Abstract
CD8+ T cells can be grouped into two different types of secretory T lymphocytes, based on the cytokine-secretion pattern upon antigen exposure: those with a T-cell cytotoxic type 1 response (Tc1), which secrete interferon-γ (IFN-γ), or those with a T-cell cytotoxic type 2 response, which secrete interleukin (IL)-4 and IL-10. We examined the CD8+ T-cell response directed against an immunodominant human leucocyte antigen (HLA)-A2-presented peptide derived from a 19-kDa Mycobacterium tuberculosis-associated antigen. T cells were examined by functional analysis and by T-cell receptor (TCR) complementarity-determining region 3 (CDR3)-spectratyping, which defines the complexity of a T-cell response. T-cell stimulation with the immunodominant VLTDGNPPEV epitope yielded a Tc2 (IL-4) cytokine-secretion pattern and resulted in oligoclonal expansion of TCR-variable beta chain (VB) families, which differed from patient to patient. Generation of T-cell clones corroborated the notion that the CD8+ T-cell response directed against the HLA-A2-presented VLTDGNPPEV epitope leads to a Tc2 cytokine-secretion pattern in CD8+ T cells, as defined by IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) release. Characterization of the cytokine-secretion profile in HLA-A2/VLTDGNPPEV-tetramer sorted T cells from patients with active tuberculosis supported this observation: peptide-specific T cells from three of three patients secreted IL-4 and only one of three patients produced IFN-γ in response to the nominal target epitope. Permutation of this T-cell epitope may aid to elicit a qualitatively different CD8+ T-cell response in patients with M. tuberculosis infection.
Introduction
The contribution of CD4+ T cells to the control of infection with Mycobacterium tuberculosis has been well documented.1,2 Recent studies support the role of CD8+ T cells in the eradication and/or containment of viable bacteria which survive in macrophages.3,4 At least two anti-M. tuberculosis-directed CD8+ T-cell subsets have been identified. One group recognizes M. tuberculosis-infected host cells by non-classical restricting antigens, including major histocompatibility complex (MHC) class Ib or CD1 molecules.5,6 The second group of ‘classical’ MHC class I-restricted T cells includes CD8+ αβ T-cell receptor-positive (TCR+) T cells, which recognize several target antigens, including the mycobacterial major secreted antigen 85,7–10 the ESAT-6 antigen,11 the CFP10/Mtb11 antigen,12 or the 19-kDa secreted lipoprotein.13 The latter antigen elicits both CD4+ and CD8+ T-cell responses in humans.13–16 A high-affinity human leucocyte antigen (HLA)-A2-binding peptide encompassing amino acid (aa) residues 88–97 (VLTDGNPPEV) in the 19-kDa lipoprotein has been identified as the immunodominant target epitope.13 T-cell precursors directed against this peptide are present in bacille Calmette–Guérin (BCG)-immunized individuals, as well as in patients with active tuberculosis. This T-cell epitope can also be implemented to drive antigen-specific and HLA-A2-restricted T cells from uninfected individuals.13 Such T cells are able to lyse HLA-2+ target cells pulsed with the nominal T-cell epitope. However, it has not yet been determined whether the T-cell response directed against this epitope is oligoclonal or polyclonal, and whether the cytokine-secretion profile of this CD8+ T-cell response can be grouped into a T-cell cytotoxic type 1 response (Tc1), secreting interferon-γ (IFN-γ), or a T-cell cytotoxic type 2 response (Tc2), secreting interleukin (IL)-4.
In other intracellular (viral) infections in which CD8+ T cells play a major role, the epitope-specific T-cell responses appear to be surprisingly oligoclonal, i.e. only a few expanded T-cell clonotypes are responsible for recognition of the nominal target epitope(s) presented by class I molecules.17,18 A focused, oligoclonal antigen-specific T-cell response has been associated with the development or maintenance of a protective cellular immune response.19 We examined the diversity, as defined by TCR complementarity determining region 3 (CDR3) spectratyping, and the quality, as defined by cytokine release, of CD8+ T-cell responses directed against the HLA-A2-presented epitope VLTDGNPPEV in six healthy subjects. T-cell clones were established in order to evaluate cytokine production upon antigen exposure.
Materials and methods
T-cell lines and cloning
Peripheral blood mononuclear cells (PBMCs) from healthy, purified protein derivative (PPD)-negative blood donors were obtained by separation over a Ficoll gradient and stored in liquid nitrogen at 1–5 × 107 cells/vial in 90% fetal calf serum (FCS) and 10% dimethylsulphoxide (DMSO). MHC class I typing was performed at the local blood bank (Dr Hitzler, University of Mainz). T cells were stimulated twice, at weekly intervals, with autologous IL-4/granulocyte–macrophage colony-stimulating factor (GM-CSF)-driven dendritic cells (DCs), in medium containing AIM-V supplemented with 10% FCS (Gibco, Eggenstein, Germany), 50 IU/ml of IL-2 and 50 ng/ml of IL-7 (Dr Natalio Vita; Sanofi, Labege, France). CD8+ T cells were then expanded by weekly restimulations using irradiated autologous PBMCs pulsed with 100 ng of peptide in the presence of 5 µg β2-microglobulin (Sigma, Deisenhofen, Germany) for 2 hr at room temperature. For cloning, T cells were seeded at 1, 10 or 100 T cells/well in U-bottom 96-well plates (10 plates for each dilution) and stimulated at weekly intervals with autologous irradiated (3000 rads) PBMCs loaded with 100 ng of peptide. T-cell clones or oligoclonal lines were expanded in 48- and then in 24-well plates and tested for peptide recognition.
Immunomagnetic cell sorting and functional assays
Prior to the generation of CD8+ T-cell lines or T-cell cloning, CD4+ T cells were separated from 3–5 × 107 PMBCs using anti-CD4-coated immunomagnetic beads (Miltenyi, Bergisch Gladbach, Germany). For functional assays, T cells were admixed with T2-cells loaded either with diluent alone (10% DMSO, 90% RPMI) or with 1 µg of the M. tuberculosis VLTDGNPPEV peptide plus 20 µg of β2-microglobulin/106 target cells. The HLA-A2-binding peptide derived from the melanoma-associated antigen gp100 (aa 154–162: KTWGQWQV) served as the negative control. Effector cells were incubated with target cells at an effector : target (E : T) ratio of 5 : 1 for 24 hr and then supernatants were harvested and tested for IFN-γ, IL-4 or GM-CSF using the enzyme-linked immunosorbent assay (ELISA) system obtained from Diaclone (Besancon, France).
Tetramer-guided cell sorting
HLA-A2 tetramers were produced as described in detail previously and loaded with either the M. tuberculosis 19-kDa peptide VLTDGNPPEV or with the HLA-A2-binding peptide NLVPMVATV provided by the cytomegalovirus (CMV) pp65 antigen.20 Peripheral blood lymphocytes (PBL) were obtained from three HLA-A2-positive patients with active pulmonary tuberculosis and evaluated for tetramer staining by flow cytometry. Briefly, CD3+ CD8+ T cells were gated using the anti-CD3 monoclonal antibody (mAb) UCHT1 (murine immunoglobulin G1 [IgG1] coupled to fluorescein isothiocyanate [FITC]) and anti-CD8 mAb B9.11 (murine IgG1 labelled with PC5) and tested for binding to phycoerythrin (PE)-labelled HLA-A2 tetramer complexes. For cell sorting, PBL were incubated with the HLA-A2 tetramer complex (1 µg/2 × 106 cells) for 1 hr at 37°, washed once in PBS, and tetramer-binding cells were isolated using anti-PE-coated immunomagnetic beads obtained from Miltenyi. T cells were rested overnight in Dulbecco's modified Eagle's minimal essential medium (DMEM) (high glucose) containing 20% FCS and 50 ng/ml of IL-7, and then tested for cytokine secretion using T2 cells loaded with the peptide VLTDGNPPEV and β2-microglobulin (100 ng of peptide and 20 µg of β2-microglobulin/105 cells/ml). One-hundred microlitres of these stimulator cells were incubated for 48 hr with 5000 tetramer-sorted T cells; the supernatants were then harvested and tested by ELISA for secretion of IFN-γ and IL-4.
TCR-CDR3 spectratyping
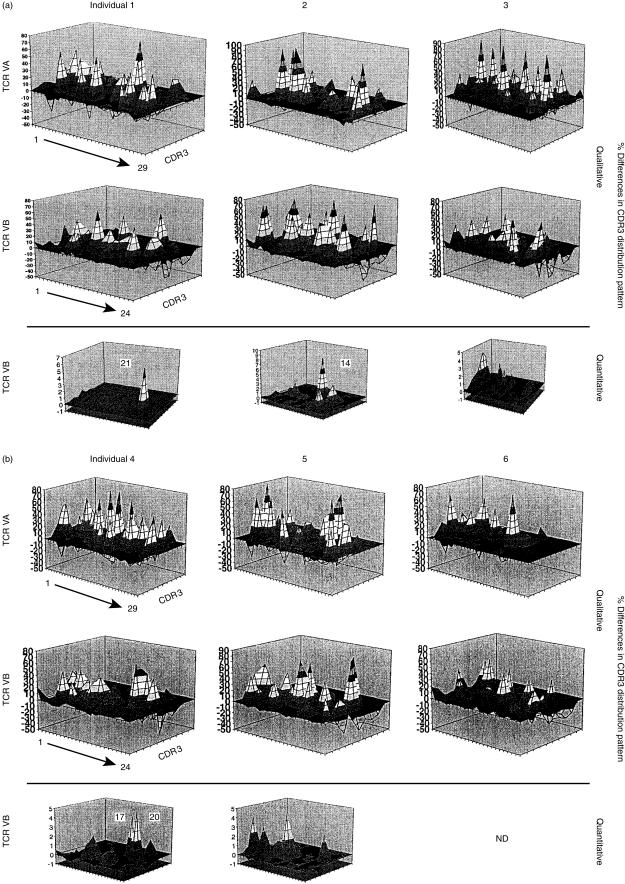
RNA was extracted and reverse transcribed into cDNA, amplified by individual TCR variable alpha chain (VA) and 24 variable beta chain (VB)-specific primer pairs, and a run-off reaction using a fluorophore-labelled TCR-CA or -CB-specific primer was performed.21 Labelled amplicons were analysed by DNA fragment analysis using appropriate size-standards and a 310 sequencer and Genescan software (ABI, Weiterstadt, Germany). In order to identify monoclonal/oligoclonal TCR transcripts, amplicons were subcloned into the TA sequencing vector (Invitrogen, Groningen, the Netherlands). TCR VA/VB were only reported as monoclonal if either direct sequencing of the polymerase chain reaction (PCR) amplicon or all subcloned PCR transcripts yielded the identical TCR sequence. If the TCR VA/VB family is oligoclonal or polyclonal, a Gauss-distribution occurs.22 Each peak represents base pairs (bp) coding for one aa residue. The area under the curve of each VA or VB amplicon represents the frequency of a distinct CDR3 length in an individual TCR VA/VB family. In order to condense the information from a single sample analysis, the individual TCR VA or VB families can be grouped into a single figure with VA1–VA29 or VB1–VB24 along with the CDR3 length expressed as the number of aa. This TCR-CDR3 landscape provides the ‘structural anatomy’, as defined by the TCR-CDR3 length for each TCR family in a T-cell subpopulation.19,22 The area under the curve of each CDR3 peak is expressed as the percentage of the entire CDR3 area (100%) for each individual VA or VB family. For clarity, each 10% value is depicted in different colours. The CDR3 pattern obtained from CD8+ T cells can be compared with a standard control TCR CDR3 analysis, which yields a Gauss-distribution of the CDR3 length composition encompassing 1–10 aa residues (z-axis). This TCR ‘perturbation’ within each CDR3 length is calculated by the areas between the CDR3 distribution in each VA or VB sample (x-axis) and the control distribution.19,22 Positive or negative perturbations may occur in each TCR VA/VB CDR3 peak and are depicted as differences versus the control sample. Each perturbation yielding a 10% difference is depicted in a different colour. Note that a ‘flat’ TCR landscape in this analysis implies that no perturbation exists, i.e. the TCR-VA/VB landscape would yield a picture identical to that of the control sample. Over- or under-representation as compared to the control distribution is indicated as percentage difference in the CDR3 distribution pattern, either as positive or negative on the y-axis, respectively.
CDR3 analysis and CCR-VB staining: quantitative TCR analysis
TCR-spectratyping yields the qualitative, but not the quantitative, assessment of a T-cell population. A panel of 21 individual mAbs directed against the TCF VB-chain (Beckman Coulter, Krefeld, Germany) were grouped to three individual anti-VB mAbs labelled with FITC, PE, or double-labelled with FITC/PE, which can be gated either on ECD-CD4 or PC5-CD8+ T cells. Thus, the frequency of 21 individual TCR VB families can be analysed in seven different tubes, which yields the percentage (X% of a VB family) in CD3+ CD8+ T cells.23 This factor can be used to correct the CDR3-VB landscape analysis. Exclusively a mAb panel directed against TCR VB-chains, but not TCR-VA-chains, is available.
Results
M. tuberculosis-specific CD8+ T cells are oligoclonal and show a Tc2 cytokine secretion pattern
PBL from six individual HLA-A2+ donors, who had not been BCG immunized or infected with M. tuberculosis, were stimulated in vitro using 7-day DC generated by stimulation with IL-4 and GM-CSF and pulsed with the peptide VLTDGNPPEV. CD8+ T cells were analysed in three individual aliquots: the first served to determine the diversity of the TCR repertoire using the TCR CDR3 spectratyping analysis; the second was used to enumerate individual T-cell TCR VB-families using a panel of 21 individual mAbs in order to gauge the quantity of the T cells in each TCR VB family; and the third aliquot was used in functional assays, including a 4-hr standard cytotoxicity assay and a 24-hr cytokine-release assay. The qualitative analysis was available for both TCR VA and VB chains in each T-cell lines, but the combination of the flow cytometry-based quantification of individual VB-families could only be used with the TCR VB-families mAb panel owing to the lack of anti-human TCR VA-specific mAbs. Note that the data are represented as TCR perturbations as compared to a typical CDR3 analysis, which represents a Gauss-distribution of each individual VA or VB families pertaining to the CDR3 length.19,22 In general, CD8+ T cells obtained from individuals 1–6 exhibited major perturbation(s) in the TCR repertoire landscape. The major percentage of the TCR VA/VB families showed an oligoclonal TCR pattern compared to non-stimulated T cells (Fig. 1a, 1b). Stimulation of the identical donor T-cell population with a different peptide (e.g. a high-affinity HLA-A2-binding peptide provided from HPV16-E7) yielded a completely different composition of T-cell expansions (data not shown). Thus, VLTDGNPPEV-driven expansion in individual T-cell lines was associated with the stimulating peptide. The combination of the quality of the T-cell response, as defined by PCR-based spectratyping analysis, in combination with the enumeration of TCR VB-families by flow cytometry, revealed that exclusively some oligoclonal TCR VB families are preferentially expanded upon peptide simulation, e.g. TCR VB21 in individual no. 1, or VB14 in individual no. 2 (Fig. 1a, 1b). We did not observe a TCR VB expansion of similar VB families in different individuals, or a shared monoclonal/oligoclonal expansion of certain T-cell subsets. Each of these T-cell populations from individuals 1–6 was tested in a 16-hr cytokine-release assay using the target antigen VLTDGNPPEV loaded onto T2 cells. CD8+ T cells from individuals 1 and 2 secreted significant amounts of GM-CSF and IL-4, but not IFN-γ, in response to the nominal antigen (Table 1). In contrast, T cells from individual no. 6 secreted IFN-γ, but not GM-CSF or IL-4. The T-cell line generated from individual no. 3 secreted IL-4 and IFN-γ. T cells from individuals 4 and 5 did not show significant cytokine release upon antigen contact. No significant cytotoxic T-cell response was observed in any of the six CD8+ T-cell lines. As GM-CSF/IL-4 secretion may represent a marker of a Tc2 response, we cloned the CD8+ T-cell lines.
Figure 1.
Oligoclonal T-cell response to the immunodominant peptide derived from the Mycobacterium tuberculosis 19-kDa antigen. CD8+ T cells from individuals 1–3 (a) and 4–6 (b) were harvested after restimulation with the nominal target epitope VLTDGNPPEV. T-cell receptor (TCR) complementarity-determining region 3 (CDR3) analysis was performed for each TCR variable alpha chain (VA) and variable beta chain (VB) family in each sample. Note that not the absolute numbers, but the qualitative over- or under-representation of each TCR family is depicted based on the CDR3-length analysis. The TCR VB data could be complemented with a qualitative assessment of individual TCR VB families obtained by flow cytometry (bottom panel: ‘quantitative’ analysis). Data are depicted as over- or under-representation of VB families as compared to normal control samples. Note the preferential expansion of certain TCR VB families. This pattern was restricted to the peptide VLTDGNPPEV; stimulation of T cells with a different peptide (e.g. YMLDLQPET, from HPV16-E7) yielded different results (data not shown). y-axis: % difference in CDR3 distribution (perturbation); z-axis: CDR3-length (1–10 amino acid residues); x-axis: individual VA (n = 29) or VB (n = 24) TCR families.
Table 1.
Cytokine-secretion pattern in Mycobacterium tuberculosis 19-kDa reactive CD8+ T-cell lines
| T2 alone | T2+ control | T2+ VLTDGNPPEV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Lines (no.) | GM-CSF | IL-4 | IFN-γ | GM-CSF | IL-4 | IFN-γ | GM-CSF | IL-4 | IFN-γ |
| 1 | 3 | 0 | 100 | 0 | 0 | 100 | 400 | 41 | 100 |
| 2 | 7 | 0 | 400 | 6 | 0 | 380 | 400 | 38 | 400 |
| 3 | 6 | 0 | 100 | 7 | 0 | 100 | 8 | 63 | 130 |
| 4 | 10 | 0 | 300 | 3 | 0 | 330 | 9 | 0 | 280 |
| 5 | 3 | 0 | 330 | 2 | 0 | 350 | 3 | 0 | 300 |
| 6 | 3 | 0 | 400 | 3 | 0 | 400 | 10 | 0 | 800 |
Cytokine release is expressed as pg/ml/24 hr.CD8+ T cells were repetitively stimulated with dendritic cells (DCs) loaded with the immunodominant peptide from the Mycobacterium tuberculosis 19-kDa peptide VLTDGNPPEV and tested for recognition of the nominal target epitope. T2 cells served as the surrogate recipient cell line. Controls were T2 cells alone, or T2 cells pulsed with an irrelevant human leucocyte antigen (HLA)-A2 binding peptide (control) from the melanoma-associated antigen gp100 (KTWGQYWQV). CD8+ T-cell lines secreted interferon-γ (IFN-γ) to T2 cells alone, or to those pulsed with the control peptide. CD8+ T-cell lines from individuals 1 and 2 secreted significant amounts of interleukin-4 (IL-4) or granulocyte–macrophage colony-stimulating factor (GM-CSF) in response to the target antigen, as compared to T2 cells alone or T2 cells pulsed with an irrelevant control peptide. Note that only CD8+ T cells obtained from individual 6 secreted significant levels of interferon-γ (IFN-γ) as compared to the control targets. T cells from individual 3 secreted IL-4 and low amounts of IFN-γ as compared to control targets. Data are representative of three experiments. No cytotoxic response was observed in a 4-hr standard
Cr-release assay (data not shown).
Values shown in bold are significant versus controls.
Generation of VLTDGNPPEV-specific and HLA-A2-restricted T-cell clones: shared TCR usage and Tc2 cytokine-secretion pattern upon antigen exposure
T cells were cloned from the CD8+ T-cell lines (Fig. 1a, 1b) from individuals 1–6, but only CD8+ T-cell lines from two of six donors (derived from individuals 2 and 3) could be successfully expanded. These T cells were examined for the usage of TCR VA/VB expression and for the pattern of cytokine secretion upon exposure to the nominal target epitope. Six individual T-cell lines obtained from CD8+ T cells obtained from individual no. 2 could be generated (compiled in Table 2). A single bona fide clone turned out to be clone no. 2.4, which exhibits the TCR VA3 chains joined with VB4 and/or VB13S6. This could indicate that two T-cell clones expressing VB4 or VB13S6 use the same TCR VA3 chain. Alternatively, a single TCR VA chain can be paired with two VB chains as a result of unsuccessful allelic exclusion.24
Table 2.
T-cell receptor usage in CD8+ T-cell lines (2.2, 2.4, 2.5, 2.15, 2.22 and 2.35)
| TCR VA chains | TCR VB chains | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA/VB | 2.2 | 2.4 | 2.5 | 2.15 | 2.22 | 2.35 | 2.2 | 2.4 | 2.5 | 2.15 | 2.22 | 2.35 |
| 1 | X | X | X | X | ||||||||
| 2 | ||||||||||||
| 3 | X | X | X | X | X | X | ||||||
| 4 | X | X | X | |||||||||
| 5 | X | |||||||||||
| 6 | X | |||||||||||
| 7 | ||||||||||||
| 8 | X | X | ||||||||||
| 9 | ||||||||||||
| 10 | ||||||||||||
| 11 | ||||||||||||
| 12 | X | X | ||||||||||
| 13 | X | |||||||||||
| 14 | ||||||||||||
| 15 | ||||||||||||
| 16 | X | X | ||||||||||
| 17 | ||||||||||||
| 18 | ||||||||||||
| 19 | ||||||||||||
| 20 | ||||||||||||
| 21 | ||||||||||||
| 22 | X | |||||||||||
| 23 | X | |||||||||||
| 24 | ||||||||||||
| 25 | ||||||||||||
| 26 | ||||||||||||
| 27 | ||||||||||||
| 28 | ||||||||||||
| 29 | X | |||||||||||
Not determined.
CD8+ peripheral blood lymphocytes (PBL) were stimulated with interleukin-4 (IL-4)/granulocyte–macrophage colony-stimulating factor (GM-CSF)-driven dendritic cells (DCs) pulsed with peptide VLTDGNPPEV, followed by cloning of T-cell lines in 96-well plates at 1, 10, or 100 T cells/well (10 plates for each concentration). T cells were stimulated weekly with 3000 rads irradiated autologous peripheral blood mononuclear cells (PBMCs) pulsed with the peptide VLTDGNPPEV in AIM-V medium containing 50 IU/ml of IL-2 and 50 ng/ml of IL-7. T cells that could be expanded were seeded in 24-well plates. RNA was isolated from each cell line and reverse transcribed into cDNA. Each T-cell receptor (TCR) variable alpha chain (VA) or variable beta chain (VB) expression was tested by reverse transcription–polymerase chain reaction (RT–PCR) analysis. Owing to the low number of cells available from T-cell line 2.2, examination of the TCR VA chains was not possible. The T-cell lines were from individual 2.
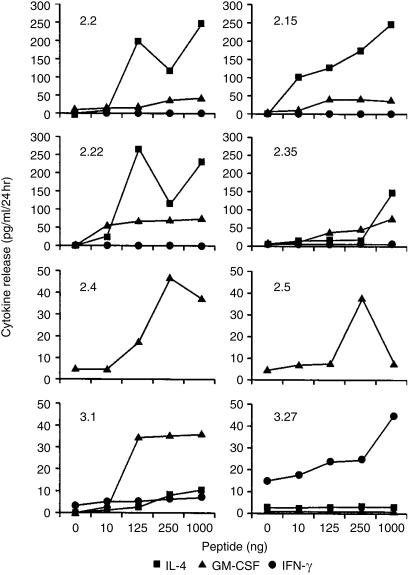
Other CD8+ T-cell lines showed an oligoclonal TCR VA/VB usage, e.g. the usage of VA1 in four of six oligoclonal T-cell lines, VA3 in three of six lines, or VB16 in two of six T-cell lines. These data indicate a common usage of certain TCR VA/VB chains, but it does not necessarily imply that these TCRs are identical. In order to address this question, we subcloned each TCR VA/VB chain for DNA-sequence analysis, compiled in Table 3. Of note, some CD8+ T-cell lines use the identical TCR VA chain, e.g. TCR VA1S1 in four of six CD8+ T-cell lines. In contrast, other T-cell lines share the identical TCR VA segment (e.g. VA3 in CD8+ T-cell lines 2.4 and 2.5), but the VA3 in the CD8+ T-cell line 2.35 is not identical owing to a different CDR3 region involved in antigen recognition. A similar situation is true for CTL lines 2.22 and 2.35: they both use VB16 as the variable segment in the TCR, but the area of antigen recognition (CDR3 region) is different. Each of these molecularly defined CD8+ T-cell lines was tested for recognition of the peptide VLTDGNPPEV in a cytokine-release assay (Fig. 2). Three groups of reactivity, as defined by cytokine release, could be identified:
Table 3.
T-cell receptor (TCR) usage in clonal/oligoclonal T cells defining a human leucocyte antigen (HLA)-A2-presented peptide from Mycobacterium tuberculosis
| T-cell clones/lines | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TCR | TCR VA/VB | CDR3 | Joining region | 2.2* | 2.4 | 2.5 | 2.15 | 2.22 | 2.35 | |
| VA1S1 | AEYFCA | VKP | GGGADGLTFGKGTJLII | AJ45 | X | X | X | X | ||
| VA3 | SYFCA | TVLSF | YNQGGKLIFGQGTE | AJ23 | X | X | ||||
| VA3 | SYFCA | TS | GGSYIPTFGRGTSL | AJ6 | X | |||||
| VA22 | QVSDSAVYFCA | LG | TGGGNKLTFGTGTQLKVN | AJ10 | X | |||||
| VA23 | QPGDSATYLCA | VSPL | NSGNTPLVFGKGT | AJ29 | X | |||||
| VA29 | QLSYSGTYFCG | TTI | GGSQGNLIFGKGTKLSVKP | AJ42 | X | |||||
| VB3 | TNQTSMYLCA | SSLQR | TEAFFGQGT | BJ1.1 | X | X | X | |||
| VB4 | PEDSSIYLCS | VGD | YGYTFGSGTRLTV | BJ1.2 | X | X | X | |||
| VB5 | ELDDSALYLCA | WGTPELP | YEQYFGPGTRLT | BJ2.7 | X | |||||
| VB6S7 | TQQEDSAVYLCA | SSNRDSPL | GELFFGEG | BJ2.2 | X | |||||
| VB8 | IQPSEPRDSYVYFCA | SSLYSGGRE | EQFFGPGTRLT | BJ2.7 | X | X | ||||
| VB12 | SSGTSVYFCA | ISESRNG | YNEQFFGPGH | BJ2.1 | X | X | ||||
| VB13S6 | LRELAAPSQTSVYFCA | SSYLPGTGETS | QHFGDGTR | BJ1.5 | X | |||||
| VB16 | LEDSGVYFCA | SSQGSG | NEQFFGPG | BJ2.1 | X | |||||
| VB16 | LEDSGVYFCA | SSQLSGQV | NEKLFFGSGTQLS | BJ1.4 | X | |||||
| GM-CSF | GM-CSF | GM-CSF | GM-CSF | GM-CSF | GM-CSF | |||||
| IL-4 | IL-4 | IL-4 | IL-4 | |||||||
Not determined for VA expression.
T cells were cloned using repetitive stimulation with the peptide VLTDGNPPEV and seeded in 96-well plates at 1, 10, or 100 cells/well. After restimulation using autologous PBMCs plus peptide, T cells were expanded and tested for T-cell receptor (TCR) variable alpha chain (VA) of variable beta chain (VB) expression. Each TCR VA/VB chain was directly sequenced. Note that some CD8+ T-cell lines utilize the identical TCR, other use the same variable region, but a different complementarity-determining region 3 (CDR3). The CDR3 region determines the antigen specificity. The cytokine-secretion pattern of these CD8+ monoclonal or oligoclonal T-cell lines is compiled as defined by interleukin-4 (IL-4) or granulocyte–macrophage colony-stimulating factor (GM-CSF) release. No interferon-γ (IFN-γ) secretion could be detected. This compilation refers to Fig. 2. Shared usage of aa within the highly polymorphic CDR3 region is indicated in bold.
Figure 2.
Monoclonal and oligoclonal CD8+ T cells secrete T-cell cytotoxic type 2 (Tc2) cytokines in response to the immunodominant peptide VLTDGNPPEV from the 19-kDa Mycobacterium tuberculosis antigen. CD8+ T cells, as defined in Table 3, were tested for recognition of the nominal target epitope in a 24-hr cytokine-release assay. T-cell lines 2.4 and 2.5 exclusively secreted granulocyte–macrophage colony-stimulating factor (GM-CSF), T-cell lines 2.2, 2.15, 2.22 and 2.35 secreted interleukin-4 (IL-4) and GM-CSF. In contrast, only a single clone, designated 3.27, secreted interferon-γ (IFN-γ), but not GM-CSF or IL-4 upon antigen exposure. The cytokine-secretion pattern could not be altered by different peptide concentrations in the assay.
T cells that respond exclusively with GM-CSF secretion, but not with IFN-γ or IL-4 release, to the stimulating peptide at different antigen concentrations: CD8+ T-cell lines 2.4. and 2.5 showed this pattern and displayed a different TCR repertoire.
In contrast, four of six CD8+ T-cell lines responded with IL-4 and GM-CSF release, but not with IFN-γ secretion. Of particular interest is the T-cell line 2.22, which exhibits a single TCR VA (VA1S1) chain, but three VB chains (VB3, VB4, and VB16). This presumably represents two CD8+ T-cell clones, one of which shares the VA1 chain paired with a single VB chain and a second VA1+ T-cell clone which coexpresses the TCR VA1 chain with different TCR VB chains owing to unsuccessful allelic TCR VB exclusion. Thus, six of six oligoclonal T-cell lines directed against the HLA-A2-presented peptide VLTDGNPPEV secrete GM-CSF, either alone or with IL-4.
Two additional T-cell clones obtained from individual no. 3 showed, at least in a single case (clone 3.27), a significant IFN-γ response to the nominal target antigen. We could not define the TCR composition in these clones due to the low cell number. Thus, the HLA-A2-binding immunodominant peptide VLTDGNPPEV is able to activate and to expand T-cell clones which predominantly secrete a T2 (IL-4) cytokine-secretion pattern. However, this is apparently not exclusive, as one of six oligoclonal T-cell lines (Table 1), or clone 3.27, secreted IFN-γ in response to the HLA-A2-presented immunodominant peptide provided by the 19-kDa lipoprotein (Fig. 2).
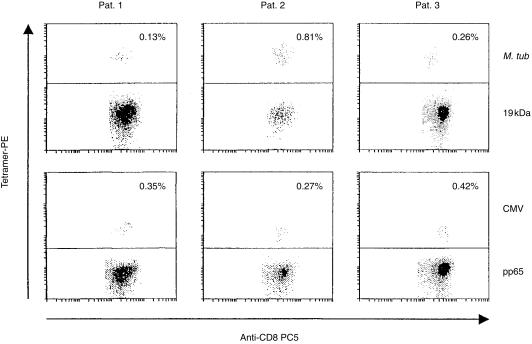
In order to gauge the anti-VLTDGNPPEV-specific cellular immune response in patients with active pulmonary tuberculosis, we used soluble HLA-A2 tetramer complexes to directly visualize antigen-specific T cells in PBL without the need for ex vivo manipulation. We were able to detect M. tuberculosis 19 kDa-specific and HLA-A2-restricted T-cell responses in PBL from three of three patients, ranging from 0·13 to 0·81% of the entire CD8+ T-cell population (Fig. 3). These HLA-A2/VLTDGNPPEV-binding T cells were sorted and tested for cytokine secretion in response to the nominal target epitope (Table 4). Three of three T-cell lines secreted IL-4. In contrast, one of these T-cell lines also secreted IFN-γ.
Figure 3.
Detection of Mycobacterium tuberculosis 19-kDa peptide-specific T cells in peripheral blood lymphocytes (PBL) from patients with tuberculosis. PBL from three human leucocyte antigen (HLA)-A2+ patients with active tuberculosis were gated for CD3+ CD8+ T cells and tested for tetramer binding by flow cytometry. The M. tuberculosis 19-kDa peptide VLTDGNPPEV (upper panel) or the cytomegalovirus (CMV) pp65 peptide NLVPMVATV (lower panel) were loaded onto phycoerythrin (PE)-labelled soluble HLA-A2 tetramer complexes. The CMV tetramer served as a positive control. Of the entire CD8+ T-cell population, 0·13–0·81% were found to bind to the M. tuberculosis 19-kDa epitope. These T cells were sorted and tested for cytokine release (Table 4). M. tuberculosis 19-kDa peptide-binding peptides exhibited a CD8+ CD45RA+ CD28− phenotype associated with ‘effector-type’ CD8+ T cells (data not shown) according to the definition of Hamann et al.47 PBL from five HLA-A2+, purified protein derivative (PPD)-negative individuals stained negative for binding to M. tuberculosis 19-kDa peptide tetramer complexes (data not shown).
Table 4.
Cytokine-secretion pattern in Mycobacterium tuberculosis 19-kDa peptide-sorted T cells
| Patient 1 | Patient 2 | Patient 3 | ||||
|---|---|---|---|---|---|---|
| Targets | IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 |
| T2-cells loaded with peptides from: | ||||||
| M. tub. 19 kDa | 0 | 60 | 0 | 64 | 108 | 52 |
| gp100 | 0 | 0 | 0 | 0 | 20 | 0 |
| Diluents | 0 | 0 | 0 | 0 | 0 | 0 |
Cytokine release is expressed as pg/ml/24 hr.
M. tuberculosis 19-kDa peptide (VLTDGNPPEV)-specific T cells were isolated from freshly harvested peripheral blood lymphocytes (PBL) from human leucocyte antigen (HLA)-A2+ patients with active pulmonary tuberculosis and tested for target recognition using the nominal T-cell epitope used for the tetramer-sorting procedure. T2 cells with diluent alone or pulsed with a control peptide from the melanoma-associated antigen gp100 served as controls. M. tuberculosis 19-kDa tetramer-sorted T cells from three of three patients secreted interleukin-4 (IL-4) and T cells from one of three patients secreted interferon-γ (IFN-γ).
Discussion
We have been able to show that a single T-cell epitope derived from a secreted M. tuberculosis 19-kDa lipoprotein leads to an oligoclonal preferential expansion of certain TCR VB families in individual subjects. This was surprising, as we would have expected a polyclonal T-cell response directed to a single T-cell epitope.25 However, recent studies examining viral infections in murine models, as well as in humans, have provided strong evidence that the peripheral T-cell response directed against single viral epitopes presented by MHC class I epitopes are indeed constituted by a few T-cell clones.26,27 These clones may constitute, at least during some time-points during the infection, the majority of T cells in the peripheral circulation. This notion can now be expanded to the bacterial M. tuberculosis epitope presented by HLA-A2. A restricted TCR repertoire or oligoclonal T-cell expansion directed against single T-cell epitopes has also been shown to be true for infection with human immunodeficiency virus (HIV),28 Epstein–Barr virus (EBV),29 cytomegalovirus (CMV)30 or hepatitis C virus (HCV).31 The biological meaning of this phenomenon pertaining to the focus of the TCR repertoire on a few T-cell clones is still to be elucidated. At least in patients with HIV infection, oligoclonal expanded CD8+ T cells most commonly represent antigen-specific effector T cells directed against a single epitope. The presence and maintenance of these clonal/oligoclonal T-cell subsets may be associated with non-progression of the disease.19 The emergence of oligoclonal expanded T cells associated with antigen-specific cellular immune reponses may also represent a marker of an effective and focused cellular immune surveillance directed against M. tuberculosis. This notion is supported by the observation that in vitro stimulation of T cells with viable mycobacteria may lead to a preferential expansion of TCR VA2.3+ CD4+ T cells in individuals with a distinct genetic background (HLA-DR17+, DQ2+).32
Only a limited number of studies have addressed the role of CD8+ T cells in patients with M. tuberculosis infection. Particularly the secreted extracellular antigens appear to represent target molecules that drive proliferation as well as IFN-γ secretion in responding T cells.33 With the advent of soluble tetramer-HLA-A2 molecules associated with peptide epitopes, the frequency of antigen-specific T cells can now be evaluated in the peripheral circulation without the need of ex vivo expansion. Tetramer-staining CD8+ T cells can also be analysed for cytokine secretion using intracellular cytokine staining. M. tuberculosis AG85-specific CD8+ T cells stain for IFN-γ, but not for IL-4, in response to the HLA-A2-restricted AG85-target epitope.12 We show in this report that most of the HLA-A2-restricted and M. tuberculosis 19-kDa lipoprotein-specific T cells do not secrete IFN-γ, but rather GM-CSF or IL-4, in an 24-hr cytokine release assay, independently of the peptide concentration.
These data could be confirmed using soluble HLA-A2 complexes loaded with the peptide VLTDGNPPEV from the M. tuberculosis 19-kDa antigen (Fig. 3 and Table 4) as a ‘bait’ to sort peptide-specific T cells from PBL from patients with pulmonary tuberculosis. It is probable that these freshly isolated T cells would reflect the ‘in vivo’ situation, as these tetramer-sorted T cells have not been expanded in vitro. Three of three T-cell lines secreted IL-4, and one of these T-cell lines also secreted IFN-γ to the nominal target epitope used for T-cell sorting. As such tetramer-sorted T cells from PBL probably do not represent a clonal population, it is not surprising that such oligo- or polyclonal T-cell populations are able to respond either with IFN-γ or IL-4 associated with the (Tc1/Tc2) composition of this polyclonal T-cell line.
The implementation of various amounts of peptide in this assay system is particularly important (Fig. 2), as increasing amounts of the target antigen may lead to differential intracellular signalling induced by serially engaged TCRs.34 This may alter the cytokine-secretion pattern. Similarly, HLA-DR haplotypes have been shown to impact on MHC class II peptide binding of mycobacterial peptides yielding either IFN-γ (high affinity) or IL-4 (low affinity) CD4+ T-cell responses.35 In addition, the source and ‘history’ of the T cells may be quite different. The latter study evaluated T cells obtained from patients who had been exposed to species of Mycobacterium.12 In contrast, the T-cell lines obtained in our study were derived from individuals who had not been exposed to M. tuberculosis or to BCG. Alternatively, the source of priming (immature DCs) may also play a role in shaping the activation of T-cell lines with a Tc2 cytokine-secretion pattern.36 In addition, VLTDGNPPEV-specific T cells have been shown to be cytotoxic.13 The lack of cytotoxic potential of T-cell clones in our report may also be a result of the source of priming antigen-presenting cells (APCs) and the implementation of IL-7 during expansion of effector T cells.
Conceptually, Tc2-type responses may not necessarily be detrimental in the course of M. tuberculosis infection. During the first period of infection with M. tuberculosis, the key cytokines associated with protection are IFN-γ and tumour necrosis factor-α (TNF-α).37,38 However, if high levels of TNF-α are maintained, they may also contribute to immunopathology. Thus, after clearance of infection, or successful containment of M. tuberculosis-infected macrophages, harmful immune responses must be eliminated. This may be mediated by anti-inflammatory cytokines including IL-10, IL-13, or in general, by a Tc2-dominated environment. How does this Tc1/Tc2 ‘switch’ occur? It could be antigen specific.39,40 Alternatively, it may be dependent on the processing and presentation of a certain set of antigen(s) expressed during the early or late phases of infection,41 which may favour the development of either Tc1 or Tc2 responses. One of these target antigens may be represented by the 19-kDa antigen, as the vast majority of the molecularly defined T-cell lines secrete GM-CSF alone or together with IL-4, but not IFN-γ, in response to the HLA-A2-presented target peptide. Thus, the nature of the APC, the quality of cytokines elicited in APCs by the 19-kDa lipoprotein42 and the availability of M. tuberculosis-associated antigens, may be instrumental in the fine tuning of the quality of the cellular immune response.
Permutation of individual aa residues in tumour-associated or viral peptides may alter the magnitude and the quality of the cellular immune response, as defined by cytokine secretion.43 This approach aims to create ‘partial agonistic’ or ‘superagonistic’ peptides. Some of these mutant ‘superagonistic peptides’ have already been successfully implemented in a clinical ‘treatment’ setting.43 Mutation(s) within the M. tuberculosis 19-kDa epitope may also be beneficial in order to drive a qualitatively different cellular immune response directed against M. tuberculosis-infected cells. This approach may be particularly useful in the rational design of novel vaccine strategies, as the immune pathogenesis of tuberculosis may be associated with an insufficient or unbalanced delivery of IFN-γ in granuloma lesions.44 In addition, the detrimental effects of a 19-kDa-based vaccine in an animal model45 support the notion that the quality of a cellular immune response is crucial for protection and clinical outcome of the infection with M. tuberculosis, as T cells obtained from mice immunized with 19-kDa antigen-pulsed DCs did not exhibit detectable antigen-specific IFN-γ responses.46
Acknowledgments
This work was supported by a grant to M.M. (SFB 490, C4), and contains sections of the doctoral thesis of K.N.
Abbreviations
- APC
antigen-presenting cell
- CDR3
complementarity determining region 3
- DC
dendritic cell; IL, interleukin
- mAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cells
- Tc1
T-cell cytotoxic type 1 response
- Tc2
T-cell cytotoxic type 2 response
- TCR
T-cell receptor
- VA
variable alpha chain
- VB
variable beta chain.
References
- 1.Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 2.Geluk A, Taneja V, van Meijgaarden KE, et al. Identification of HLA class II-restricted determinants of Mycobacterium tuberculosis-derived proteins by using HLA-transgenic, class II-deficient mice. Proc Natl Acad Sci USA. 1998;95:10797–802. doi: 10.1073/pnas.95.18.10797. 10.1073/pnas.95.18.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tascon RE, Colston MJ, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–92. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 4.Huygen K, Content J, Denis O, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–8. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 5.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med. 1998;187:1633–40. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165:925–30. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- 7.Lozes E, Huygen K, Content J, et al. Immunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complex. Vaccine. 1997;15:830–3. doi: 10.1016/s0264-410x(96)00274-5. 10.1016/s0264-410x(96)00274-5. [DOI] [PubMed] [Google Scholar]
- 8.Geluk A, van Meijgaarden KE, Franken KL, Drijfhout JW, D'Souza S, Necker A, Huygen K, Ottenhoff TH. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8(+) T cells in HLA-transgenic mice and humans. J Immunol. 2000;165:6463–71. doi: 10.4049/jimmunol.165.11.6463. [DOI] [PubMed] [Google Scholar]
- 9.Smith SM, Malin AS, Pauline T, Lukey Atkinson SE, Content J, Huygen K, Dockrell HM. Characterization of human Mycobacterium bovis bacille Calmette–Guerin-reactive CD8+ T cells. Infect Immun. 1999;67:5223–30. doi: 10.1128/iai.67.10.5223-5230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SM, Brookes R, Klein MR, et al. Human CD8(+) CTL specific for the mycobacterial major secreted antigen 85A. J Immunol. 2000;165:7088–95. doi: 10.4049/jimmunol.165.12.7088. [DOI] [PubMed] [Google Scholar]
- 11.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1998;95:270–5. doi: 10.1073/pnas.95.1.270. 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewinsohn DM, Zhu L, Madison VJ, Dillon DC, Fling SP, Reed SG, Grabstein KH, Alderson MR. Classically restricted human CD8(+) T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J Immunol. 2001;166:439–46. doi: 10.4049/jimmunol.166.1.439. [DOI] [PubMed] [Google Scholar]
- 13.Mohagheghpour N, Gammon D, Kawamura LM, van Vollenhoven A, Benike CJ, Engleman EG. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J Immunol. 1998;161:2400–6. [PubMed] [Google Scholar]
- 14.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med. 1999;189:1973–80. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashbridge KR, Backstrom BT, Liu HX, Vikerfors T, Englebretsen DR, Harding DR, Watson JD. Mapping of T helper cell epitopes by using peptides spanning the 19-kDa protein of Mycobacterium tuberculosis. Evidence for unique and shared epitopes in the stimulation of antibody and delayed-type hypersensitivity responses. J Immunol. 1992;148:2248–55. [PubMed] [Google Scholar]
- 17.Bousso P, Levraud JP, Kourilsky P, Abastado JP. The composition of a primary T cell response is largely determined by the timing of recruitment of individual T cell clones. J Exp Med. 1999;189:1591–600. doi: 10.1084/jem.189.10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maryanski JL, Jongeneel CV, Bucher P, Casanova JL, Walker PR. Single-cell PCR analysis of TCR repertoires selected by antigen in vivo: a high magnitude CD8 response is comprised of very few clones. Immunity. 1996;4:47–55. doi: 10.1016/s1074-7613(00)80297-6. [DOI] [PubMed] [Google Scholar]
- 19.Gorochov G, Neumann AU, Kereveur A, et al. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat Med. 1998;4:215–21. doi: 10.1038/nm0298-215. [DOI] [PubMed] [Google Scholar]
- 20.Bodinier M, Peyrat MA, Tournay C, Davodeau F, Romagne F, Bonneville M, Lang F. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat Med. 2000;6:707–10. doi: 10.1038/76292. [DOI] [PubMed] [Google Scholar]
- 21.Hohn H, Reichert T, Neukirch C, Pilch H, Maeurer MJ. Monoclonal TCR mRNA transcripts are preferentially detected in the TCR variable alpha chain in CD8(+) T lymphocytes: implications for immunomonitoring. Int J Mol Med. 1999;3:139–44. doi: 10.3892/ijmm.3.2.139. [DOI] [PubMed] [Google Scholar]
- 22.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–81. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 23.Jager E, Maeurer M, Hohn H, et al. Clonal expansion of Melan A-specific cytotoxic T lymphocytes in a melanoma patient responding to continued immunization with melanoma-associated peptides. Int J Cancer. 2000;86:538–47. doi: 10.1002/(sici)1097-0215(20000515)86:4<538::aid-ijc16>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Padovan E, Giachino C, Cella M, Valitutti S, Acuto O, Lanzavecchia A. Normal T lymphocytes can express two different T cell receptor beta chains: implications for the mechanism of allelic exclusion. J Exp Med. 1995;181:1587–91. doi: 10.1084/jem.181.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busch DH, Pilip I, Pamer EG. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J Exp Med. 1998;188:61–70. doi: 10.1084/jem.188.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moss PA, Moots RJ, Rosenberg WM, Rowland-Jones SJ, Bodmer HC, McMichael AJ, Bell JI. Extensive conservation of alpha and beta chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci USA. 1991;88:8987–90. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sourdive DJ, Murali-Krishna K, Altman JD, et al. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JD, Ogg GS, Allen RL, et al. Oligoclonal expansions of CD8(+) T cells in chronic HIV infection are antigen specific. J Exp Med. 1998;188:785–90. doi: 10.1084/jem.188.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Argaet VP, Schmidt CW, Burrows SR, et al. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein–Barr virus. J Exp Med. 1994;180:2335–40. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esin S, Batoni G, Saruhan-Direskeneli G, et al. In vitro expansion of T-cell-receptor Valpha2.3(+) CD4(+) T lymphocytes in HLA-DR17(3), DQ2(+) individuals upon stimulation with Mycobacterium tuberculosis. Infect Immun. 1999;67:3800–9. doi: 10.1128/iai.67.8.3800-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baldwin SL, D'Souza C, Roberts AD, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–9. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13:665–75. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 35.Agrewala JN, Wilkinson RJ. Influence of HLA-DR on the phenotype of CD4+ T lymphocytes specific for an epitope of the 16-kDa alpha-crystallin antigen of Mycobacterium tuberculosis. Eur J Immunol. 1999;29:1753–61. doi: 10.1002/(SICI)1521-4141(199906)29:06<1753::AID-IMMU1753>3.0.CO;2-B. 10.1002/(sici)1521-4141(199906)29:06<1753::aid-immu1753>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 36.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–16. doi: 10.1038/79758. 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 37.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Pando R, Rook GA. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–5. [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990;145:149–54. [PubMed] [Google Scholar]
- 40.Barnes PF, Chatterjee D, Abrams JS, Lu S, Wang E, Yamamura M, Brennan PJ, Modlin RL. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–7. [PubMed] [Google Scholar]
- 41.Skeiky YA, Ovendale PJ, Jen S, et al. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J Immunol. 2000;165:7140–9. doi: 10.4049/jimmunol.165.12.7140. [DOI] [PubMed] [Google Scholar]
- 42.Post FA, Manca C, Neyrolles O, Ryffel B, Young DB, Kaplan G. Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits Mycobacterium smegmatis-induced cytokine production by human macrophages in vitro. Infect Immun. 2001;69:1433–9. doi: 10.1128/IAI.69.3.1433-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson LB, Waldner H, Carrizosa AM, Sette A, Collins M, Kuchroo VK. Heteroclitic proliferative responses and changes in cytokine profile induced by altered peptides: implications for autoimmunity. Proc Natl Acad Sci USA. 1998;95:264–9. doi: 10.1073/pnas.95.1.264. 10.1073/pnas.95.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orme IM. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–7. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 45.Yeremeev VV, Lyadova IV, Nikonenko BV, Apt AS, Abou-Zeid C, Inwald J, Young DB. The 19-kDa antigen and protective immunity in a murine model of tuberculosis. Clin Exp Immunol. 2000;120:274–9. doi: 10.1046/j.1365-2249.2000.01212.x. 10.1046/j.1365-2249.2000.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baird MA, Hart DN, Abernethy N, Watson JD. Dendritic cell presentation of PPD and 19 kDa protein of Mycobacterium tuberculosis and emergent T helper cell phenotype. Immunol Cell Biol. 1995;73:537–43. doi: 10.1038/icb.1995.84. [DOI] [PubMed] [Google Scholar]
- 47.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–18. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]