Abstract
Mouse allograft inflammatory factor-1 (AIF-1) cDNA was cloned and the AIF-1-specific monoclonal antibodies were established to examine its tissue distribution. The mouse AIF-1 was highly conserved among all reported AIF-1 from a variety of species, from invertebrates to mammals, and the cloned cDNA was in good accordance with putative expressed regions of genomic sequences in the mouse major histocompatibility complex (MHC) class III region. The messages of mouse AIF-1 were abundantly expressed in the testis, moderately in the spleen and lymph nodes and slightly in the liver and thymus of normal BALB/c mice. Immunohistological examination revealed that differentiating germ cells in the testis and presumably macrophages in the red pulp of the spleen were positive for AIF-1. To analyse the function of the AIF-1, a macrophage cell line, RAW 264.7, was transfected with mouse AIF-1 cDNA. Upon stimulation with bacterial lipopolysaccharide, the transfectants that overexpressed AIF-1 showed marked morphological changes and produced significantly large amounts of interleukin (IL)-6, IL-10 and IL-12p40 but not IL-12p70 compared with control cells. No difference was noted in production of tumour necrosis factor-α, transforming growth factor-β1 and IL-1α. These results suggest that AIF-1 plays an important role in cells of a monocyte/macrophage lineage upon stimulation with inflammatory stimuli by augmenting particular cytokine production.
Introduction
Allograft inflammatory factor-1 (AIF-1) was originally identified as a gene product expressed in infiltrating macrophages in heterotopic cardiac allografts of a rat model.1,2 The expression of AIF-1 was mostly limited to cells of a monocyte/macrophage lineage, and augmented by interferon-γ (IFN-γ). Thus, it was assumed that AIF-1 was a novel molecule involved in allogeneic responses. However, it has been reported that AIF-1 is also expressed in macrophages and microglial cells in experimental autoimmune encephalomyelitis, neuritis and uveitis models,3,4 in devascularized skeletal muscles in rat systems5 and in cerebral infarctions in humans.6 Vascular smooth muscle cells also expressed AIF-1 when injured with balloon angioplasty.7,8 Thus, it seems that AIF-1 plays a pivotal role not only in immune responses to alloantigens but also in various host responses to inflammatory stimuli. However, little is known concerning exact cell type(s) expressing AIF-1 and the functions of AIF-1 in these lesions.
To date, AIF-1 or AIF-1-like genes have been cloned from a wide range of organisms, such as invertebrates (sponge9) and vertebrates (bony fish – carp10 and bream; mammals – rat,1 human11 and pig12). The similarity of AIF-1 sequences among the sponge and vertebrates reaches 70%,9 which suggests that AIF-1 is an essential molecular component functioning across species barriers. Indeed, it was shown in two species of sponges, Suberites domuncula and Geodia cydonium, that AIF-1 was induced in allografts but not in autografts.9 Thus, it seems that AIF-1 functions even in allogeneic responses of the lowest metazoan phylum. However, how AIF-1 functions in the rejection process among these sponges remains to be elucidated.
Interestingly, AIF-1 is expressed in the rat testis under normal conditions.1 Since a set of genes expressed in the cells of a monocyte/macrophage lineage in various lesions appears to be quite different from those in germ cells of the testis, the induced expressions of AIF-1 proteins in the mononuclear cells and the physiological expressions of AIF-1 in the germ cells may reflect the different biological functions.
In the present study, we first cloned mouse AIF-1 cDNA and established monoclonal antibodies (mAb) against recombinant mouse AIF-1 proteins to study AIF-1 functions in mouse models, which have advantages to investigate further the role of AIF-1 under various genetic backgrounds. We then analysed the effects of the AIF-1 overexpression on certain functions of a mouse macrophage cell line, RAW 264.7, upon stimulation with bacterial lipopolysaccharide (LPS). Possible pathophysiological roles of AIF-1 in macrophages are discussed.
Materials and methods
Mice
BALB/c mice were purchased from SLC Japan (Hamamatsu, Japan). All experiments were performed on 12-week-old male mice. Mice were maintained under specific pathogen-free conditions. The animal care and experimental procedures conformed to the regulations of Hokkaido University Animal Care and Use Committee.
Cell culture
A mouse myeloma cell line P3X63Ag8U.1 and a mouse macrophage cell line RAW 264.7 were obtained from the American Type Culture Collection (Rockville, MD) and cultured in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 µg/ml streptomycin and 50 µm 2-mercaptoethanol.
Cloning and sequencing of the mouse AIF-1 cDNA
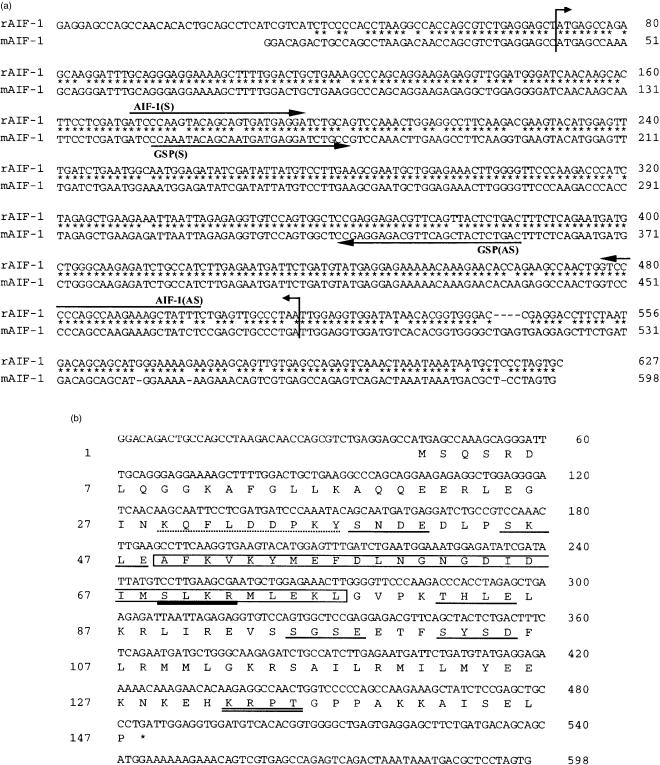
Total RNA was isolated from the spleens of BALB/c mice as reported.13 Ten micrograms of RNA was reverse-transcribed using random hexamers (Takara Shuzo, Ohtsu, Japan) and Moloney murine leukaemia virus reverse-transcriptase (Super-Script II; Life Technologies, Rockville, MD). One-twentieth of reverse transcript was used directly as a template for the amplification reaction. Primers were designed from the rat AIF-1 cDNA sequence1 (sense primer: 5′-ATCCCAAGTACAGCAGTGATGAGGA-3′; antisense primer: 5′-AAATAGCTTTCTTGGCTGGGGGAC-3′) to amplify mouse AIF-1 cDNA. Conditions for polymerase chain reaction (PCR) were as follows. Reaction mixture (50 µl) contained 200 nm of each primer, 0·4 mm dNTP, 20 mm Tris–HCl (pH 8·4), 50 mm KCl, 2·5 mm MgCl2 and 2 units Taq DNA polymerase (Life Technologies). PCR amplification was performed with 30 cycles at 94° for 1 min, at 58° for 1 min and at 72° for 1 min, and a final extension step at 72° for 10 min. The PCR product was directly subcloned into a pCR2 vector (Invitrogen, Carlsbad, CA) and sequenced. Based on this sequence, 5′- and 3′-rapid amplification of cDNA ends (RACE) were performed using a mouse spleen cDNA library (Marathon-Ready cDNA; Clontech, Palo Alto, CA) according to the manufacturer's instruction. The 5′- and 3′-ends were amplified with AIF-1-specific primers described in Fig. 1(a) and were sequenced to obtain the complete sequence of the mouse AIF-1 cDNA. Sequencing analysis was performed using a DNA sequencing system with dye-terminator cycle sequencing kit (PE Applied Biosystems, Foster City, CA) with an ABI Prism 373A sequencer (PE Applied Biosystems). Sequence alignment and database searches were performed with Genetyx-Mac Version 10.1 (Software Development Co., Ltd, Tokyo, Japan).
Figure 1.
Complementary DNA and amino acid sequences of mouse AIF-1. (a) Alignment of the nucleotide sequences of rat and mouse AIF-1 cDNA. The upper row indicates the rat AIF-1 cDNA (accession number U17919) and the lower row indicates the mouse AIF-1 cDNA (AB013745). A region between two arrows represents the coding sequence. The locations of a primer pair deduced from the rat sequence to amplify mouse AIF-1 are indicated as AIF-1 (S; sense) and AIF-1 (AS; antisense). Gene-specific primers (GSP) used for 5′- and 3′-RACE are indicated as GSP (AS) and GSP (S), respectively. Numbers on the right indicate the position of nucleotide. (b) Nucleotide and deduced amino acid sequences of mouse AIF-1 cDNA. Numbers on the right indicate the position of nucleotide and numbers on the left indicate the position of amino acid. Each bar represents putative amino acid motifs: (single line) casein kinase II phosphorylation site; (broken line) tyrosine kinase phosphorylation site; (double line) protein kinase A and (bold line) protein kinase C phosphorylation site. The EF hand-like motif is indicated by an open box.
Northern blot
Total RNA was isolated from various tissues of BALB/c mice. Twenty micrograms of total RNA was electrophoresed through formaldehyde/agarose gel and transferred to a nitrocellulose membrane (Hybond N; Amersham Pharmacia, Little Chalfont, UK) by capillary transfer. A probe for AIF-1 was generated by labelling the PCR product [a segment of cDNA ≈440 base pairs (bp) long was used] with [32P]dCTP (Megaprime DNA labelling systems; Amersham Pharmacia) according to the manufacturer's instructions and the hybridization was performed at 42° overnight. The blot was washed with 1× sodium saline citrate (SSC), 0·1% sodium dodecyl sulphate (SDS) and 0·5× SSC, 0·1% SDS, then exposed to autoradiography films (Hyperfilm MP; Amersham Pharmacia).
Production of mAb
Mouse AIF-1 cDNA was inserted into a pTYB2 expression vector (New England Biolabs, Inc., Beverly, MA) to synthesize a fusion protein with a chitin-binding domain and an intein, a protein-splicing element. The fusion protein was produced in Escherichia coli strain ER2566 induced with isopropyl-β-d-thiogalactopyranoside (IPTG) and the crude extract of E. coli was affinity-purified on a chitin column. The recombinant AIF-1 was obtained by cleaving fusion protein with dithiothreitol and dialysed against phosphate-buffered saline (PBS). The concentration of the protein was measured by the method of Bradford assay with bovine serum albumin as a standard (Bio-Rad, Hercules, CA). Hybridomas were established according to a method by Kishiro et al.14 In brief, a 10-week-old female WKY/NCrj rat was immunized with an emulsion of 150 µg of recombinant AIF-1 and complete adjuvant H37 Ra (Difco, Detroit, MI). After 2 weeks, iliac lymph node (LN) cells were fused with P3X63Ag8U.1 myeloma cell line using polyethylene glycol (MW 1500; Roche Diagnostics, Mannheim, Germany) and selected with hypoxanthine aminopterin thymidine (HAT) medium (Roche Diagnostics). The culture supernatants were screened primarily by enzyme-linked immunosorbent assay (ELISA) against recombinant AIF-1 proteins. Then each ELISA-positive culture supernatant was screened by immunoblot and immunohistology.
Immunohistology
Each tissue from BALB/c mice was snap-frozen in Tissue Tek® O.C.T. (optimal cutting temperature) compound (Sakura, Tokyo, Japan) and sectioned at 5 µm as described elsewhere.15 Sections were fixed in acetone for 10 min and the endogenous peroxidase activity was blocked with PBS containing 0·1% NaN3 and 0·3% hydrogen peroxide. Culture supernatants of hybridoma containing anti-AIF-1 mAb were directly used as a primary antibody. The primary antibody was detected with a peroxidase-conjugated rabbit anti-rat immunoglobulin (Dako Japan, Kyoto, Japan) followed by development with 3,3′-diaminobenzidine substrate. Haematoxylin was used for counter staining.
Transfection
Mouse AIF-1 cDNA was subcloned into a pRc/CMV plasmid vector (Invitrogen) at a BstXI site. Transfection of the vectors containing mouse AIF-1 cDNA or vector alone into RAW 264.7 cells was performed with Lipofectamine (Life Technologies), according to the manufacturer's protocol. Stable transfectants were established by selection of G418 (Life Technologies) -resistant colonies and each clone was examined for expression level of AIF-1 by immunoblot analysis.16
Immunoblot analysis
Cells (1 × 106) were lysed with buffer containing 1% Nonidet-P40, 10 mm Tris–HCl (pH 7·6), 100 mm NaCl, 1 mm ethylenediaminetetraacetic acid and 1 mm orthovanadate supplemented with protease inhibitor mixture (Complete™; Roche Diagnostics). The lysates were centrifuged at 17 000 g for 10 min, followed by separation on a 15% SDS–polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Membranes were blocked in 5% non-fat dry milk in PBS and then incubated with an anti-AIF-1 mAb. After washing, primary antibody was detected by peroxidase-conjugated rabbit anti-rat immunoglobulin antibody and visualized with a chemiluminescence detection kit (ECL™; Amersham). Three clones which overexpressed AIF-1 were chosen and light microscopy pictures of wild-type, vector control and these three AIF-1 transfectant cells were taken after these cells were cultured with either medium alone or stimulated with a 1-µg/ml LPS from E. coli 055:B5 (Difco) at 37° for 24 hr. The dilution of LPS was determined by preliminary experiments where it was shown that 1 µg/ml LPS was optimal for morphological changes and cytokine productions as described below.
Cytokine assays
Cells were cultured in medium with or without 1 µg/ml LPS at 37° for 24 hr. Total RNA was isolated from each cell and preparation for reverse transcription (RT) -PCR was carried out as mentioned above. Primers for tumour necrosis factor-α (TNF-α), TNF-β, transforming growth factor-β1 (TGF-β1), Mac-1, interleukin-1α (IL-1α), IL-6, IL-10 and IL-12p40 were described previously.17–19 Primers used for analysis of tissue factor were 5′-GGCAACCCAAACCCACCAA-3′ (sense) and 5′-TGTAAATGGCGGCTCCTCC-3′ (antisense). β-actin was used as an internal reference (sense: 5′-ATCCCAAGTACAGCAGTGATGAGGA-3′; antisense: 5′-AAATAGCTTTCTTGGCTGGGGGAC-3′). PCR amplification was performed with 30 cycles at 94° for 1 min, at 55° for 1 min and at 72° for 1 min, and a final extension step at 72° for 10 min.
The concentrations of IL-6, -10, -12p40 and -12p70 in culture supernatants were determined using a mouse IL-6, -10, -12p40 or -12p70 ELISA kit (BioSource International, Inc., Camarillo, CA), respectively, according to the manufacturer's instructions. Each cytokine level was expressed as mean ± SEM and analysed by one-way analysis of variance (anova). Multiple comparisons between each of AIF-1 transfectant and vector control were performed with Bonferroni's method. A P-value less than 0·05 was considered statistically significant.
Results
Cloning of mouse AIF-1
It was assumed that the mouse coding sequence for AIF-1 showed a high degree of homology to that of rat, since homology between the rat AIF-1 and the human AIF-1 is 87·4% at the nucleotide level.1,11 Thus, we utilized a primer pair based on the rat cDNA sequence to amplify mouse AIF-1 cDNA (Fig. 1a). A pair of primers amplified a putative coding sequence of the mouse AIF-1 (330 bp long). The complete cDNA sequence was then determined with 5′- and 3′-RACE methods based on this partial cDNA sequence (Fig. 1a). The full-length of mouse AIF-1 cDNA was 598 bp long, containing a coding sequence of 444 bp. The deduced polypeptide was composed of 147 amino acids (Fig. 1b) and the calculated molecular mass was 16 910. These sequence data are incorporated into the EMBL/Genbank/DDBJ database under accession number AB013745. The nucleotide and amino acid sequences were 92·1% and 93·9% identical, respectively, between rat and mouse. As revealed in the rat AIF-1 protein, the mouse AIF-1 possessed a single EF hand-like motif in residue 49–77, a characteristic structure conserved among the Ca2+-binding protein family.20,21 Motif analysis of the amino acid sequence also revealed potential phosphorylation sites for protein kinase A (132–135), protein kinase C (69–72), casein kinase II (38–41, 45–48, 82–85, 95–98, 102–105), and tyrosine kinases (29–37) as depicted in Fig. 1(b). Mouse AIF-1 cDNA also revealed complete alignment with genomic sequences in the mouse major histocompatibility complex (MHC) class III region as indicated in human AIF-1.11
Establishment of anti-AIF-1 mAb
Mouse cDNA for AIF-1 was subcloned into an expression vector to generate the fusion protein and the recombinant AIF-1 protein was obtained by cleaving fusion protein. Recombinant AIF-1 protein was used as an immunogen to establish mAb against mouse AIF-1 proteins as described in the Materials and Methods. Several mAb were established for immunoblot analysis or immunohistology and these isotypes were determined. Two representative clones [#19-6 for immunohistology: rat immunoglobulin M (IgM); #18-A for immunoblot analysis: rat IgG2a] were employed for further experiments.
Tissue distribution of AIF-1
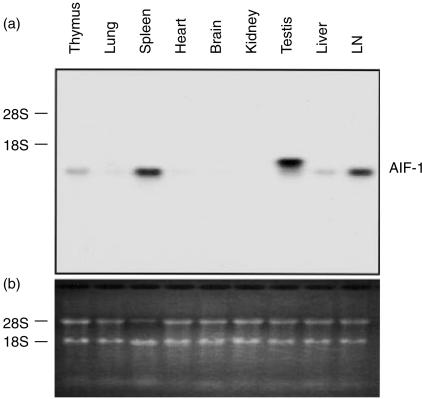
To evaluate the relative transcription level of AIF-1 in various tissues, Northern blot analysis was performed using various organs from BALB/c mice. As shown in Fig. 2(a), a considerable AIF-1 expression was detected in the testis, whereas moderate to slight expressions were observed in the spleen, LN, liver and thymus under normal conditions. Faint expressions were also observed in the lung, heart and brain. In the resident peritoneal cells (macrophages), only a little expression was detected with RT-PCR analysis (data not shown). In the testis, the molecular size of AIF-1 mRNA was larger than those in other organs.
Figure 2.
Expression of AIF-1 mRNA in various mouse tissues. (a) Northern blot analysis of mouse tissues. Total RNA (20 µg) from each tissue was electrophoresed on an agarose/formaldehyde gel, transferred to a nitrocellulose membrane and hybridized with a specific probe as described. A considerable AIF-1 expression was detected in the testis. Expressions were moderate in the spleen and LN and slight in the liver and thymus. (b) RNA gel stained with ethidium bromide.
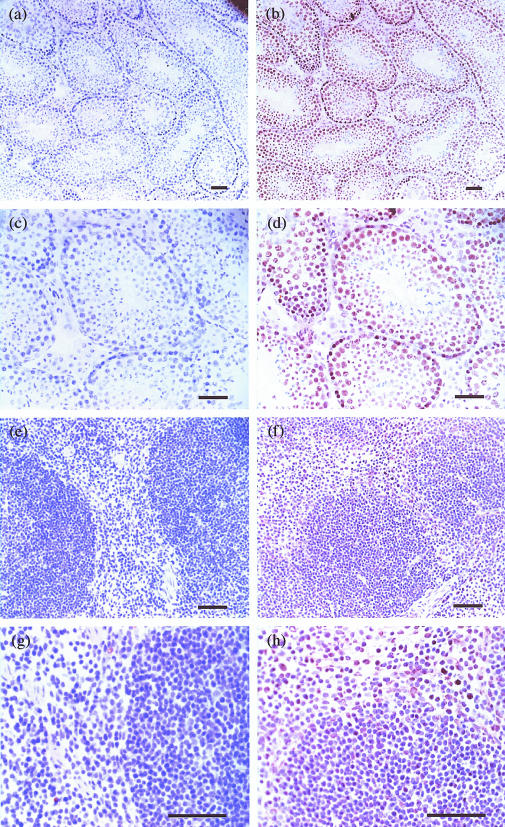
To examine the cell populations that express AIF-1 at protein level, immunohistological analyses were performed with the testis and spleen where AIF-1 mRNA were abundantly detected. In the testis, strong expression of AIF-1 was shown in differentiating germ cells, and the AIF-1 expression disappeared when these germ cells matured into spermatozoa (Fig. 3b,d). In the spleen, mononuclear cells, presumably macrophages in the red pulp, were positive for AIF-1 (Fig. 3f,h). However, no significant signals were detected in marginal metallophilic macrophages and marginal zone macrophages. These results were consistent with the previous report on rat AIF-1.1 Staining without the primary mAb exhibited no specific positive signal (Fig. 3a,c,e,g).
Figure 3.
Immunohistology of the testis and spleen for AIF-1 expression. Sections of the testis (b, d) and spleen (f, h) from naive BALB/c mice were stained with anti-AIF-1 mAb. Sections of the testis (a, c) and spleen (e, g) were stained without the primary mAb (control). In the testis, differentiating germ cells are positive for AIF-1 antigen (b, d). Expression of AIF-1 is not detected in spermatozoa. In the spleen, a subset of mononuclear cells in the red pulp are positive for AIF-1 antigen (f, h). Original magnifications: a, b: ×100; c, d, e, f: ×200; g, h: ×400. Each bar represents 10 µm.
Establishment of AIF-1 transfected cells
To analyse the functions of AIF-1, RAW 264.7 cells, a mouse cell line of monocyte/macrophage lineage that expresses a very low level of AIF-1 message in unstimulated conditions (data not shown), were transfected with expression vectors, pRc/cytomegalovirus (CMV) alone (mock transfectants), or pRc/CMV-mAIF-1 (AIF-1 transfectants). The stable transfectants with or without AIF-1 insert were established and analysed for the level of AIF-1 expression with immunoblot (Fig. 4a). Distinct bands of 17 000 MW were detected with an anti-AIF-1 mAb, #18-A, in lysates of AIF-1 transfectants (#182 < #24 = #203), whereas trace amounts of AIF-1 bands were detectable in wild-type RAW 264.7 cells and mock transfectant cells. Then, these cells were cultured in the presence or absence of 1 µg/ml LPS. No increases in the transcription level of AIF-1 were observed in these wild-type and transfectant cells after LPS-stimulation (data not shown). However, it should be noted in Fig. 4(b) that AIF-transfectants were adhesive to a plastic dish and exhibited a dendritic shape compared to mock or wild-type cells, especially when these cells were stimulated with LPS.
Figure 4.
Immunoblot analysis and morphology of transfectants that overexpress AIF-1. (a) Relative expression levels of AIF-1 proteins in RAW 264.7 (wild-type: wt), mock transfectant (mock) and three AIF-1 transfectants (#182, #24, #203) were quantified by immunoblot. Distinct bands of 17 000 MW are detected in AIF-1 transfectants (#182 < #24 = #203), whereas trace amounts of AIF-1 bands are detectable in wt and mock cells. (b) Influence of AIF-1 overexpression on the cell morphology. Cells were cultured with either 1 µg/ml of LPS (right panel) or medium alone (left panel) for 24 hr (× 50).
Enhanced cytokine production in AIF-1 transfectants
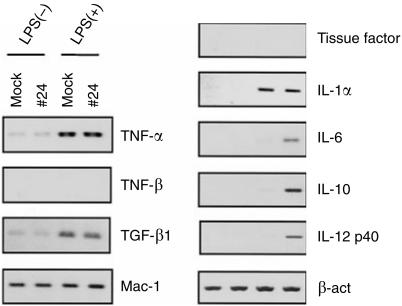
To examine the functional alterations of RAW 264.7 cells by overexpressing AIF-1, relative transcription levels of several cytokine genes were compared between an AIF-1 transfectant cell line, #24, and mock transfectants. Cells were cultured with either 1 µg/ml LPS or medium alone for 24 hr. Since the expression of AIF-1 is augmented by IFN-γ,1 LPS alone was used as a stimulant. In comparison with mock transfectants, transcription levels of IL-6, -10, and -12p40 were markedly enhanced in #24 cells upon stimulation with LPS (Fig. 5). On the other hand, no difference was noted in the transcription levels of TNF-α, TNF-β, TGF-β1, Mac-1, tissue factor and IL-1α between #24 and mock cells that had been either stimulated with LPS or unstimulated. Similar observations were obtained with other transfectants, #182 and #203, although expression levels of the cytokine genes showed a variation (data not shown).
Figure 5.
Influence of AIF-1 overexpression on transcription levels of various cytokines. A vector control (mock) and an AIF-1 transfectant (#24) were cultured with or without 1 µg/ml of LPS and total RNA was extracted after 24 hr. Transcription levels of cytokine genes typically expressed in macrophages were compared between mock and #24 cells by RT-PCR. Transcription of β-actin was examined as a control. Relative transcription levels of IL-6, -10 and -12p40 are augmented in #24 cells after LPS stimulation compared to mock cells.
To examine whether the enhanced expressions of the cytokine messages were reflected on the protein level, amounts of IL-6, -10, and -12p40 in the culture supernatants of LPS-stimulated AIF-1 transfectants and control cells were quantified. After stimulation with LPS, wild-type and mock transfectant cells produced no detectable levels of cytokines except that the mock transfectants produced substantial levels of IL-10 (Fig. 6). On the other hand, three lines of AIF-1 transfectants produced significantly large amounts of IL-6, -10, and -12p40 upon stimulation with LPS (Fig. 6a,b,c). Functional IL-12, IL-12p70, is a heterodimer that consists of two subunits of p40 and p35. It has been reported that p40 homodimer antagonizes the function of IL-12p70.22 Then, we quantified the production of IL-12p70 by AIF-1 transfectants and control cells. Figure 6(d) shows that no IL-12 p70 was detected in the culture of AIF-1 transfectants after LPS stimulation. This finding suggests that only p40 subunits were produced by these transfectants.
Figure 6.
Influence of AIF-1 overexpression on the production of cytokines. RAW 264.7 (wt), a vector control (mock) and three AIF-1 transfectants (#182, #24, #203) were cultured in triplicate with or without 1 µg/ml of LPS for 24 hr. Concentrations of (a) IL-6, (b) IL-10, (c) IL-12p40 and (d) IL-12p70 in the culture supernatants were quantified (mean ± SEM). *P < 0·0001 compared to mock transfectant; ND, not detected.
Discussion
AIF-1 was originally identified in rat cardiac allografts with chronic rejection.1,2 Studies of endomyocardial biopsy specimens from human heart transplants11 and allogeneic grafts in two marine sponge species9 also showed that the expression of AIF-1 was augmented in these allografts. These reports suggested that AIF-1 expression was induced in response to allogeneic antigens. However, the subsequent studies demonstrated that the expression of AIF-1 was increased in various host responses to inflammatory stimuli.3,5,8,12 Thus far, however, AIF-1 gene and product have not been identified in a mouse system. The mouse system has an advantage of availability of a number of genetically manipulated stocks.
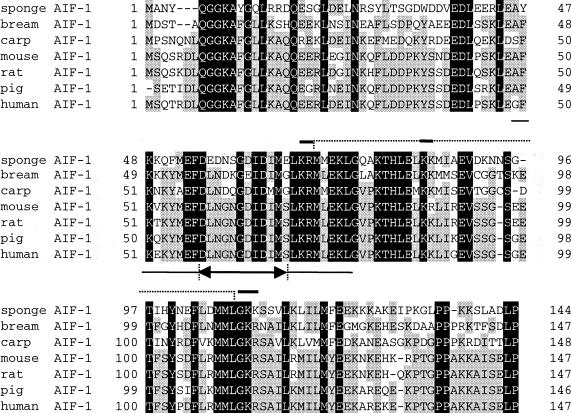
In the present study we first cloned a complete sequence of mouse AIF-1 cDNA. To date, AIF-1 and AIF-1-like genes have been cloned from a wide range of organisms and there have been several reports of functional implication for AIF-1.6,9,12 Figure 7 shows alignment of the deduced amino acid sequences of AIF-1 reported from different sources,1,9–12 including the present study. It will be seen in this figure that the sequences of AIF-1 are highly conserved across the species barrier, from the lowest metazoan phylum to humans. Thus, it seems that AIF-1 is an essential molecule for the subsistence of these species. Although the sequence homology is evident around the EF hand-like motif, AIF-1 possesses only a single repeat of the motif which contains amino acid substitutions in several residues. These findings suggest that AIF-1 may not function as a Ca2+-binding protein.20,21 In humans, two splice variants of AIF-1 have been reported and both of these gene products possess no, or incomplete, EF hand-like motif.23 It is unknown whether these two variants possess differential functions compared to full-length AIF-1. In a mouse system signals of splice variants have not been detected by RT-PCR (data not shown). Detailed examination, however, is required to reach the final conclusion.
Figure 7.
Alignment of deduced amino acid sequences of mouse AIF-1 and AIF-1 related genes. Sponge AIF-1 (accession number Y18439), bream AIF-1 (AB019540), carp AIF-1 (AB012309), mouse AIF-1, rat AIF-1, pig AIF-1 (P81076) and human AIF-1 (U19713) are presented and compared. Amino acid residues conserved among all species are shadowed in black; those present in at least four species are shaded in grey. The EF hand-like motif is underlined and the region between two arrows represents the putative Ca2+-binding loop. The putative post-translational processing motif of peptide hormones is indicated above by a broken line and the recognition sites for pro-hormone convertases and amidation enzymes are emphasized with a bold line.
Another amino acid motif common to almost all AIF-1 is the -KR-KK-GKR- pattern, a characteristic sequence for peptide hormone precursors,12 which is a putative cleavage site for prohormone convertase and peptide amidation enzymes.24 Chen et al.12 reported that AIF-1 influenced glucose-stimulated insulin secretion when injected intravenously in rat. It has been shown that recombinant AIF-1 proteins abrogate proliferation and differentiation of cultured satellite cells of skeletal muscles.5 These findings suggest that AIF-1 proteins are secreted to extracellular compartments. On the other hand, human vascular smooth muscle cells which had been transfected AIF-1 cDNA showed enhanced proliferation. It remains to be elucidated whether AIF-1 has a secretory form and whether receptors for AIF-1 are present and expressed on the cell surface.
Thus far, several genes have been identified as homologues of AIF-1. It was postulated that microglial response factor-1 (MRF-1) which was cloned from microglial cells played certain roles in the apoptosis of neurons.25 Ionized calcium-binding adapter molecule-1 (Iba-1) was also identified in microglias.26,27 Expression of these molecules, MRF-1 and Iba-1, was up-regulated in microglias surrounding the injured motoneuron nucleus after the motor nerve axotomy.25,27 Thus, it was suggested that AIF-1, a homologue of MRF-1 and Iba-1, exerted certain influences on apoptosis or protection of injured cells. It is noteworthy that AIF-1 is most abundant in testis where apoptosis and proliferation of cells constantly take place.28,29
In the present study, immunohistological analysis revealed that AIF-1-positive cells were observed among mononuclear cells residing in the red pulp of the spleen, as well as in differentiating germ cells in the testis. These results were consistent with a previous report on rat AIF-1.1 Substantial levels of AIF-1 messages were also detected in the LN, liver and thymus by Northern blot analysis. Chen et al.12 reported that macrophages, Kupffer cells and dendritic cells in these tissues expressed AIF-1.
We found that the molecular size of AIF-1 mRNA in the testis was different from those of the spleen, LN, liver and thymus. It seems that transcriptional regulation and biological function of AIF-1 are different between the immunocytes and germ cells. It should be noted that AIF-1 was stained in the nucleus as well as in the cytosol of germ cells in the testis. Although sponge AIF-1 possessed a putative classical basic-type nuclear localization signal,9,30 this sequence is partially altered in the AIF-1 of other species. It should be pursued in further studies how mouse AIF-1 proteins relocate from cytosol to nucleus.
Resident peritoneal cells, most of which are macrophages, expressed only a little amount of AIF-1 (data not shown). It seems that AIF-1 expression is confined to a subpopulation of macrophages in the spleen in a resting condition. Thus, precise histological analysis of AIF-1 expression in tissues other than the spleen and testis and examination of the conditions that induce AIF-1 expression should be carried out to determine the exact cell subpopulation(s) expressing AIF-1 and to pursue functional roles of AIF-1 in vivo.
To analyse functions of AIF-1 in cells of a monocyte/macrophage lineage, we generated transfectants that constitutively express AIF-1 proteins. The parental cell line RAW 264.7 expressed little AIF-1. Although no detrimental influences of AIF-1 overexpression on RAW 264.7 cells were noted, these AIF-1 transfectants showed significant morphological changes especially upon stimulation with LPS. In addition, we could show that the LPS-stimulated transfectants produced significantly larger amounts of IL-6, -10 and -12p40 than control cells. The productions of IL-10 and IL-12p40 showed a direct and an inverse correlation to the amount of AIF-1, respectively. No apparent correlation was observed between the IL-6 production and the amount of AIF-1. These cytokine productions are regulated by the cytokine network and the present finding may be related to the regulation of the cytokine network as well as to differential influence of AIF-1 on various cytokine productions. Indeed, when these AIF-1 transfectants were stimulated with LPS in the presence of anti-IL-10 mAb, production of IL-12p40 were augmented (data not shown). To elucidate the precise mechanism underlying the complex patterns of cytokine productions by AIF-1 transfectants, it is necessary to determine what cytokine production is directly and primarily regulated by AIF-1. This point should be pursued in further investigations.
Recently it has been reported that IL-10 and IL-12 regulate the functions of helper T cells.31 In the present study, AIF-1-transfected cells produced not only IL-10, but also the IL-12p40 subunit that functions as an antagonist for functional IL-12p70 after activation with LPS.22 These results suggest that AIF-1 functions in the development of Th2 type T cells. It seems necessary to determine whether the characteristic pattern of cytokine production observed in AIF-1 transfectant represents the immune or inflammatory responses in vivo and results in alteration of the Th1/Th2 balance. To address this issue studies employing transgenic mice that overexpress AIF-1 with a macrophage-specific promoter are being undertaken in our laboratories.
When immune responses to allogeneic antigens are induced in mammals, activation of T cells and the subsequent macrophage activation play a major role.32 However, allogeneic responses are also observed in organisms like the sponge, which lacks T cells. Common features in these different types of alloresponses seen in mammals and sponges appear to be the activation of cells of a monocyte/macrophage lineage. It was reported in the sponge system that grey cells infiltrating into the contact zone with the allograft were activated immunocytes.33,34 These grey cells appear to correspond to macrophages in mammals. Indeed upon stimulation with allogeneic antigens, enhanced AIF-1 expressions were observed both in the macrophages of mammals and in the boundary zone (perhaps grey cells) of sponges.1,9,11 It has been postulated that products of AIF-1-related genes activate immunocytes that are stimulated with alloantigens.34 The present finding that AIF-1 overexpression resulted in the enhanced cytokine production may be concordant with this postulate. Thus, when the transplantation of allograft is clinically taken into consideration, regulation of AIF-1 expression appears to be important for prolongation of the graft survival. It seems important to study how AIF-1 expression is regulated, not only for the better understanding of AIF-1 biology but also for development of a new treatment that improves engraftment of allogeneic transplants.
Abbreviations
- AIF-1
allograft inflammatory factor-1
- ELISA
enzyme-linked immunosorbent assay
- IL
interleukin
- LPS
lipopolysaccharide
- LN
lymph node(s)
- mAb
monoclonal antibody(ies)
- TGF
transforming growth factor
- TNF
tumour necrosis factor.
References
- 1.Utans U, Arceci RJ, Yamashita Y, Russell ME. Cloning and characterization of allograft inflammatory factor-1: a novel macrophage factor identified in rat cardiac allografts with chronic rejection. J Clin Invest. 1995;95:2954–62. doi: 10.1172/JCI118003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utans U, Liang P, Wyner LR, Karnovsky MJ, Russell ME. Chronic cardiac rejection: identification of five upregulated genes in transplanted hearts by differential mRNA display. Proc Natl Acad Sci USA. 1994;91:6463–7. doi: 10.1073/pnas.91.14.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schluesener HJ, Seid K, Kretzschmar J, Meyermann R. Allograft-inflammatory factor-1 in rat experimental autoimmune encephalomyelitis, neuritis, and uveitis: expression by activated macrophages and microglial cells. Glia. 1998;24:244–51. doi: 10.1002/(sici)1098-1136(199810)24:2<244::aid-glia9>3.0.co;2-3. 10.1002/(sici)1098-1136(199810)24:2<244::aid-glia9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Pashenkov M, Efendic S, Zhu J, Zou LP, Ostenson CG, Mustafa M. Augmented expression of daintain/AIF-1 is associated with clinical disease: dynamics of daintain/AIF-1 expression in spleen, peripheral nerves and sera during experimental autoimmune neuritis. Scand J Immunol. 2000;52:117–22. doi: 10.1046/j.1365-3083.2000.00682.x. 10.1046/j.1365-3083.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuschel R, Deininger MH, Meyermann R, Bornemann A, Yablonka-Reuveni Z, Schluesener HJ. Allograft inflammatory factor-1 is expressed by macrophages in injured skeletal muscle and abrogates proliferation and differentiation of satellite cells. J Neuropathol Exp Neurol. 2000;59:323–32. doi: 10.1093/jnen/59.4.323. [DOI] [PubMed] [Google Scholar]
- 6.Postler E, Rimner A, Beschorner R, Schluesener HJ, Meyermann R. Allograft-inflammatory-factor-1 is upregulated in microglial cells in human cerebral infarctions. J Neuroimmunol. 2000;104:85–91. doi: 10.1016/s0165-5728(99)00222-2. 10.1016/s0165-5728(99)00222-2. [DOI] [PubMed] [Google Scholar]
- 7.Autieri MV. cDNA cloning of human allograft inflammatory factor-1: tissue distribution, cytokine induction, and mRNA expression in injured rat carotid arteries. Biochem Biophys Res Commun. 1996;228:29–37. doi: 10.1006/bbrc.1996.1612. [DOI] [PubMed] [Google Scholar]
- 8.Autieri MV, Carbone C, Mu A. Expression of allograft inflammatory factor-1 is a marker of activated human vascular smooth muscle cells and arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1737–44. doi: 10.1161/01.atv.20.7.1737. [DOI] [PubMed] [Google Scholar]
- 9.Kruse M, Steffen R, Batel R, Muller IM, Muller WE. Differential expression of allograft inflammatory factor 1 and of glutathione peroxidase during auto- and allograft response in marine sponges. J Cell Sci. 1999;112:4305–13. doi: 10.1242/jcs.112.23.4305. [DOI] [PubMed] [Google Scholar]
- 10.Fujiki K, Shin DH, Nakao M, Yano T. Molecular cloning of carp (Cyprinus carpio) CC chemokine, CXC chemokine receptors, allograft inflammatory factor-1, and natural killer cell enhancing factor by use of suppression subtractive hybridization. Immunogenetics. 1999;49:909–14. doi: 10.1007/s002510050573. [DOI] [PubMed] [Google Scholar]
- 11.Utans U, Quist WC, McManus BM, Wilson JE, Arceci RJ, Wallace AF, Russell ME. Allograft inflammatory factor-1. A cytokine-responsive macrophage molecule expressed in transplanted human hearts. Transplantation. 1996;61:1387–92. doi: 10.1097/00007890-199605150-00018. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZW, Ahren B, Ostenson CG, et al. Identification, isolation, and characterization of daintain (allograft inflammatory factor 1), a macrophage polypeptide with effects on insulin secretion and abundantly present in the pancreas of prediabetic BB rats. Proc Natl Acad Sci USA. 1997;94:13879–84. doi: 10.1073/pnas.94.25.13879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Kishiro Y, Kagawa M, Naito I, Sado Y. A novel method of preparing rat-monoclonal antibody-producing hybridomas by using rat medial iliac lymph node cells. Cell Struct Funct. 1995;20:151–6. doi: 10.1247/csf.20.151. [DOI] [PubMed] [Google Scholar]
- 15.Ato M, Iwabuchi K, Matsuki N, et al. Delayed clearance of zymosan-induced granuloma and depressed phagocytosis of macrophages with concomitant up-regulated kinase activities of Src-family in a human monocyte chemoattractant protein-1 transgenic mouse. Immunobiology. 2000;201:432–49. doi: 10.1016/s0171-2985(00)80096-0. [DOI] [PubMed] [Google Scholar]
- 16.Iwabuchi K, Hatakeyama S, Takahashi A, et al. Csk overexpression reduces several monokines and nitric oxide productions but enhances prostaglandin E2 production in response to lipopolysaccharide in the macrophage cell line J774A.1. Eur J Immunol. 1997;27:742–9. doi: 10.1002/eji.1830270324. [DOI] [PubMed] [Google Scholar]
- 17.Murray LJ, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur J Immunol. 1990;20:163–70. doi: 10.1002/eji.1830200124. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Kobayashi S, Inobe M, Jia WY, Tamakoshi M, Miyazaki T, Uede T. In vivo cytokine gene expression in various T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Autoimmunity. 1994;17:49–57. doi: 10.3109/08916939409014658. [DOI] [PubMed] [Google Scholar]
- 19.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–42. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 20.Heizmann CW, Hunziker W. Intracellular calcium-binding proteins: more sites than insights. Trends Biochem Sci. 1991;16:98–103. doi: 10.1016/0968-0004(91)90041-s. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama S, Kretsinger RH. Evolution of the EF-hand family of proteins. Annu Rev Biophys Biomol Struct. 1994;23:473–507. doi: 10.1146/annurev.bb.23.060194.002353. [DOI] [PubMed] [Google Scholar]
- 22.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits the effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–8. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 23.Hara H, Ohta M, Ohta K, Nishimura M, Obayashi H, Adachi T. Isolation of two novel alternative splicing variants of allograft inflammatory factor-1. Biol Chem. 1999;380:1333–6. doi: 10.1515/BC.1999.170. [DOI] [PubMed] [Google Scholar]
- 24.Steiner DF, Smeekens SP, Ohagi S, Chan SJ. The new enzymology of precursor processing endoproteases. J Biol Chem. 1993;267:23435–8. [PubMed] [Google Scholar]
- 25.Tanaka S, Suzuki K, Watanabe M, Matsuda A, Tone S, Koike T. Upregulation of a new microglial gene, mrf-1, in response to programmed neuronal cell death and degeneration. J Neurosci. 1998;18:6358–69. doi: 10.1523/JNEUROSCI.18-16-06358.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the MHC class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–62. doi: 10.1006/bbrc.1996.1112. 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- 27.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 28.Allan DJ, Harmon BV, Kerr JFR. Cell death in spermatogenesis. In: Potten CW, editor. Perspectives on Mammalian Cell Death. London: Oxford University Press; 1987. pp. 229–58. [Google Scholar]
- 29.Print CG, Loveland KL. Germ cell suicide: new insights into apoptosis during spermatogenesis. Bioessays. 2000;22:423–30. doi: 10.1002/(SICI)1521-1878(200005)22:5<423::AID-BIES4>3.0.CO;2-0. 10.1002/(sici)1521-1878(200005)22:5<423::aid-bies4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Christophe D, Christophe-Hobertus C, Pichon B. Nuclear targeting of proteins. how many different signals? Cell Signal. 2000;12:337–41. doi: 10.1016/s0898-6568(00)00077-2. 10.1016/s0898-6568(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 31.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 32.Kindred B. Immunological unresponsiveness of genetically thymusless (nude) mice. Eur J Immunol. 1971;1:59–61. doi: 10.1002/eji.1830010114. [DOI] [PubMed] [Google Scholar]
- 33.Humphreys T. Rapid allogeneic recognition in the marine sponge Microciona prolifera. Implications for evolution of immune recognition. Ann NY Acad Sci. 1994;712:342–5. doi: 10.1111/j.1749-6632.1994.tb33590.x. [DOI] [PubMed] [Google Scholar]
- 34.Müller WE, Blumbach B, Müller IM. Evolution of the innate and adaptive immune systems: relationships between potential immune molecules in the lowest metazoan phylum (Porifera) and those in vertebrates. Transplantation. 1999;68:1215–27. doi: 10.1097/00007890-199911150-00001. [DOI] [PubMed] [Google Scholar]