Abstract
The cytokine interleukin-12 (IL-12) is essential for resistance to Trypanosoma cruzi infection because it stimulates the synthesis of interferon-γ (IFN-γ), a major activator of the parasiticidal effect of macrophages. A less studied property of IL-12 is its ability to amplify the proliferation of T or natural killer (NK) lymphocytes. We investigated the role of endogenously produced IL-12 in the maintenance of parasite antigen (T-Ag)-specific lymphoproliferative responses during the acute phase of T. cruzi infection. We also studied whether treatment with recombinant IL-12 (rIL-12) would stimulate T-Ag-specific or concanavalin A (Con A)-stimulated lymphoproliferation and abrogate the suppression that is characteristic of the acute phase of infection. Production of IL-12 by spleen-cell cultures during T. cruzi infection increased in the first days of infection (days 3–5) and decreased as infection progressed beyond day 7. The growth-promoting activity of endogenous IL-12 on T-Ag-specific proliferation was observed on day 5 of infection. Treatment of cultures with rIL-12 promoted a significant increase in Con A-stimulated proliferation by spleen cells from normal or infected mice. Enhanced T-Ag-specific proliferation by rIL-12 was seen in spleen cell cultures from infected mice providing that nitric oxide production was inhibited by treatment with the competitive inhibitor NG-monomethyl-l-arginine (NMMA). Enhancement of proliferation promoted by IL-12 occurred in the presence of neutralizing anti-interleukin-2 (IL-2) antibody, suggesting that this activity of IL-12 was partly independent of endogenous IL-2. Thymidine incorporation levels achieved with rIL-12 treatment of the cultures were ≈ 50% of those stimulated by rIL-2 in the same cultures.
Introduction
The protozoan Trypanosoma cruzi is the aetiological agent of Chagas' disease, which is endemic in South and Central America. The acute phase of experimental T. cruzi infection in mice is characterized by progressive and intense suppression of the lymphoproliferative responses of spleen cells stimulated with polyclonal activators or with parasite antigens.1,2 The suppression is largely mediated by nitric oxide (NO) produced by interferon-γ (IFN-γ)- and tumour necrosis factor-α (TNF-α)-activated macrophages,3 by prostaglandins4,5 and by decreased interleukin-2 (IL-2) synthesis accompanied with reduced expression of IL-2 receptors (IL-2R) in lymphocytes.6–8 In spite of the several suppressor mechanisms that directly or indirectly affect proliferation, and the response to IL-2 during infection, lymphocyte proliferation is partially or completely restored (depending on the degree of suppression) when NO and/or prostaglandin synthesis are blocked,3–5 when IFN-γ and/or TNF-α are neutralized by monoclonal antibodies (mAbs),3,5 or when rIL-2 is added to cultures.3,4
Interleukin-12 (IL-12) participates in the resistance of mice to T. cruzi infection by innate and acquired immunity.9 The neutralization of this cytokine by mAbs leads to aggravation of the infection,9,10 accompanied with a reduction of IFN-γ and NO synthesis by spleen cells from infected mice,9 indicating that IL-12 is produced during infection and is important in the control of T. cruzi parasitism. IL-12 can also act as a growth factor, promoting the proliferation of preactivated T and natural killer (NK) cells, and enhancing the generation of cytotoxic T lymphocytes (CTLs) and lymphokine-activated killer (LAK) cells.11–13 However, IL-12 induces little or no proliferation of resting T and NK cells, but increases T-cell proliferation to phytohaemagglutinin (PHA), alloantigens, anti-CD3 antibody or IL-2.11,14,15 Moreover, treatment of peripheral blood mononuclear cell (PBMC) cultures from patients with Chagas' disease with recombinant IL-12 (rIL-12) stimulated parasite-specific lymphoproliferation.16 Bearing in mind the described activity of IL-12 as growth factor of preactivated T and NK cells, and that this cytokine is synthesized during T. cruzi infection, we investigated the role of endogenously produced IL-12 in the maintenance of lymphoproliferative responses during infection and whether rIL-12 would stimulate parasite antigen-specific lymphoproliferation and abrogate suppression.
This article reports that IL-12 is produced during T. cruzi infection and that endogenously produced IL-12 exerts a significant role in antigen-specific lymphocyte proliferation in the first week of infection. We also show that addition of rIL-12 increases parasite antigen- and concanavalin A (Con A)-stimulated specific proliferation in culture, partially reducing suppression. Moreover, IL-12 was capable of stimulating proliferation, even when endogenous IL-2 was neutralized by mAb.
Materials and methods
Animals
Female C57BL/6 mice, (6–8-weeks old, specific pathogen-free [SPF]), were bred in the SPF mouse breeding facilities (Biotério de Camundongos Isogênicos) of the Department of Immunology, ICB, USP. The mice were housed at a density of five per microisolator cage and offered food and water ad libitum. All animal procedures were performed in accordance with the principles of the Brazilian Code for the Use of Laboratory Animals. For each experiment, the spleen cells of three mice were pooled.
Infection and preparation of parasite antigen
T. cruzi (strain Y) was maintained by weekly intraperitoneal (i.p.) inoculation of BALB/c mice. Trypomastigote-containing blood was drawn from these mice and injected (5000 forms i.p.) into C57BL/6 mice. T. cruzi tissue-culture trypomastigote antigen (T-Ag) was prepared from parasites harvested from infected cultures of LLC-MK2 cells (CCL7.1; American Type Culture Collection [ATCC], Bethesda, MD) by 10 freeze–thaw cycles, as previously described.1
Cell cultures
Spleen-cell suspensions were prepared from normal (NSC) or infected (ISC) mice on days 5, 7, 9 and 12 after infection. The cells were cultured in 96-well culture plates at a density of 4 × 105/well, in RPMI-1640 containing 10% fetal calf serum (FCS), 2 mm l-glutamine, 0·05 mm 2-mercaptoethanol (2-ME), and penicillin and streptomycin (100 U/ml and 100 µg/ml, respectively) (Sigma Chemical Co., St Louis, MO). The cultures were stimulated with Con A (2·5 µg/ml), T-Ag (4 × 105 parasites, prepared by the freeze–thaw procedure stated above) or maintained in culture medium alone, for a total period of 72 hr. NG-monomethyl-l-arginine (NMMA) (Sigma Chemical Co.), a competitive inhibitor of NO synthesis, was added to cultures at a concentration of 0·5 mm, after titration for a dose that induced the maximum increase of antigen-specific proliferation without toxicity. Methyl-[3H]thymidine ([3H]TdR, specific activity 5 Ci/mmol; Amersham Pharmacia, Biotech UK Ltd, Little Chalfont, Bucks., UK) was added (0·5 µCi/well) 18 hr before termination of the cultures. Radioactivity was measured by scintillation counting and the results were expressed as mean counts per minute (c.p.m.) ±standard deviation (SD) of triplicate cultures.
Treatment of cultures with rIL-12, rIL-2 or anti-IL-12 mAb
The activity of purified murine rIL-12 (a gift from Dr M. Gately, Hoffman-La Roche Inc., Nutley, NJ) on cell proliferation was titrated in the range of 150–2500 pg/ml. The different concentrations of rIL-12 were added 36 hr after starting the cultures, which were then harvested 36 hr later.
In some experiments, cultures were also treated with 50 µl of a supernatant from rat S4B6.31 hybridoma cells that produce immunoglobulin 2a (IgG2a) anti-mouse IL-2 mAb. Previous titration of this supernatant showed that, at the dilution of 1 : 4 (v/v) used in the cultures, it completely inhibited growth of the IL-2-dependent HT-2 cell line.17 The anti-IL-2-containing supernatant was added together with rIL-12 at 36 hr of culture.
The effects of rIL-12 and rIL-2 on cell proliferation were compared. To achieve this, murine rIL-2 (a gift from Dr Robert L. Coffman, DNAX Research Institute Inc., Palo Alto, CA) was added at 50 U/ml, alone or in combination with rIL-12 (2500 pg/ml), 36 hr after initiation of the cultures.
In order to verify the effect of endogenously produced IL-12 in the proliferation of spleen cells, the cultures were treated with the neutralizing IgG2a rat anti-mouse IL-12 (p-40) mAb, C17.8 (a gift from Dr Giorgio Trinchieri, Wistar Institute, Philadelphia, PA), at 100 µg/ml. As a control, the rat anti-mouse β-galactosidase mAb, GL 117 (of the same isotype), was added at the same concentration to the cultures. The mAbs were added at the start of culture, with or without NMMA.
Detection of IL-12
Production of IL-12 (p-40) was measured in 48-hr culture supernatants of spleen cell cultures by using a two-site sandwich enzyme-linked immunosorbent assay (ELISA). This method was standardized in our laboratory using rat anti-mouse IL-12 (p-40) mAbs: C17.15 as capture antibody and C15.6 as biotinylated antibody. The lower limit of IL-12 detectable in our assay was 0·31 ng/ml. The mAbs were purified from ascites fluid donated by Dr Giorgio Trinchieri.
Data analysis
Means of control and experimental groups were compared by using the Student's t-test. Comparisons of multiple groups were performed by analysis of variance (anova) and Bonferroni's test. Differences were considered to be significant when the P-value was < 0·05. Statistical analysis was performed using GraphPad Instat.
Results
Production of IL-12 by T. cruzi-infected mice
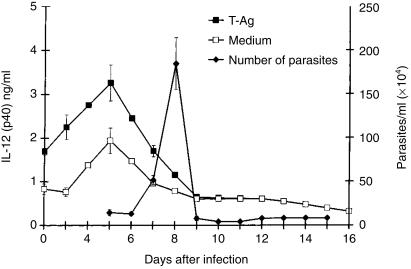
The levels of IL-12 produced by spleen-cell cultures from infected mice, stimulated or not stimulated by parasite antigen, were measured by ELISA detection of the p-40 chain (Fig. 1). Levels of IL-12 were highest on day 5 postinfection (p.i.) when parasites became detectable in the blood. As parasitaemia increased from day 7 p.i. onwards, a progressive decrease of IL-12 synthesis by ISC was observed and preinfection levels were reached on day 9 p.i.
Figure 1.
Production of interleukin-12 (IL-12) by spleen cell cultures, and corresponding blood parasite counts, after intraperitoneal (i.p.) infection of C57Bl/6 mice with 5000 Trypanosoma cruzi blood forms. The cultures were stimulated with parasite antigen (T-Ag) or incubated in culture medium alone. Values are expressed as arithmetic means from duplicate cultures ±standard deviation (for IL-12) or from six mice (parasite counts). The results are representative of four experiments.
Neutralization of IL-12 at the start of infection reduces Ag-specific proliferation
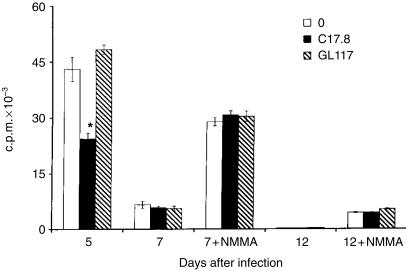
Proliferative responses to T. cruzi Ag by ISC were present on day 5 p.i., and treatment of the cultures with neutralizing anti-IL-12 mAb, C17.8, but not with the isotype-matched control anti-β-galactosidase mAb, GL-117, reduced Ag-specific lymphocyte proliferation by ≈ 50% (Fig. 2).
Figure 2.
Effect of neutralization of endogenously produced interleukin-12 (IL-12) by monoclonal antibody (mAb) anti-IL-12 (C17.8, 100 µg/ml) on parasite antigen (T-Ag)-stimulated spleen cell proliferation from Trypanosoma cruzi-infected mice. As an isotype control the rat anti-β-galactosidase mAb GL 117 (IgG2a) was used at the same concentration as C17.8. *P < 0·01. Values are expressed as arithmetic means from triplicate cultures ±standard deviation. The results are representative of three experiments.
On day 7 of infection, Ag-specific proliferation by ISC was intensely suppressed, but still detectable, whereas on day 12 p.i., suppression was almost complete, with [3H]TdR incorporation levels below 500 c.p.m. Antigen-specific proliferation could be enhanced by treating ISC from days 7 or 12 p.i. with NMMA, a competitive inhibitor of NO synthesis (Fig. 2). Neutralizing endogenously produced IL-12 with anti-IL-12 mAb had no effect on Ag-specific proliferation by ISC (treated or not treated with NMMA) from days 7 or 12 p.i. No effect of anti-IL-12 mAb treatment was observed on Con A-stimulated proliferative responses by ISC which were treated or not treated with NMMA (data not shown). The results indicate that endogenous IL-12 acts as a costimulator of Ag-specific lymphocyte proliferation early in the course of infection.
Treatment with rIL-12 stimulates parasite Ag-specific or Con A-induced proliferation
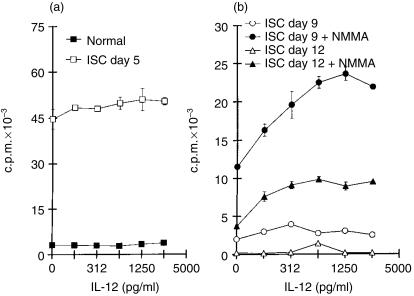
We next asked whether Ag-specific proliferation by ISC could be enhanced by the addition of rIL-12. Proliferation was markedly suppressed in ISC from days 9 and 12 p.i. (Fig. 3b) compared to ISC cultures from day 5 p.i. (Fig. 3a). The addition of NMMA increased the suppressed proliferative responses to parasite Ag by ISC from days 9 and 12 p.i. (Fig. 3b) but not from day 5 p.i. (results not shown). Treatment with rIL-12 in doses up to 2500 pg/ml did not further increase Ag-specific proliferation by ISC from day 5 p.i. or by NSC (Fig. 3a). However, addition of rIL-12 to NMMA-treated ISC from days 9 or 12 p.i. resulted in a marked dose-dependent increase of Ag-specific lymphocyte proliferation (Fig. 3b). This enhancing effect of IL-12 on proliferation was not observed in ISC that were not treated with NMMA (Fig. 3b). Thus, rIL-12 was capable of further stimulating Ag-specific proliferation provided that NO production was blocked by NMMA.
Figure 3.
Effect of recombinant interleukin-12 (rIL-12) on parasite antigen (T-Ag)-stimulated spleen cell proliferation from Trypanosoma cruzi-infected (ISC) or from normal control (NSC) mice. Cultures were obtained on days 5, 9 or 12 after infection and were treated (or not treated) with NG-monomethyl-l-arginine (NMMA). Values are expressed as arithmetic means of triplicate cultures ±standard deviation. (a) NSC and ISC on day 5 of infection. (b) ISC on days 9 and 12 of infection, treated or not treated with NMMA. The results are representative of four experiments.
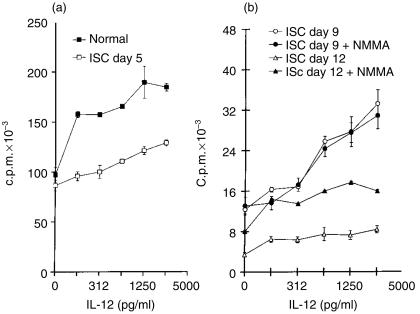
Lymphocyte proliferation induced by the polyclonal activator Con A was also enhanced by IL-12 in either NSC or ISC (Fig. 4a, 4b). The maximal effect of rIL-12 varied between 50% and 100% increase and occurred regardless of whether the cultures had been treated with NMMA.
Figure 4.
Effect of interleukin-12 (IL-12) on concanavalin A (Con A)-stimulated spleen cell proliferation from Trypanosoma cruzi-infected (ISC) or from normal control (NSC) mice. Cultures were obtained on days 5, 9 or 12 after infection and were treated (or not treated) with NG-monomethyl-l-arginine (NMMA). Values are expressed as arithmetic means of triplicate cultures ±standard deviation. (a) NSC and ISC on day 5 of infection. (b) ISC on days 9 and 12 of infection, treated or not treated with NMMA. The results are representative of four experiments.
Stimulation of parasite Ag-specific proliferation by IL-12 is not affected by anti-IL-2 treatment
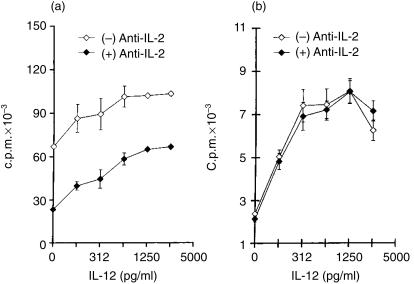
It was important to determine whether the stimulation of lymphocyte proliferation by IL-12 resulted from a direct effect of this cytokine or indirectly from an IL-12-dependent effect on the synthesis/activity of IL-2. To achieve this, the cultures were treated with rIL-12 in the presence or absence of anti-IL-2 neutralizing mAb, S4B6.31. As shown in Fig. 5(a), anti-IL-2 treatment significantly reduced NSC proliferation to Con A; however, the addition of rIL-12 was still capable of enhancing proliferation to anti-IL-2-treated cultures. In fact, proliferation achieved with the highest dose of rIL-12 (2500 pg/ml) in the presence of anti-IL-2-blocking mAb reached values found in Con A-stimulated, but untreated, cultures. However, the maximal stimulation of proliferation by IL-12 in Con A-stimulated cultures was seen when endogenous IL-2 was not neutralized. Regarding parasite Ag-specific responses, proliferation by NMMA-treated ISC was not decreased by IL-2 neutralization; supplementing IL-12 markedly increased proliferation, not being affected by neutralizing endogenous IL-2 (Fig. 5b). The possibility that lymphocytes proliferating to parasite-Ag in the presence of NMMA could use a cytokine other than IL-2 as a growth factor was investigated. To achieve this, cultures were treated with anti-IL-4 mAb, 11B11 (20 µg/ml), or with anti-granulocyte–macrophage colony-stimulating factor (GM-CSF) mAb (20 µg/ml); no inhibition of proliferation was observed (data not shown), indicating that these cytokines do not participate as growth factors in the observed proliferation.
Figure 5.
Effect of treatment with anti-interleukin-2 (IL-2) monoclonal antibody (mAb) S4B6.31 on concanavalin A (Con A)-stimulated spleen cell proliferation from normal mice in cultures treated with interleukin-12 (IL-12) (a), and the effect of the same treatments on parasite antigen (T-Ag)-stimulated proliferation in NG-monomethyl-l-arginine (NMMA)-treated cultures of spleen cells obtained from day-12 Trypanosoma cruzi-infected mice (b). Values are expressed as arithmetic means of triplicate cultures ±standard deviation. The results are representative of three experiments.
Comparison between the proliferation-stimulating ability of IL-12 and IL-2
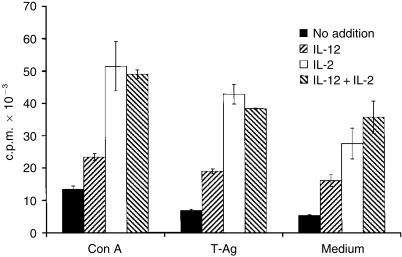
The effect of supplementing cultures with IL-12 or IL-2 on lymphocyte proliferation was tested by treating ISC from day 12-infected mice with these cytokines. The results shown in Fig. 6 were obtained for the maximal concentrations of the tested cytokines. These were 50 U/ml for IL-2, as previously titrated to induce maximal proliferation of Con A-stimulated NSC or HT-2 cells, and 2500 pg/ml for IL-12, as shown in Figs 4 and 5. Neither IL-2 nor IL-12 stimulated Ag-specific proliferation when NO production was not blocked (data not shown). When cultures were treated with NMMA, the levels of proliferation obtained with IL-12 treatment were ≈ 50% of those obtained with IL-2, as seen in either Con A- or parasite-Ag-stimulated cultures (Fig. 6). No synergistic effect was observed by adding both cytokines together to the cultures.
Figure 6.
Comparison of interleukin-2 (IL-2) and interleukin-12 (IL-12) treatment on spleen cell proliferation in concanavalin A (Con A)- or parasite antigen (T-Ag)-stimulated cultures, or in cultures incubated in medium alone, from Trypanosoma cruzi-infected mice. The cultures were performed on day 12 of infection, treated with NG-monomethyl-l-arginine (NMMA) and supplemented with IL-12 (2·5 ng/ml), IL-2 (50 U/ml) or both cytokines. Values are presented as arithmetic means of triplicate cultures ±standard deviation. The results are representative of two experiments.
Discussion
The cytokine IL-12 is essential for resistance to T. cruzi infection because it stimulates the synthesis of IFN-γ, a major activator of the parasiticidal activity of macrophages. A less investigated role of IL-12 is its ability to amplify T or NK lymphocyte proliferation. Indeed, IL-12 was originally described as having a co-mitogenic effect on human T cells.18 Most studies have investigated the stimulatory activity of IL-12 on proliferation when the recombinant cytokine is added to mitogen- or anti-CD3-stimulated lymphocyte cultures.18,19 Although the role of endogenous IL-12 on antigen-stimulated T-cell proliferation has been studied in much less detail, this cytokine clearly participates in enhancing T-cell proliferation to allostimulation in mixed leucocyte cultures.20 The results presented in this report show that IL-12 is mainly produced early in the course of T. cruzi infection in the mouse and that it has an important role of promoting parasite–antigen-specific proliferation early in infection. This activity of IL-12 was inferred from the data showing significant inhibition of Ag-specific proliferation by anti-IL-12 p40 mAb, 17.8, on the first week of infection. Recently, however, IL-23, a heterodimer formed by the IL-12 chain p40 and a protein designated p19, was described as stimulatory for anti-CD3- and anti-CD28-induced proliferation of mouse or human memory lymphocytes.21 Proliferation of murine lymphocytes induced by the hybrid Hy-p40-p19 molecule, or by conditioned medium of p19p40-cotransfected cells, was markedly suppressed by treating the cultures with the neutralizing anti-IL-12p40 mAb 17.8.21 Therefore, as we also used mAb 17.8, this antibody could block the effect of both IL-12 and IL-23 in the cultures. At this point, we cannot be sure whether endogenous IL-23 stimulates T. cruzi Ag-specific proliferation in our system.
To our knowledge this is the first time that the proliferation-enhancing ability of endogenous IL-12 has been shown to occur in a situation of in vivo infection or immunization. The participation of endogenous IL-12 as a growth-promoting factor for T. cruzi-specific T cells was restricted to the first week of infection. Nevertheless, it may compensate for suboptimal IL-2 synthesis and/or expression of IL-2 receptors that have been reported to occur in early T. cruzi infection.22 As infection progressed beyond day 7, there was a progressive decline in IL-12 production, and parasite Ag- and mitogen-specific proliferative responses became gradually suppressed, reaching the lowest levels by day 12 of infection; endogenous IL-12 had no demonstrable participation in proliferation, even when suppression was partially abrogated by inhibiting NO synthesis with NMMA (cf. Figure 2). The decline in IL-12 production correlated with increased IL-10 and NO production and progressive immunesuppression during this time-period of infection (A. P. Galvão da Silva & I. A. Abrahamsohn, unpublished).
Treatment with rIL-12 had no effect on Ag-specific proliferation on day 5 after infection (when endogenous IL-12 acts as a T-cell growth factor), suggesting that there was no shortage of growth factors for the relatively limited numbers of Ag-specific T cells that are induced to proliferate by parasite antigen. In contrast, IL-12 treatment clearly enhanced Con A-stimulated proliferation in normal or day-5-infected cultures. The polyclonal nature of the stimulation that affects most T cells would require supplementation with growth-promoting factors to achieve optimal T-cell proliferation.
Proliferation enhancement by IL-12 in the phase of high suppression was seen in parasite Ag-stimulated cultures, provided that NO synthesis was blocked by the addition of NMMA. Treatment with NMMA alone stimulated proliferation by 300%, while addition of IL-12 to these cultures further doubled the incorporation rates. In addition, inhibition of NO synthesis is also required in order to observe a proliferation-enhancing effect by IL-2 in similar cultures.3 Two mechanisms may explain this requirement. First, the high levels of NO produced in the cultures cause cell death that can be reversed by NMMA treatment,23 and a higher number of cells expressing IL-12 receptors would be available in culture. Second, T. cruzi-infected mice treated with an inhibitor of NO synthesis have increased expression of IL-2R, suggesting that NO down-regulates IL-2R expression during infection.24 The proliferation of Con A-stimulated cultures, however, was enhanced by IL-12, regardless of whether NO synthesis was blocked. As Con A is a polyclonal activator, the frequency of T cells activated by Con A, even in the presence of NO, would be higher than in antigen-stimulated cultures, and the cultures would respond to IL-12 supplementation.
The mechanisms by which IL-12 stimulates proliferation are not completely clear. The effect requires previous activation of the cells, as IL-12 is not capable of activating resting T cells. In fact, IL-12 was not capable of inducing resting T cells from normal mice to proliferate, even when exposed to parasite antigen, and IL-12 addition was most effective at stimulating proliferation in Ag- or Con A-stimulated cultures when added at 36 hr of culture. A similar behaviour was seen for PBMC from Chagas' disease patients stimulated with parasite antigen.16 Activated T cells express high-affinity IL-12R, which is a requirement for IL-12-induced proliferation, but not for induced cytokine production.25
Our results indicate that IL-12 enhances proliferation to Con A and parasite antigen, even when endogenous IL-2 has been neutralized by a specific mAb. This is in accordance with previous observations using other antigens or mitogens.11 Although IL-12 can promote proliferation independently from IL-2, these two cytokines can have an additive effect on each other, optimizing proliferation as IL-2 induces the expression of IL-12 receptor26,27 and IL-12 induces IL-2Rα.28,29 In fact, our results show that maximal proliferation to Con A induced by IL-12 is dependent on the availability of endogenous IL-2, whereas Ag-specific proliferation is stimulated by IL-12, but not affected by neutralization of endogenous IL-2.
Regarding the relative efficacy of IL-12 and IL-2 in promoting cell proliferation, our results show that IL-12 is less efficient at enhancing proliferation than IL-2 when both cytokines are used at concentrations that induce maximal cell proliferation. These cytokines also do not synergize and these results agree with previous published reports on the subject.11,19 In addition, whereas IL-2 enhances proliferation of both resting and preactivated T cells, IL-12 does not stimulate resting or normal cells (Fig. 3 and data not shown) and thus is capable of preferentially expanding parasite Ag-specific clones. This latter observation agrees with the effect of IL-12 on T. cruzi Ag-specific proliferation by human T cells.16
In summary, we have shown that endogenous IL-12 acts as a T-cell growth factor for parasite Ag-specific proliferation in acute T. cruzi infection, and that in vitro treatment with IL-12 contributes to restoring cell proliferation to suppressed parasite-specific and Con A lymphocyte responses of the acute phase.
Acknowledgments
The authors are grateful to Dr Maurice Gately (Hoffman-La Roche, Nutley, NJ) and to Dr Giorgio Trinchieri (Wistar Institute, Philadelphia, PA) for the gift of recombinant IL-12 and monoclonal anti-IL-12 antibodies. The authors also thank Dr Mahasti S. de Macedo for reading of the manuscript. Mr Ademir Veras da Silva provided expert technical help. A. P. Galvão da Silva was supported by an MSc fellowship from FAPESP. This work was supported by FAPESP and CNPq.
References
- 1.Curotto-de-Lafaille MA, Barbosa-De-Oliveira LC, Lima GC, Abrahamsohn IA. Trypanosoma cruzi: maintenance of parasite-specific T cell responses in lymph nodes during the acute phase of infection. Exp Parasitol. 1990;70:164–74. doi: 10.1016/0014-4894(90)90097-v. [DOI] [PubMed] [Google Scholar]
- 2.Ramos C, Lamoyi E, Feoli M, Rodriguez M, Perez M, Ortiz-Ortiz L. Trypanosoma cruzi: immunosuppressed response to different antigens induced in the infected mouse. Exp Parasitol. 1978;45:190–9. doi: 10.1016/0014-4894(78)90059-0. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamsohn IA, Coffman RL. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–63. [PubMed] [Google Scholar]
- 4.Harel-Bellan A, Joskowicz M, Fradelizi D, Eisen H. T lymphocyte function during experimental Chagas' disease: production of and response to interleukin 2. Eur J Immunol. 1985;15:438–42. doi: 10.1002/eji.1830150505. [DOI] [PubMed] [Google Scholar]
- 5.Pinge-Filho P, Tadokoro CE, Abrahamsohn IA. Prostaglandins mediate suppression of lymphocyte proliferation and cytokine synthesis in acute Trypanosoma cruzi infection. Cell Immunol. 1999;193:90–8. doi: 10.1006/cimm.1999.1463. 10.1006/cimm.1999.1463. [DOI] [PubMed] [Google Scholar]
- 6.Tarleton RL. Trypanosoma cruzi-induced suppression of IL-2 production. II. Evidence for role for suppressor cells. J Immunol. 1988;140:2769–73. [PubMed] [Google Scholar]
- 7.Kierszenbaum F, Sztein MB. Mechanism underlying immunosuppression induced by Trypanosoma cruzi. Parasitol Today. 1990;6:261–4. doi: 10.1016/0169-4758(90)90187-9. [DOI] [PubMed] [Google Scholar]
- 8.Rottenberg M, Lindquist C, Koman A, Segura EL, Orn A. Modulation of both interleukin-2 receptor expression and interleukin-2 production during experimental murine Trypanosoma cruzi. Scand J Immunol. 1989;30:65–72. doi: 10.1111/j.1365-3083.1989.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamsohn IA, Coffman RL. Trypanosoma cruzi: IL-10, TNF, IFN-γ and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol. 1996;84:231–44. doi: 10.1006/expr.1996.0109. 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 10.Aliberti JC, Cardoso MA, Martins GA, Gazzinelli RT, Vieira LQ, Silva JS. Interleukin-12 mediates resistance to Trypanosoma cruzi in mice and is produced by murine macrophages in response to live trypomastigotes. Infect Immun. 1996;64:1961–7. doi: 10.1128/iai.64.6.1961-1967.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147:874–82. [PubMed] [Google Scholar]
- 12.Trinchieri G, Kubin M, Bellone G, Cassatella MA. Cytokine cross-talk between phagocytic cells and lymphocytes: relevance for differentiation/activation of phagocytic cells and regulation of adaptive immunity. J Cell Biochem. 1993;53:301–8. doi: 10.1002/jcb.240530406. [DOI] [PubMed] [Google Scholar]
- 13.Trinchieri G, Scott P. Immunoregulation by interleukin-12. Res Immunol. 1995;146:423–31. doi: 10.1016/0923-2494(96)83011-2. [DOI] [PubMed] [Google Scholar]
- 14.Valiante NM, Rengaraju M, Trinchieri G. Role of natural killer cell stimulatory factor (NKSF/IL-12) in the ability of B cell lines to stimulate T and NK cell proliferation. Cell Immunol. 1992;145:187–98. doi: 10.1016/0008-8749(92)90322-g. [DOI] [PubMed] [Google Scholar]
- 15.Bertagnolli MM, Lin BY, Young D, Hermann SH. IL-12 augments antigen-dependent proliferation of activated T lymphocytes. J Immunol. 1992;149:3778–83. [PubMed] [Google Scholar]
- 16.de Barros-Mazon S, Abrahamsohn IA. IL-12 enhances proliferation of peripheral blood mononuclear cells from Chagas' disease patients to Trypanosoma cruzi antigen. Immunol Lett. 1997;57:39–45. doi: 10.1016/s0165-2478(97)00079-5. 10.1016/s0165-2478(97)00079-5. [DOI] [PubMed] [Google Scholar]
- 17.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 18.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer all stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–46. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perussia B, Chan SH, D'Andrea A, et al. Natural killer (NK) cell stimulatory factor or IL-12 has differential effects on proliferation of TCR-αβ, TCR-γδ T lymphocytes and NK cells. J Immunol. 1992;149:3495–502. [PubMed] [Google Scholar]
- 20.Chouaib S, Chehimi J, Bani L, Genetet N, Tursz T, Gay F, Thinchieri G, Mami-Chouaib F. Interleukin 12 induces the differentiation of major histocompatibility complex class I-primed cytotoxic T-lymphocyte precursors into allo-specific cytotoxic effectors. Proc Natl Acad Sci USA. 1994;91:12659–63. doi: 10.1073/pnas.91.26.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well distinct from IL-12. Immunity. 2000;13:715–25. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 22.Soong L, Tarleton RL. Trypanosoma cruzi infection suppresses nuclear factors that bind to specific sites om the interleukin-2 enhance. Eur J Immunol. 1994;24:16–23. doi: 10.1002/eji.1830240104. [DOI] [PubMed] [Google Scholar]
- 23.Martins GAC, Aliberti JC, Silva JS. Nitric oxide-induced apoptotic cell death in the acute phase of Trypanosoma cruzi infection in mice. Immunol Lett. 1998;63:113–20. doi: 10.1016/s0165-2478(98)00066-2. 10.1016/s0165-2478(98)00066-2. [DOI] [PubMed] [Google Scholar]
- 24.Millar AE, Kahn SJ. Trypanosoma cruzi: the effect of nitric oxide synthesis inhibition on the CD4 T cell response to the trans-sialidase superfamily. Exp Parasitol. 2000;94:84–91. doi: 10.1006/expr.1999.4472. 10.1006/expr.1999.4472. [DOI] [PubMed] [Google Scholar]
- 25.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 26.Chizzonite R, Truitt T, Desai B, Nunes P, Podlaski FJ, Ster AS, Gately MK. IL-12 receptor. I. Characterization of the receptor on PHA-activated human lymphoblast. J Immunol. 1992;148:3117–24. [PubMed] [Google Scholar]
- 27.Wu C, Warrier RR, Wang X, Presky DH, Gately MK. Regulation of interleukin-12 receptor beta 1 chain expression and interleukin-12 binding by human peripheral blood mononuclear cells. Eur J Immunol. 1997;27:147–54. doi: 10.1002/eji.1830270122. [DOI] [PubMed] [Google Scholar]
- 28.Yanagida T, Kato T, Igarashi O, Inoue T, Nariuchi H. Second signal activity of IL-12 on the proliferation and IL-2R expression of T helper cell-1 clone. J Immunol. 1994;152:4919–28. [PubMed] [Google Scholar]
- 29.Ueta C, Kawasumi HA, Fujiwara H, Miyagwa T, Kida H, Ohmoto Y, Kishimoto S, Tsuyuguchi I. Interleukin-12 activates human gamma delta T cells: synergistic effect of tumor necrosis factor-alpha. Eur J Immunol. 1996;26:3066–73. doi: 10.1002/eji.1830261237. [DOI] [PubMed] [Google Scholar]