, Gilchrist M, et al. Novel CD8 molecule on macrophages and mast cells: expression, function and signaling. Int Arch Allergy Immunol. 1999;118:180. doi: 10.1159/000024060. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Huang HWEP. NK1.1+ CD4+ T cells lose NK1.1 expression upon in vitro activation. J Immunol. 1997;158:5112. [PubMed] [Google Scholar]
- 40.Yrlid U, Petersson E, Dohlsten M, Hedlund G. TCR alpha beta+ anti-tumor cytolytic T lymphocytes express NKR-P1 while the anti-tumor activity of TCR gamma delta+ T lymphocytes is not correlated to NKR-P1 expression. Cell Immunol. 1996;173:287. doi: 10.1006/cimm.1996.0280. 10.1006/cimm.1996.0280. [DOI] [PubMed] [Google Scholar]
- 41.Kimura Y, Tomida S, Matsumoto Y, Hiromatsu K, Yoshikai Y. Evidence for the early recruitment of T-cell receptor γδ+ T cells during rat listeriosis. Immunology. 1996;87:21. [PMC free article] [PubMed] [Google Scholar]
- 42.Kempf MC, Cesbron-Delauw M-F, Deslee D, Groß U, Herrmann T, Sutton P. Different manifestations of Toxoplasma gondii infection in F344 and LEW rats. Med Microbiol Immunol. 1999;187:137. doi: 10.1007/s004300050085. [DOI] [PubMed] [Google Scholar]
- 43.Helgeland L, Brandtzaeg P, Rolstad B, Vaage J. Sequential development of intraepithelial gamma delta and alpha beta T lymphocytes expressing CD8 alpha beta in neonatal rat intestine: requirement for the thymus. Immunology. 1997;92:447. doi: 10.1046/j.1365-2567.1997.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]