Abstract
The open environment of the eye is continuously subject to an influx of foreign agents that can activate complement. Decay-accelerating factor (DAF), membrane cofactor protein (MCP) and CD59 are regulators that protect self-cells from autologous complement activation on their surfaces. They are expressed in the eye at unusually high levels but their physiological importance in this site is unstudied. In the rat, a structural analogue termed 5I2 antigen (5I2 Ag) has actions overlapping DAF and MCP. In this investigation, we injected F(ab′)2 fragments of 5I2 mAb into the conjunctiva and aqueous humor, in the latter case with and without concomitant blockage of CD59. Massive neutrophilic infiltration of the stroma and iris resulted upon blocking 5I2 Ag activity. Frank necrosis of the iris occurred upon concomitant intraocular blockage of CD59. C3b was identified immunohistochemically, and minimal effects were seen in complement-depleted animals and in those treated with non-relevant antibody. The finding that blockage of 5I2 Ag function in periocular tissues and within the eye causes intense conjunctival inflammation and iritis demonstrates the importance of intrinsic complement regulators in protecting ocular tissues from spontaneous or bystander attack by autologous complement.
Introduction
Intrinsic membrane regulators of complement are species-specific proteins that protect self-cells from activation of autologous complement on their surfaces (reviewed in refs 1 and 2). In humans, these proteins consist of the decay-accelerating factor (DAF or CD55),3,4 the membrane cofactor protein (MCP or CD46)4 and CD59 (homologous restriction factor 20 [HRF20] or the membrane inhibitor of reactive lysis [MIRL]).5,6 DAF and MCP act early in the activation sequence to disable the classical and alternative pathway C3 convertases,3,4,7 the central amplification enzymes of the cascade. CD59 functions later in the cascade to prevent binding of C9 to C5b-85,6 and consequent formation of membranolytic poly C9 channels that bring about cell lysis.
These three critical regulatory proteins were initially described on blood elements and on the vascular endothelium,8 i.e. cells that are in constant contact with high concentrations of serum complement proteins. Subsequently they were identified on ocular cells,9–12 specifically on epithelium and fibroblasts of the cornea and conjunctiva, as well as on multiple other cell types within the eye and in periocular tissues. Surprisingly, the levels of the proteins on some ocular cell types were found to be among the highest in the body.9
In blood, where complement is at functionally optimal levels, the essential protective activities of these three regulators are well understood.3–7 They prevent complement activation on self-cells initiated by autologous C3b fragments that spontaneously deposit as a result of the natural tickover of C3 (see the Discussion), or deposit in a bystander fashion during focused complement activation on targets. This is because nascent C3b-activation fragments condense with free hydroxyl and amino groups wherever present and consequently bind indiscriminately to host tissues as well as to foreign agents. In the absence of DAF, MCP and CD59, these bound fragments would initiate amplification of complement activation, eventuating in host cell injury. Their physiological importance has been documented in that loss of the activities of DAF and CD59 results in blood cell destruction.1,6,13 In contrast, in the eye, where complement levels are much lower than those in blood,14,15 what roles they play in limiting autologous complement-mediated injury to ocular tissues is unstudied. The fact that the eye is a site which is continuously exposed to exogenous agents that can potentially activate complement, and the finding that these regulators are expressed at high levels, argue that their activities in this site should be physiologically important.
In view of the inability to study the functions of these regulators in humans, an animal model has been developed.16 In the rat, a 44000-molecular weight (MW) protein designated 5I2 antigen (5I2 Ag)17 (Crry/p65 in the mouse), with potent complement regulatory activity, has been shown to be a functional analogue of MCP, possessing overlapping activity with that of DAF. Similarly, a 19000-MW protein (initially termed rat inhibitory protein, or RIP), recognized by the antibody TH9, has been shown to be the rat homologue of human CD59.18 Previous studies by ourselves16 and others17 have shown that in the rat, expression of 5I2 Ag and CD5919 on ocular surface cells, the iris and choroid, eyelid, and orbital tissues, in general parallels that of DAF, MCP and CD59 in humans.
In order to understand the role of the regulators in ocular homeostasis, i.e. whether the eye is at risk for damage from complement activation by the tickover phenomenon, we examined the effect of blocking 5I2 Ag function with specific monoclonal antibodies (mAb) or F(ab′)2 fragments in the absence of disease. Because other defence mechanisms, including blinking, tear flow and the overlying mucin layer, complicate direct access to the ocular surface, we blocked 5I2 Ag function in the conjunctival substantia propria immediately below the epithelium and in the aqueous humor, where antibody has access to the iris.
Materials and methods
Antibodies
Mouse 5I2 mAb (IgG1) was prepared as described previously.17 Murine OX-18 mAb (IgG1) was purchased from Pharmingen Inc. (La Jolla, CA), anti-ciliary neurotrophic factor mAb (IgG2b) from R & D Systems (Minneapolis, MN) and non-relevant murine RPC 5 (IgG2a) from Sigma Chemical Co. (St. Louis, MO). Murine anti-rat TH918 mAb (IgG1) was kindly provided by Dr Paul Morgan (University of Wales College of Medicine, Cardiff, UK). 5I2 and OX-18 F(ab′)2 fragments were prepared as described previously.19
Antibody injections
For all injections, adult rats underwent xylazine/ketamine anaesthesia and topical proparacaine anaesthesia. For conjunctival injections, each rat had the lower lid distracted outward and was subepithelially injected using a 30-gauge needle attached to a Hamilton syringe. In initial blocking studies using intact mAbs, rats underwent injection with 25 µl of a 1 µg/µl solution of murine 5I2 mAb, OX-18 anti-major histocompatibility complex (MHC) class I mAb, non-relevant control mAbs, or saline solution. Subsequent titrations showed similar inflammatory effects with the use of 10 µl (10 µg) of antibody while causing less tissue distortion, whereas with further dilution the response tapered. Therefore, to work at saturating conditions, 10 µg was employed for all subsequent conjunctival studies, including those with F(ab′)2 fragments.
For analysis of the effects of intraocular regulator blockage, 10 µl of intact mAb or F(ab′)2 fragments of 5I2 or OX-18 were injected into the aqueous humor. To allow analysis of the effects of combined blockage of complement regulation at the C3 and C8 steps in the cascade, F(ab′)2 fragments of 5I2, in combination with either anti-CD59 mAb or OX-18 mAb, were injected. To keep the total amount of injected protein constant, the injection volume was decreased to 5 µl for each antibody.
Tissue processing and analysis
Twenty-four hours after antibody administration, the rats were killed and orbital tissues excised. The excised tissues were either immediately snap-frozen in liquid nitrogen for immunohistochemical analysis or fixed in 10% formalin for morphological and cytological analyses. Formalin-fixed tissues were embedded in paraffin, sectioned and stained with haematoxylin and eosin. Sections were examined histologically by two of the authors in a blinded fashion. Within the area injected, sections with the greatest amount of inflammation were analysed.
Immunohistochemical analyses of C3b deposition were performed on frozen sections following fixation in cold acetone for 10 min. Tissues were incubated with fluorescein isothiocyanate (FITC)-conjugated goat mAb to rat C3 (Cappel Laboratories, Cochranville, PA; 1:500 and 1:1000 dilutions) at room temperature for 30 min. Sections were examined using either an Olympus BHT (Tokyo, Japan) or a Nikon Optiphot-2 (Tokyo, Japan) fluorescence microscope and a Chroma Technologies (Brattleboro, VT) FITC HYQ filter with an excitation wavelength of 480 nm.
Complement depletion
Systemic complement depletion was performed by administering 0·5 ml of cobra venom factor (CoVF; Quidel, Inc., La Jolla, CA) intraperitoneally 48 and 24 hr prior to injection of 5I2 F(ab′)2. Two injections were given based on preliminary studies showing a 90% reduction of complement 24 hr after a single injection of CoVF. Tail vein blood specimens were obtained prior to and after CoVF treatment to quantify the extent of complement depletion. After subconjunctival injection of either 5I2 F(ab′)2 or control F(ab′)2 fragments, orbits were exenterated at either 24 hr, for analysis of inflammation, or at 3 hr (see the Results) for analysis of C3b deposition.
The total haemolytic complement activity was determined by incubating 100 µl of antibody-sensitized sheep erythrocytes (EshA) (1–108/ml) with 1·4 ml of rat serum sequentially diluted in dextrose gelatin veronal buffer (DGVB++ 2·47 mm sodium veronal buffer, pH 7·3, 72·7 mm NaCl, 2·5% dextrose, 0·1% gelatin, 0·15 mm CaCl2 and 0·5 mm MgCl2) at 37° for 30 min.20 Following centrifugation, the percentage haemolysis was determined by assaying the supernatant absorbance at 412 nm.
Results
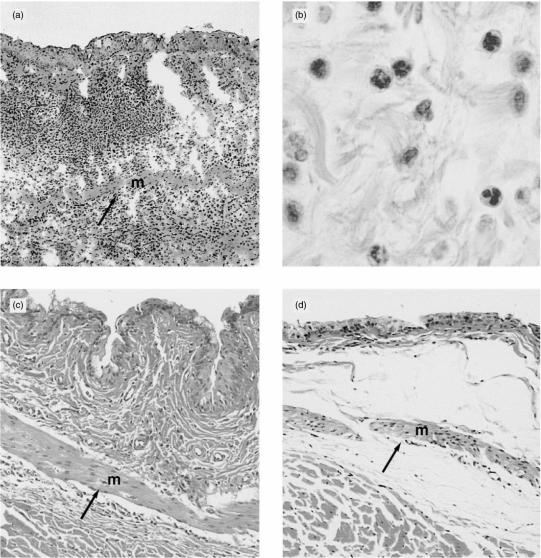
In the first series of experiments, the effects of administering intact antibodies were assessed. The results are shown in Fig. 1. All animals injected with 5I2 antibody demonstrated intense inflammatory cell infiltration, thickening and oedema (Fig. 1a). The infiltrate consisted primarily of neutrophils (Fig. 1b). No mast cells retaining intact granules were seen. In contrast, animals injected with OX-18 antibody against class I MHC protein, which is expressed at high levels, showed less intense changes than those induced by 5I2 antibody (Fig. 1c). Animals injected with non-relevant RPC 5 or anti-ciliary neurotrophic factor antibody (control mAbs of isotypes that are inherently more efficient complement activators)21,22 showed only mild inflammation and oedema (Fig. 1d). These studies with intact IgG extended previous work by ourselves with intact mABs.23
Figure 1.
(a) Rat conjunctival tissue injected with 5I2 monoclonal antibody (mAb). Extensive inflammation is present with muscle displacement (m→). (b) Neutrophilic nature of the infiltrate (original magnification ×1000). (c) Rat conjunctival tissue injected with OX-18 anti-major histocompatibility complex (MHC) class I mAb. Less inflammation and muscle (m) displacement (→) is seen (original magnification ×40). (d) Rat conjunctival tissue injected with anti-ciliary neurotrophic factor mAb. Inflammation and oedema are mild. Muscle (m) is minimally displaced but the oedema results in its displacement (→) (original magnification ×40).
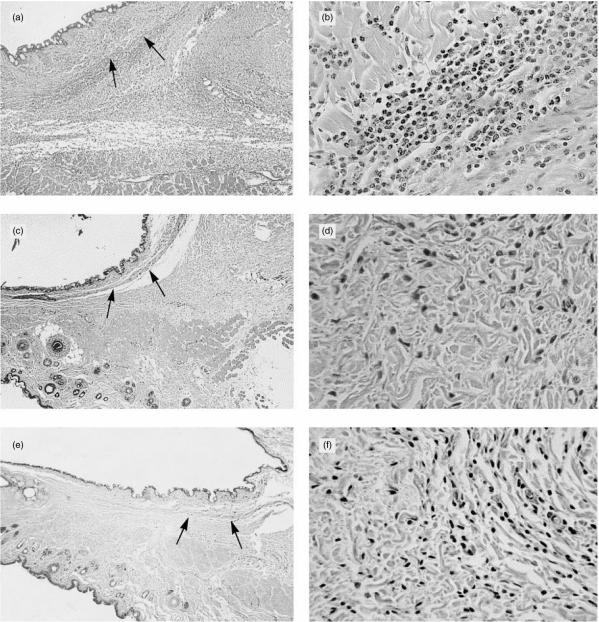
To exclude the effects of Fc-mediated complement activation by intact 5I2 or OX-18 antibody, i.e. to isolate the effect of blockage of the regulatory function of 5I2 Ag, studies were next performed with purified F(ab′)2 fragments that are unable to fix C1. The results of these studies are shown in Fig. 2. Following injection of 5I2 F(ab′)2, tissues showed oedema, swelling and widespread intense infiltration by neutrophils, approaching that seen with intact antibody (Fig. 2a, 2b). As with the intact mAb, the inflammation was diffuse and not in a perivascular distribution. In contrast, tissues injected with OX-18 F(ab′)2 showed mild focal inflammation with only post-injection oedema (Fig. 2c, 2d).
Figure 2.
(a) Rat conjunctiva injected with 5I2 F(ab′)2 fragments show diffuse and extensive oedema and inflammation approaching that of intact 5I2 monoclonal antibody (mAb). Muscle (indicated by the arrows) is displaced from epithelium by inflammation (original magnification ×25). (b) High-power photomicrograph of (a) showing the virtually complete neutrophilic character of the infiltrate (original magnification ×400). (c) Conjunctiva from an animal injected with OX-18 F(ab′)2, showing sparse inflammation. Muscle (indicated by the arrows) is not displaced (original magnification ×25). (d) High-power photomicrograph of (c) showing few inflammatory cells of a primarily mononuclear nature, presumably lymphocytes (original magnification ×400). (e) Conjunctiva from rat injected with 5I2 F(ab′)2 fragments after systemic complement depletion. Inflammation is minimal (original magnification ×25). (f) High-power photomicrograph of (e) showing sparse inflammation which is mononuclear in nature (original magnification ×400).
To establish that the inflammatory changes induced by blocking 5I2 Ag were complement-mediated, control studies were performed in animals that had undergone systemic complement depletion with CoVF prior to administration of F(ab′)2 fragments. Haemolytic assays showed that their total haemolytic complement activity in plasma was reduced by > 95%. As seen in Fig. 2(e), 2(f), the cellular inflammatory response was mild and focal, without significant oedema.
To determine the longevity of the inflammatory changes and ascertain the pathological sequelae, tissues were analysed 1, 2, 3 and 6 days after injection of F(ab′)2 fragments of 5I2 mAb. The analyses showed a completely neutrophilic inflammation within the first 24 hr with transition to a primarily mononuclear infiltrate by days 2 and 3. By day 6, the intensity of the response tapered in that only scattered mononuclear cells remained.
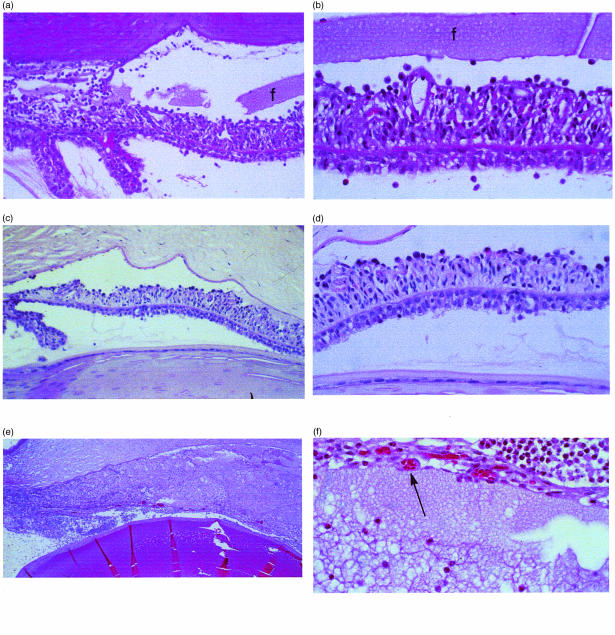
To assess the effects of blocking intrinsic complement regulatory function intraocularly in the anterior segment, injections into the aqueous humor were performed. Directed administration of 5I2 F(ab′)2 fragments into the aqueous induced extensive inflammation of the iris and an associated inflammatory cell response in the aqueous humor (Fig. 3a, 3b), which was markedly different from that following identical administration of OX-18 anticlass I MHC F(ab′)2 (Fig. 3c, 3d). In 5I2 F(ab′)2-injected eyes there was marked cellular inflammation and thickening of the iris and ciliary body, and protein accumulation in the anterior chamber. When mAb against the downstream cell regulator, CD59, was injected concurrently, in addition to the above changes obliteration of anterior chamber anatomy by massive leucocyte infiltration occurred and frank iris necrosis was seen (Fig. 3e, 3f). This contrasted with concurrent injection of intact OX-18 with 5I2 F(ab′)2, which showed much less inflammation and no necrosis.
Figure 3.
(a) Anterior chamber of rat injected into the aqueous humor with 5I2 F(ab′)2 fragments. Marked inflammation of the iris and anterior chamber, equivalent to that of intact 5I2 IgG, is seen. Moderate fibrin (f) is seen in the anterior chamber (original magnification ×200). (b) High-power view of (a) showing thickening and inflammatory cells throughout the iris. The overlying fibrin is seen across the entirety of the field (original magnification ×400). (c) The anterior chamber injected identically with anti-OX-18 F(ab′)2 fragments. Inflammation is minimal and proteinaceous material is absent (original magnification ×200). (d) High-power view of (c) showing few inflammatory cells on the anterior iris surface (original magnification ×400). (e) Anterior chamber injected into the aqueous humor simultaneously with 5I2 and anti-rat CD59 monoclonal antibodies (mAbs). The inflammation totally obscures the underlying anatomy (original magnification ×100). (f) High-power view of (e) showing necrosis and thinning of iris tissue resulting from the 5I2 and anti-CD59-induced inflammation (original magnification ×400).
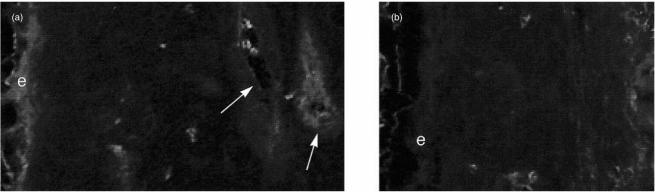
To document that complement activation in fact resulted from blocking 5I2 Ag, and that it paralleled the observed inflammatory changes, tissues from untreated animals (and those that were complement depleted with CoVF) were examined for deposited C3b fragments. Kinetics studies of C3b deposition showed that maximal changes were observed 3 hr after 5I2 F(ab′)2 injection and consequently this time-point was employed for the analyses. As seen in Fig. 4, immunohistochemical labelling of tissues after treatment with 5I2 F(ab′)2 fragments showed that deposition of C3b antigen was most pronounced on the epithelium and on endothelial cell surfaces (Fig. 4a). This contrasted with studies using control F(ab′)2, where labelling was absent (Fig. 4b). In animals pretreated with CoVF and then injected with 5I2 F(ab′)2, greatly reduced C3b deposition was observed. As demonstrated previously, markedly decreased cellular infiltrate was also seen.
Figure 4.
(a) Fluorescein isothiocyanate (FITC)–anti-C3 labelling of rat conjunctival and subconjunctival tissues injected with 5I2 F(ab′)2 fragments. Label is seen in the epithelium (e) and in vessel walls (arrow). (b) FITC–anti-C3 labelling of rat conjunctival and subconjunctival tissues injected with control F(ab′)2. Neither the epithelium nor the vessels show label.
Discussion
DAF, MCP and CD59 are cell-associated regulators of complement that function intrinsically in the plasma membranes of self-cells to prevent activation of autologous complement on their surfaces. A large body of evidence has established that their activities are essential physiologically.3–8,24,25 Important among this evidence are studies of acquired haemolytic anaemia and paroxysmal nocturnal hemoglobinuria (PNH) (reviewed in ref. 1), in which deficiency of two of the regulators, DAF4,13,26,27 and CD59,6,28 leads to increased uptake of autologous C3b13,29 and C930 on affected erythrocytes, eventuating in their lysis in vivo. Surprisingly, it has been found that expression levels of DAF are many-fold9,10 and of MCP12 and CD5911,12 more than twofold higher on corneal, conjunctival and uveal cells than on most blood cells. The high levels of these proteins on these anterior segment cells argues that they must be functionally important at this site.
While numerous locations in the body, e.g. the gastrointestinal and respiratory systems, are exposed to foreign agents, the eye is unique in that minor levels of inflammation, which would be easily tolerated by other mucous membrane sites, may cause significant alterations in visual acuity that can result in great functional disability. It therefore is critical that bystander damage to host tissues resulting from inflammation at the ocular surface and within the eye, especially the intense inflammation that is associated with attack against offending pathogens, be rigorously controlled. This is especially so because most inflammatory processes involving the eye affect the anterior segment more than the posterior globe and orbit. In systemic autoimmune inflammatory conditions associated with complement activation, typically the eye is less affected than other sites.31,32 It is noteworthy that the need of ocular cells for protection has not been widely appreciated previously as there are limited data regarding the complement system in ocular disease. With the exception of ocular cicatricial pemphigoid33–35 and bacterial keratitis,36 only scattered reports37–39 of the role of complement in their pathology are available.
The key question that has remained unanswered regarding the importance of intrinsic cell-associated complement regulators on the ocular surface and in the eye is what would happen in their absence. 5I2 Ag is a rat analogue of MCP, which has activity overlapping that of DAF. The direct rat MCP homologue is significantly expressed only in testes.40 Previous studies in rats41 have shown that intravenous injection of 5I2 F(ab′)2 fragments (which, as indicated, cannot activate complement themselves but are able to block the function of 5I2 Ag) results in vascular collapse and massive deposition of C3b on vascular endothelial cells. The present study addresses this issue as it relates to the anterior segment. An advantage of conducting the studies in the manner described in this report, i.e. under constitutive conditions, is that it excluded the confounding effects of other inflammatory mediators which invariably accompany disease.
In our conjunctival injection system, introduction of antibody to 5I2 Ag, which normally functions to prevent the amplification phase of the complement cascade, led to massive infiltration of neutrophils. Neutrophil and eosinophil chemotaxis results from the local generation of C5a and C3a fragments.42,43 The difference between the 5I2 Ag and OX-18 or non-relevant antibody-injected animals thus reflects the markedly increased production of these anaphylatoxins resulting from C3 convertase formation in the former case. Evidence in support of this mechanism is: (1) the abrogation of the effect in animals depleted of complement by CoVF administration, and (2) the finding of deposition of C3b fragments on the anterior segment cells.
Our demonstration that the inflammatory changes were reproduced following injection of purified 5I2 F(ab′)2, but not following the administration of OX-18 F(ab′)2, verified that the process was a consequence of disabling the function of the 5I2 Ag rather than as a result of complement activation by 5I2 antibody itself. This excludes a proinflammatory effect of the injected 5I2 antibody. This finding is consistent with the C3 ‘tickover’ process.44 C3 molecules, which contain metastable internal thioester bonds, are constantly condensing (in small numbers) with free hydroxyl or amino acceptors on host cell surfaces. In the presence of 5I2 Ag, these bound C3 molecules are prevented from serving as sites for the stable assembly of the alternative pathway C3 convertase, C3bBb, which would lead to amplification of C3b deposition and further progression of the cascade. The demonstration that interfering with the function of 5I2 Ag permitted complement activation to proceed indicates that C3 tickover is operating constitutively in periocular tissues.
Having studied the effects of interfering with regulation of the C3 step of the cascade by blocking 5I2 Ag activity in both subconjunctival tissue and the iris, we next investigated the combined effects of blocking regulation in the latter site at both the C3 step and the pathway's C9 end-point. In this most demonstrative investigation of the functional importance of these regulators, the combined inactivation of both early (5I2 Ag) and late (rat CD59) cascade regulation led to a degree of inflammation more extensive than that of inactivating 5I2 Ag alone or in conjunction with administration of OX-18 antibody to highly expressed class I MHC protein. In the absence of protection against autologous cytolytic C5b-9 complex formation as well as amplified C3b deposition, frank tissue necrosis occurred.
With the use of intact antibodies, similar findings resulting from blocking 5I2 Ag intraocularly have concurrently been obtained45,46 by another group. Consistent with the above premise that tickover operates constitutively, in their studies they obtained Western blot evidence for low levels of C3 fragments in normal aqueous humor.47 While they were unable to show an effect of blocking CD59 when studied alone, we found that blocking CD59 in conjunction with 5I2 Ag eventuated in greatly enhanced uveal damage, highlighting the importance of the combined functions of both intrinsic regulators.
Taken together, the results of this study, our previous work23 and that of the above study47 demonstrate that intrinsic membrane regulators of complement, present in periocular tissues and within the anterior chamber, are vital for protecting ocular tissues from complement-mediated inflammation resulting from C3b deposition (whether spontaneous or induced) at these sites. In the case of the internal anterior segment, their absence leads to the development of iritis.
Acknowledgments
This work was supported by NEI grant EY11288 (to M.E.M. and D.S.B.), NEI Core Grant EY11373 (to M.E.M. and D.S.B.) and Research to Prevent Blindness (to D.S.B.). The authors thank Dr Paul Morgan for providing TH9 anti-rat CD59 mAb, Hidechika Okada for critical reading, and Sara Cechner for manuscript preparation. This work was presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL, USA.
References
- 1.Medof ME. The molecular basis for paroxysmal nocturnal hemoglobinuria. Transfusion. 1993;33:852–73. doi: 10.1046/j.1537-2995.1993.331094054626.x. [DOI] [PubMed] [Google Scholar]
- 2.Lachmann PJ. The control of homologous lysis. Immunol Today. 1991;12:312–5. doi: 10.1016/0167-5699(91)90005-E. [DOI] [PubMed] [Google Scholar]
- 3.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–78. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicholson-Weller A, March JP, Rosen CE, Spicer DB, Austen KF. Surface membrane expression by human leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985;65:1237–44. [PubMed] [Google Scholar]
- 5.Okada N, Harada R, Fujita T, Okada H. A novel membrane glycoprotein capable of inhibiting membrane attack by homologous complement. Int Immunol. 1989;1:205–8. doi: 10.1093/intimm/1.2.205. [DOI] [PubMed] [Google Scholar]
- 6.Holguin MH, Fredrick LR, Bernshaw NJ, Wilcox LA, Parker CJ. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J Clin Invest. 1989;84:7–17. doi: 10.1172/JCI114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seya T, Turner JR, Atkinson JP. Purification and characterization of a membrane protein (gp45–70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986;163:837–55. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asch AS, Kinoshita T, Jaffe EA, Nussenszweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986;163:221–6. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medof ME, Walter EI, Rutgers JL, Knowles DM, Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987;165:848–64. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lass JH, Walter EI, Burris TE, et al. Expression of two molecular forms of the complement decay-accelerating factor in the eye and lacrimal gland. Invest Ophthalmol Vis Sci. 1990;31:1136–48. [PubMed] [Google Scholar]
- 11.Bardenstein DS, Dietz Y, Lass JH, Medof E. Localization of the complement membrane attack complex inhibitor (CD59) in human conjunctiva and lacrimal gland. Curr Eye Res. 1994;13:851–5. doi: 10.3109/02713689409015085. [DOI] [PubMed] [Google Scholar]
- 12.Bora NS, Gobleman CL, Atkinson JP, Pepose JS, Kaplan HJ. Differential expression of the complement regulatory proteins in the human eye. Invest Ophthalmol Vis Sci. 1993;34:3579–84. [PubMed] [Google Scholar]
- 13.Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci USA. 1983;80:5430–4. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondino BJ, Brady KJ. Distribution of hemolytic complement in the normal cornea. Arch Ophthalmol. 1981;99:1430–3. doi: 10.1001/archopht.1981.03930020304022. [DOI] [PubMed] [Google Scholar]
- 15.Mondino BJ, Hoffman DB. Hemolytic complement activity in normal human donor corneas. Arch Ophthalmol. 1980;98:2041–4. doi: 10.1001/archopht.1980.01020040893021. [DOI] [PubMed] [Google Scholar]
- 16.Bardenstein DS, Cheyer C, Okada N, Morgan BP, Medof ME. Cell surface regulators of complement, 5I2 antigen, and CD59, in the rat eye and adnexal tissues. Invest Ophthalmol Vis Sci. 1999;40:519–24. [PubMed] [Google Scholar]
- 17.Takizawa H, Okada N, Okada H. Complement inhibitor of rat cell membrane resembling mouse Crry/p65. J Immunol. 1994;152:3032–8. [PubMed] [Google Scholar]
- 18.Hughes TR, Piddlesden SJ, Williams JD, Harrison RH, Morgan BP. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992;284:169–76. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funabashi K, Okada N, Matsuo S, Yamamoto T, Morgan BP, Okada H. Tissue distribution of complement regulatory membrane proteins in rats. Immunology. 1994;81:444–51. [PMC free article] [PubMed] [Google Scholar]
- 20.Brodbeck WG, Liu D, Sperry J, Mold C, Medof ME. Localization of classical and alternative pathway regulatory activity within the decay-accelerating factor. J Immunol. 1996;156:2528–33. [PubMed] [Google Scholar]
- 21.Neuberger MS, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–6. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 22.Dangl JL, Wensel TG, Morrison SL, Stryer L, Herzenberg LA, Oi VT. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–94. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bardenstein DS, Medof ME. Blocking of conjunctival complement regulatory proteins causes inflammatory cell infiltration. Invest Ophthalmol Vis Sci. 1997;38:S1075. (Abstr.). [Google Scholar]
- 24.Cosio FG, Sedmak DD, Mahan JD, Nahman NS. Localization of decay accelerating factor in normal and diseased kidneys. Kidney Int. 1989;36:100–7. doi: 10.1038/ki.1989.167. [DOI] [PubMed] [Google Scholar]
- 25.Lederman MM, Purvis SF, Walter EI, Carey JT, Medof ME. Heightened complement sensitivity of acquired immunodeficiency syndrome lymphocytes related to diminished expression of decay-accelerating factor. Proc Natl Acad Sci USA. 1989;86:4205–9. doi: 10.1073/pnas.86.11.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinoshita T, Medof ME, Silber R, Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985;162:75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson-Weller A, March JP, Rosenfeld SI, Austen KF. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci USA. 1983;80:5066–70. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitlow MB, Iida K, Stefanova I, Bernard A, Nussenzweig V. H19, a surface membrane molecule involved in T-cell activation, inhibits channel formation by human complement. Cell Immunol. 1990;126:176–84. doi: 10.1016/0008-8749(90)90310-n. [DOI] [PubMed] [Google Scholar]
- 29.Medof ME, Kinoshita T, Silber R, Nussenzweig V. Amelioration of the lytic abnormalities of paroxysmal nocturnal hemoglobinuria with decay-accelerating factor. Proc Natl Acad Sci USA. 1985;82:2980–4. doi: 10.1073/pnas.82.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenfeld SI, Jenkins DE, Leddy JP. Enhanced reactive lysis of paroxysmal nocturnal hemoglobinuria erythrocytes by C5b-9 does not involve increased C7 binding or cell-bound C3b. J Immunol. 1985;134:506–11. [PubMed] [Google Scholar]
- 31.McGavin DD, Williamson J, Forrester JV, et al. Episcleritis and scleritis. A study of their clinical manifestations and association with rheumatoid arthritis. Br J Ophthalmol. 1976;60:192–226. doi: 10.1136/bjo.60.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinberg AD, Talal N. The coexistence of Sjogren's syndrome and systemic lupus erythematosus. Ann Intern Med. 1971;74:55–61. doi: 10.7326/0003-4819-74-1-55. [DOI] [PubMed] [Google Scholar]
- 33.Proia AD, Foulks GN, Sanfilippo FP. Ocular cicatricial pemphigoid with granular IgA and complement deposition. Arch Ophthalmol. 1985;103:1669–72. doi: 10.1001/archopht.1985.01050110063026. [DOI] [PubMed] [Google Scholar]
- 34.Mondino BJ, Ross AN, Rabin BS, Brown SI. Autoimmune phenomena in ocular cicatricial pemphigoid. Am J Ophthalmol. 1977;83:443–50. doi: 10.1016/0002-9394(77)90546-3. [DOI] [PubMed] [Google Scholar]
- 35.Foster CS, Wilson LA, Ekins MB. Immunosuppressive therapy for progressive ocular cicatricial pemphigoid. Ophthalmology. 1982;89:340–4. doi: 10.1016/s0161-6420(82)34791-0. [DOI] [PubMed] [Google Scholar]
- 36.Mondino BJ, Brown SI, Rabin BS, Bruno J. Alternate pathway activation of complement in a Proteus mirabilis ulceration of the cornea. Arch Ophthalmol. 1978;96:1659–61. doi: 10.1001/archopht.1978.03910060285021. [DOI] [PubMed] [Google Scholar]
- 37.Mondino BJ, Nagata S, Glovsky MM. Activation of the alternative complement pathway by intraocular lenses. Invest Ophthalmol Vis Sci. 1985;26:905–8. [PubMed] [Google Scholar]
- 38.Kahaly G, Hansen C, Felke B, Dienes HP. Immunohistochemical staining of retrobulbar adipose tissue in Graves' ophthalmopathy. Clin Immunol Immunopathol. 1994;73:53–62. doi: 10.1006/clin.1994.1169. [DOI] [PubMed] [Google Scholar]
- 39.Antonelli A, Palla R, Casarosa L, Fallahi P, Baschieri L. IgG, IgA and C3 deposits in the extra-thyroidal manifestations of autoimmune Graves' disease: their in vitro solubilization by intravenous immunoglobulin. Clin Exp Rheumatol. 1996;14(Suppl. 15):S31–S5. [PubMed] [Google Scholar]
- 40.Tsujimura A, Shida K, Kitamura M, et al. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. Biochem J. 1998;330:163–8. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo S, Ichida S, Takizawa H, Okada N, Baranyi L, Iguchi A, Morgan B, Okada H. In vivo effects of monoclonal antibodies that functionally inhibit complement regulatory proteins in rats. J Exp Med. 1994;180:1619–27. doi: 10.1084/jem.180.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugli TE. The structural basis for anaphylatoxin and chemotactic functions of C3a, C4a, and C5a. CRC Crit Rev Immunol. 1981;1:321–66. [PubMed] [Google Scholar]
- 43.Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–27. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abbas AK, Lichtman AH, Pober JS. The complement system. In: Abbas AK, Lichtman AH, Pober JS, editors. Cellular and Molecular Immunology. 3. Philadelphia: W.B. Saunders; 1997. pp. 313–38. [Google Scholar]
- 45.Bardenstein DS, Cheyer C, Lee C, Okada N, Morgan BP, Medof ME. Inflammation of periocular and ocular tissues after blockage of complement regulatory protein function. Invest Ophthalmol Vis Sci. 2000;41:S673. (Abstr.). [Google Scholar]
- 46.Sohn JH, Kaplan HJ, Suk HJ, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41:s673. (Abstr.). [PMC free article] [PubMed] [Google Scholar]
- 47.Sohn JH, Kaplan HJ, Suk HJ, Bora PS, Bora NS. Chronic low level complement activation within the eye is controlled by intraocular complement regulatory proteins. Invest Ophthalmol Vis Sci. 2000;41:3492–502. [PMC free article] [PubMed] [Google Scholar]