Abstract
Many marine fish and invertebrates show a dual life history where settled adults produce dispersing larvae. The planktonic nature of the early larval stages suggests a passive dispersal model where ocean currents would quickly cause panmixis over large spatial scales and prevent isolation of populations, a prerequisite for speciation. However, high biodiversity and species abundance in coral reefs contradict this panmixis hypothesis. Although ocean currents are a major force in larval dispersal, recent studies show far greater retention than predicted by advection models. We investigated the role of animal behavior in retention and homing of coral reef fish larvae resulting in two important discoveries: (i) Settling larvae are capable of olfactory discrimination and prefer the odor of their home reef, thereby demonstrating to us that nearby reefs smell different. (ii) Whereas one species showed panmixis as predicted from our advection model, another species showed significant genetic population substructure suggestive of strong homing. Thus, the smell of reefs could allow larvae to choose currents that return them to reefs in general and natal reefs in particular. As a consequence, reef populations can develop genetic differences that might lead to reproductive isolation.
Keywords: coral reef, olfaction, population genetics
The planktonic life history phase of many larval marine organisms suggests wide distribution by ocean currents and genetic homogeneity of the population within the dispersal area. This appears contradicted by rich phylogenetic diversity, which requires at least some form of isolation of populations, be it physical, caused by geographic distance and barriers (1) that make populations in remote areas less accessible, or behavioral, based on habitat preferences (2) or social preferences (3), leading to assortative mating.
The persistence of reef fish populations on isolated oceanic islands (4, 5) demonstrates that larvae can be retained in the natal environment despite pelagic dispersal. Such “self-recruitment” has been shown by using otolith microchemistry and tagging (6) and genetic markers (7). Lee-side eddies can “passively” trap larvae and keep them near reef habitat, whether reefs are isolated (4) or connected in seasonally stable currents (8, 9) or daily tidal flow (10). Because not finding a reef is lethal, one might expect in addition the evolution of “active” behavioral adaptations facilitating retention and homing (5). Dispersal models better predict observed recruitment when they include a behavioral component (11, 12). From an extensive study on the scaling of marine populations, Paris and Cowen (9) proposed a biophysical retention mechanism based on ontogenetic larval vertical migration to take advantage of favorable currents at different depths. Indeed, impressive larval swimming capabilities have been shown (13), and for sensory guidance both acoustic (14–16) and olfactory (17) mechanisms have been suggested. However, no plausible mechanism has yet been demonstrated that could allow these centimeter-sized larvae to choose between ocean water masses and to keep them in the appropriate current regime for successful recruitment either to reefs in general or to the natal reef in particular.
We chose a multidisciplinary approach using hydrodynamic modeling, population genetics, and sensory/behavioral experiments to tackle this question. We developed a hydrodynamic model to serve as a null hypothesis describing the passive distribution pattern of organisms among five closely spaced reefs. We developed genetic markers to evaluate the actual dispersal patterns exhibited by three species of coral reef fish chosen for their different larval pelagic duration and swimming capabilities. Finally, we developed a miniflume for olfactory preference tests with settlement-stage larvae to test their ability to smell the difference between water of the five reefs and their possible preference for the settlement reef.
Results
Hydrodynamic Model.
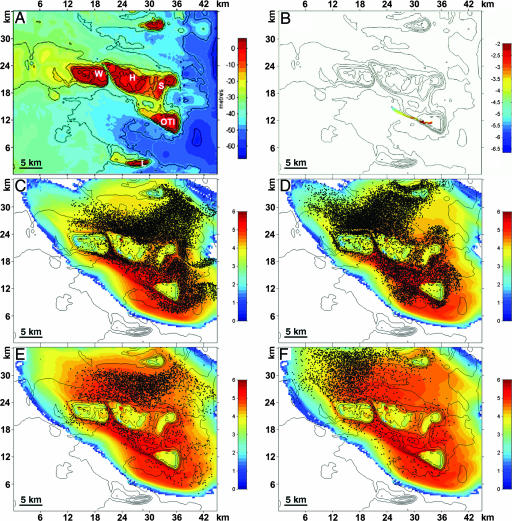
To test sensory/behavioral modification of passive dispersal we selected a geographically and hydrodynamically linked group of reefs where genetic mixing would be expected. The study site consisted of five adjacent reefs separated by 3–23 km in the Capricorn/Bunker group of the Great Barrier Reef, Australia: One Tree Island (OTI), Sykes (S), and Heron (H) in the north and Lamont (L) and Fitzroy (F) in the south (Fig. 1A). A surface current model of the area was generated based on high-resolution bathymetry, known tidal flow, and wind forcing. Tidal flow in this area is predominantly southeast to northwest; the prevailing wind direction year-round is (and during the actual model run was) primarily from the east/southeast at ≈5 m/s (10 knots). The model was calibrated for particle dispersal with current meters on three sides of the island and by tracking the concentration of neutrally buoyant particles released from the northwest side of OTI reef for 3 days (Fig. 1B), taking water samples over a wide area. After an 8-day model run, the period when developing larvae are most planktonic, particles had dispersed in a northwesterly direction engulfing OTI, H, and S reefs; to the southeast, across a deep channel, L and F reefs did not receive particles from OTI (Fig. 1 C and D). After a 20-day model run (Fig. 1 E and F), which covers the mean larval dispersal period, the distribution of particles extended even farther northwest. The 8- and 20-day dispersal averages depict the odor retention “halo” we discussed earlier (17) and shows mean particle retention near the reef of origin (Fig. 1 C–F, color contours). Overall, the passive dispersal model predicts that larvae dispersing from OTI become fully mixed among the three northern reefs (OTI, S, and H) and less so among the two southern reefs (L and F). Larvae from the southern reefs would be passively distributed to the northern cluster but not the reverse. Genetic differences, if any, would be expected between the northern and southern reef groups.
Fig. 1.
Bathymetric map and hydrodynamic modeling of the study area. (A) Bathymetric map including OTI, S, H, Wistari (W), and L reefs. F reef is located to the southeast of L just outside the map area of this model; Wistari is not included among our study reefs. Color scale: water depth in meters. (B) Snapshot of particle positions at 6 h, the initial flood tide carrying particles away from OTI; color is log concentration, with red highest. Particles were released at a frequency of 250 particles per model time step (=50 s) for 1.4 h, giving a total of 25,200 particles. (C–F) Particle concentration (log10) averaged over 8 and 20-day periods (ebb and flood, respectively) shows strong connectivity between reefs and high concentrations near the reef of origin (OTI); superimposed on these color images are snapshots of particle positions at ebb and flood times. Heron Island is located exactly on the Tropic of Capricorn, at 151° 55′ longitude.
Population Genetic Analysis.
We tested the hydrodynamic model predictions by evaluating across the five test reefs the genetic substructure of three species of reef fishes with different larval dispersal and swimming capabilities: (i) spiny damselfish Acanthochromis polyacanthus [no pelagic larvae (18)], (ii) cardinal fish Ostorhinchus (formerly Apogon) doederleini [larval pelagic stage 16–27 days (19), relatively weak swimmers (13)], and (iii) neon damselfish Pomacentrus coelestis [larval pelagic stage 18–20 days (20), strong swimmers (13)]. We developed DNA microsatellite markers for all three species (21–23); more detailed information about these loci is shown in supporting information (SI) Tables 3–5.
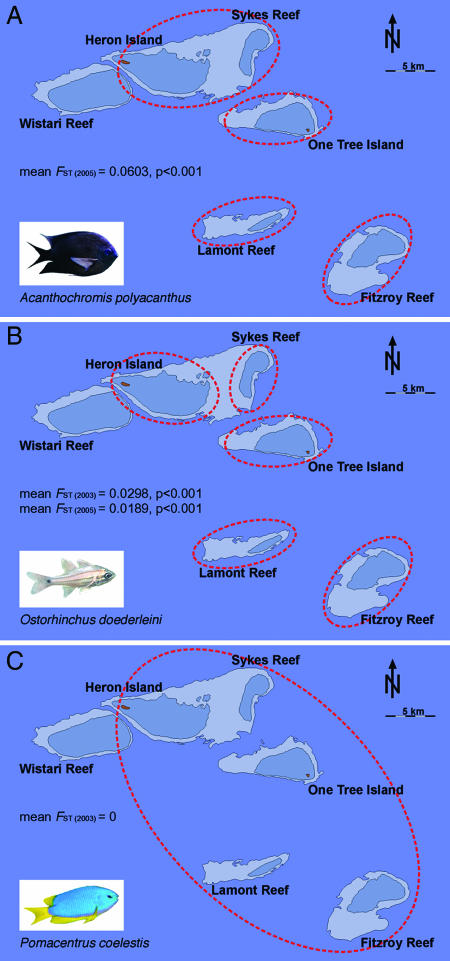
As expected from the absence of a larval dispersal stage in this species, genetic differences between populations were largest for A. polyacanthus (Fig. 2A). Among all five reefs the mean FST value, used as an indicator for genetic difference between populations, was FST = 0.0603 (P < 0.001). The pairwise comparison of H and S reefs (FST = 0.0077, P = 0.03) was not significant after Bonferroni correction, suggesting that H/S populations are linked despite nondispersing larvae (Table 1 and Fig. 2A).
Fig. 2.
Genetic substructure of three fish populations across five test reefs. (A) A. polyacanthus without pelagic larval dispersal stage. (B) O. doederleini with 3-week larval dispersal. (C) P. coelestis with 3-week larval dispersal. Red dotted circles enclose genetically different populations. Mean FST values among reefs are provided on the graph.
Table 1.
Reef distances and genetic population structure of three species of coral reef fishes
| Reefs | OTI | S | H | L | F |
|---|---|---|---|---|---|
| OTI | 3.9 | 6.3 | 8.1 | 12 | |
| S | 0.0393* | ||||
| 0.0134* | 2.4 | 15 | 22.5 | ||
| 0.0028 | |||||
| H | 0.0400* | 0.0077 | |||
| 0.0198* | 0.0260* | 15 | 22.5 | ||
| 0.0039 | 0.0045 | ||||
| L | 0.0585* | 0.0789* | 0.0800* | ||
| 0.0173* | 0.0130* | 0.0160* | 7.5 | ||
| 0.0015 | 0.0024 | 0.0027 | |||
| F | 0.0375* | 0.0506* | 0.0655* | 0.0650* | |
| 0.0114* | 0.0226* | 0.0294* | 0.0174* | ||
| −0.0004 | −0.0005 | −0.0004 | 0.0002 |
Data show A. polyacanthus 2005 (Roman), O. doederleini 2005 (italic), and P. coelestis 2003 (bold) at five reefs. Below diagonal: pairwise FST values (an asterisk indicates statistical significance after Bonferroni correction for multiple comparisons). Above diagonal: nearest distance between reefs (in kilometers).
Despite dispersing larvae, O. doederleini showed significant genetic substructure among all five reefs. The genetic differences between reefs were stable over 2 years (mean FST 2003 = 0.0298, P < 0.001; mean FST 2005 = 0.0189, P < 0.001) (Table 1, Fig. 2B, and SI Table 6); no genetic differences existed between populations of the same reef between different years (mean FST values = 0.0025, P > 0.30).
Among 66 freshly settled larval O. doederleini, caught at OTI, 38 (58%) were assigned to the adult population of OTI with a mean probability of 82%, and 24 (36%) with a statistical probability of ≥95%; 28 larvae could not be assigned unequivocally to any reef population. Together, the genetic substructure of the adult population and larval assignment are indicative of strong and persistent larval homing.
In contrast, P. coelestis, despite dispersal in the same tidal current regime, including lee-side retention eddies, showed no genetic substructure among any of the reefs including the reefs across the 60-m-deep channel (mean FST value = 0) (Fig. 2C). To further evaluate the potential barrier effect of this channel, we performed a hierarchical genetic analysis. The result (SI Table 7) showed for all three species that genetic differences between and within the groups of the northern and southern reefs were not different. Therefore, neither self-recruitment of O. doederleini (and A. polyacanthus) nor dispersal of P. coelestis was significantly affected by this channel.
Olfactory Choice Tests.
To evaluate whether fish might use odor differences between nearby reefs for homing, we designed very small, two-channel choice flumes for shipboard use (SI Fig. 3). In these flumes, single larvae can be tested while using small amounts of water, a logistical necessity. Fish larvae were caught before settling (with light traps) or while settling (with crest nets) on OTI reef (24) and tested individually for olfactory preference of “home” reef water (OTI) versus “foreign” reef water (H, S, L, or F). To evaluate whether OTI might be exceptional, we did similar tests at F, a location where shipboard tests with locally caught larvae are possible. Merely for convenience we call “home” the reef where a fish was caught. We calculated odor preference from time spent in one or the other reef water in the choice flume. Selected from each day's catch we tested settling stage apogonids (O. doederleini, Apogonid sp. 1, and eight other unidentified apogonid species 2–9) and pomacentrids (P. coelestis and four other identified pomacentrid species); early juvenile A. polyacanthus were caught with hand nets at OTI.
The results show that O. doederleini differentiated between and expressed significant preference for their home reef (i.e., OTI or F) versus all other reefs; even stronger olfactory preference was found in other species of apogonids (Table 2 and SI Table 8). However, we chose O. doederleini for genetic analysis because, unlike several other cardinal fishes, in the settling stage it is clearly identifiable to species. Freshly settled O. doederleini, caught at OTI, expressed a significant preference for OTI reef water (Table 2). These larvae could be genetically assigned to the OTI adult population with high probability (see above). P. coelestis and other dispersing damsel fishes also expressed significant olfactory preference for home reef water (Table 2 and SI Table 8). In the nondispersing damsel fish A. polyacanthus sample size (n = 6) is too small to determine olfactory preference (Table 2).
Table 2.
Olfactory preference of larval reef fish for water from home versus foreign reefs
| Species | Home reef preference ± SE, % | n | P |
|---|---|---|---|
| O. doederleini | 7.0 ± 2.3 | 66 | 0.023 |
| O. doederleini OTI | 12.4 ± 5.7 | 22 | 0.05 |
| Apogon sp. 1 | 17.1 ± 3.7 | 55 | 0.000 |
| Apogonid sp. 2–9 | 9.0 ± 2.6 | 114 | 0.000 |
| P. coelestis | 8.5 ± 3.3 | 66 | 0.002 |
| Pomacentrus sp. 1–4 | 15.3 ± 6.2 | 21 | 0.016 |
| A. polyacanthus | −16.7 ± 7.3 | 6 | 0.094 |
Preference is expressed as mean difference in time spent in home versus foreign water during odor-choice test in a two-channel flume. Positive values indicate preference for home reef odor, and negative values indicate preference for foreign reef odor. Animals were caught at two different home reefs (OTI and F) and tested against water from four other reefs. O. doederleini OTI refers to the larvae that were later genetically assigned to the OTI adult population. Unidentified Apogon sp. 1 was common in our catches and could be analyzed separately.
Discussion
During the settlement stage of their dispersal, coral reef fish larvae of several species discriminated the odors of water from several nearby reefs, preferring the settlement reef. This shows that even nearby reefs smell different and that reef fish larvae can tell the difference. Their odor preference suggests that they might use this sensory capability to guide their behavior toward reefs in general and the home reef in some instances. The latter would enhance homing and genetic substructure. Olfactory-driven homing and the resulting genetic differences between populations might be the first step to reproductive isolation that might lead to speciation.
The genetic results showed that, in response to the same physical dispersal environment, the cardinal fish (O. doederleini) expressed strong evidence for homing by pelagic larvae, whereas our two damsel fish species showed two opposite responses: avoiding larval dispersal altogether (A. polyacanthus) and broad dispersal among all study reefs, including the reefs across the 60-m-deep channel (P. coelestis).
The stable genetic substructure of the O. doederleini population of the Capricorn/Bunker group must result from a strong predominance of self-recruitment overriding the dispersal potential of the prevailing tidal and wind-driven current regime that links these reefs hydrodynamically already after 8 days but even more strongly after 20 days. Because the P. coelestis population is panmictic in the same study area experiencing the same current regime, we suggest that, in addition to retention in lee-side eddies (4) and via ontogenetic vertical migration in stratified currents (8, 9), sensory-guided behavior could be a component of the homing retention of O. doederleini as well as the dispersal of P. coelestis.
O. doederleini would increase the probability of larvae staying close to the source of home odor if among mixing water masses they consistently choose during their entire larval development the freshest home reef water in both the vertical and horizontal planes. When the larvae developmentally reach the settlement stage, they could then locate or already be in fresh ebb currents from the home reef. Nighttime ebb currents are typically cooler than the surrounding ocean (17); therefore, they sink providing olfactory information at some depth for typical nighttime settling. We know of no other sensory modality that could allow animals to identify favorable water masses. In addition, reef sounds (14, 16) might help direct larvae once in the reef's close proximity. Daily tidal currents in our study area are at a 10-km scale; reef sounds have been estimated to be detectable within 1 km (25).
P. coelestis, in contrast, may use its home odor knowledge to recognize reef odors in general as a way to remain in the wider reef environment but avoid open ocean water. It is a stronger swimmer than O. doederleini. In flume tests it may still prefer one reef over another either because in a forced choice test it remembers home odor or because it became adapted to the location where it was caught. To distinguish between these hypotheses we should know the reef of origin of individuals, but because this species is panmictic in our study area we cannot perform genetic assignment tests and further work is required.
The hydrodynamic model did not predict that P. coelestis released from OTI would cross the 60-m channel either within the first 8 planktonic larval days or within the 20 days of the entire pelagic stage of this species. Based on the same tidal flow and current regime, larval release from the southern reefs could easily reach the northern reefs. In addition, occasional large storms could further facilitate wider dispersal. However, the same hydrodynamics would also disperse O. doederleini. To explain their different population structure, we suggest that apogonids, which are weaker swimmers than pomacentrids (13), have adopted a more rigorous homing behavior and, in addition, may be less successful in finding and/or settling on another reef after being carried away by storm events. Finally, post-settlement selection could further act to isolate apogonids more than pomacentrids. O. doederleini typically settles in dense groups deep within the reef where competition may be severe, whereas P. coelestis settles loosely along the outer reefs. This would put greater selection pressure on apogonids than pomacentrids to develop strategies to stay close to home, including home odor preference and their prevalence in deeper water layers (26), which would reduce advective transport. Phylogenetic differences between apogonids and pomacentrids probably do not explain our results, because some pomacentrids show extreme homing behavior, e.g., Amphiprion polymnus (6). Homing as an active retention mechanism might also explain why in different species of coral reef fish the scale of genetic structure does not increase with the pelagic larval duration (27).
The genetic differences among subpopulations in O. doederleini on such a small geographic scale are, to our knowledge, the largest ever found in a species with larval dispersal phase. Supported by the assignment test, this emphasizes a high rate of self-recruitment in this species.
The A. polyacanthus populations of H and S reefs, which are physically connected by a shallow submerged platform, were genetically similar. This similarity could be the result of genetic exchange due to migration of adults or subadults or to a unique founder event in H and S from genetically similar animals. The H–S gene flow levels of A. polyacanthus were similar to those of black surfperch, Embiotoca jacksoni (28), a species that also lacks a pelagic phase and lives on almost continuous reefs along the California and Baja California coasts (29).
Olfaction is commonly involved with associative learning where odor is the marker for important biological events in the animal's life. Many animals can learn odors rapidly and use them for feeding efficiency, social organization, homing, etc. For homing, in our context, perhaps best known is the classical research showing that juvenile salmon learn odors associated with their home stream before seaward migration and use the memory of this signature smell to relocate their birth stream for spawning as adults many years later (30, 31). Among reef fishes, anemonefish imprint on the odor of their specific anemone host, demonstrating that imprinting can occur in the reef context (32). Fish olfaction can develop very early [zebrafish 48 h after fertilization (33), anemonefish 2–3 days after fertilization (34)], and at settlement, olfactory organs can have receptor densities similar to adults as shown in the spangled emperor, Lethrinus nebulosus (35). The species we studied have well developed noses at settlement (17). At the time of hatching they are still in the reef, and, similar to the dye particles used to calibrate the hydrodynamic model, they will subsequently be planktonically embedded for several days in the reef-flavored water mass that carries them into the pelagic environment (Fig. 1 B–D). These days of home odor exposure may result in recognition of and preference for water flavored by their natal reef (e.g., O. doederleini). This imprinting could also allow for later generalization to generic reef odor to facilitate return to any reef during settlement (e.g., P. coelestis).
We can only speculate about the chemical substances that trigger olfactory preferences in larval fish. Either they originate from the environment, suggesting that larvae recognize their natal reef by the odor of its unique species assemblage, or that larvae use “pheromones,” i.e., the odor of their own species and population. The latter theory is supported by Doving et al.'s study on cardinal fish (36). He showed that five-lined cardinalfish (Cheilodipterus quinquelineatus) and ochre-striped cardinalfish (Apogon compressus) preferred artificial reef sites that had previously been occupied by conspecifics. Individual C. quinquelineatus preferred the scent of conspecifics from their own reef site to that from another site. This indicates that larval reef fish might be imprinted on their own population as has been suggested in perch (3) and zebrafish (37). Compounds of the MHC released via gills and urine might shape this olfactory template (38).
Other senses besides olfaction can contribute to homing behavior. Several sense organs of settlement stage reef fishes are well developed (see ref. 39), but their physiological and behavioral functions are poorly studied. For reef identification and localization, only olfaction and hearing appear a priori useful. Horizontal visual distances are limited by light scatter and are estimated to be ≈50 m in daytime Great Barrier Reef conditions. A magnetic sense is not known in reef fishes, but this information may be more useful for animals that cover great distances (40). Otoliths are present and can provide useful directional signals in conditions where waves, both surface and internal, are predictably shore-directed (41). Reef sounds show promise for reef orientation (14, 16, 42, 43), but we still need to know how far the acoustic reef signal extends above the noise or how it relates to the hearing frequency range of these fishes (44); it may be limited to relatively short distances (≈1 km) (25). Superficial lateral line is useful for rheotactic responses (45, 46), particularly in shear layers near solid surfaces and among water masses. However, although directional per se, none of these acoustic and hydrodynamic senses would allow animals suspended in the water column without external frame of reference to select favorable currents. Only olfaction can provide information on the identity of the water mass encountered. (Compare navigating the New York subway system with no external frame of reference: it works only when one knows what train to take. Human's trains are labeled with visual signs whereas the water currents are labeled with olfactory signals.)
For practical application, our results suggest that olfactory imprinting of natal reef odor may play an important role in limiting dispersal of reef fishes. This would support isolation of populations and cause genetic population divergence, which ultimately may lead to speciation. The relative autonomy of some populations of reef fishes should be considered by managers: the location and spacing of Marine Protected Areas needs to incorporate species-specific requirements.
Methods
Hydrodynamic Model.
The five reefs selected for hydrodynamic connectivity (OTI, S, H, L, and F) are located at the eastern end of the Capricorn/Bunker group of Australia's Great Barrier Reef (Fig. 1). Bathymetry (Fig. 1A) was derived from laser swath-mapping (provided by Australian Defense, unpublished data). Average water depth around these reefs is 40 m. The northern (OTI, S, and H) and southern (L and F) reefs are separated by a 60-m-deep channel that could act as a physical barrier for dispersal. A similarly deep channel separates L and F. H and S are connected reefs emerging from a single platform and linked by a shallow ridge, 5 m deep. OTI is linked to the H/S platform across a shallow area, 20 m deep. Linear geographic separation of the reefs varies from 2.4 to 22.5 km.
To simulate the circulation, the field-calibrated hydrodynamic model 3DD was used, which is a depth-integrated numerical model of tidal circulation around OTI and surrounding reefs (47–49). Sea levels were generated from tidal simulations by using a coarse grid (750-m cell size) consisting of the Queensland coastline, the edge of the continental shelf, and the Capricorn/Bunker group. This grid was used to generate boundary conditions for nested fine grid (300-m cell size) simulations. Tidal circulation patterns around OTI, therefore, incorporated the influence of neighboring reefs. Wind data were obtained from the Heron Island weather station. The influence of wind was added as 3% of the wind strength at the free surface with decay by depth in accordance with an imposed velocity profile of a cubic form.
Population Genetics.
For population genetic analyses, adults of the three species selected for this study were caught at four different sites (separated by ≈0.5–5 km) at each of the five reefs: O. doederleini in 2003 (n = 325) and 2005 (n = 314), P. coelestis in 2003 (n = 193), and A. polyacanthus in 2005 (n = 236). For sample sizes per reef see SI Tables 3–5. Fin clips were stored in 99% ethanol until DNA extraction.
We developed DNA microsatellite markers for each species (21–23) and tested for variation in allele frequencies among individuals from the five different reefs. Each individual was tested for five to six loci. Compliance with Hardy–Weinberg expectations was calculated in GENETIX (50). All data were controlled and balanced for null alleles by using the program MICROCHECKER (51). Fragment length was transferred into number of base pairs to fit the requirements of the analyses for pairwise FST values in ARLEQUIN3.0 (52) for genetic population differences between reefs and for a hierarchical analyses (AMOVA) grouping the northern (OTI, S, and H) and southern (L and F) reefs. We assigned larvae to adult populations using GENECLASS (53) using the Bayesian method by Rannala and Mountain (54). This method removes the individual being assigned (“leave one out” method) and uses the exclusion–simulation approach to obtain a level of certainty (P value) for each individual assignment to a single population.
Odor Choice.
For odor choice tests, larval fish at the stage of potential settlers were collected with light traps, channel nets, or hand nets. From daily catches of many species of settling larvae, we selected apogonids and pomacentrids for our experiments. Light traps were set overnight just outside a major reef entrance (trapping presettling fish), and channel nets were set for several hours during incoming high tides in a reef crest entrance channel (trapping fish entering the lagoon). In addition, we caught newly settled O. doederleini at patches of coral rubble we had located in the sandy parts of the lagoon close to one of the reef entrances. Settling apogonids initially accept low-quality habitat before settling in better reef structures (26). We checked these patches each morning and caught all newly settled larvae with hand nets. With some exceptions, apogonid classification is still uncertain, particularly at the larval stage. In many cases, apogonid larvae could be identified only to family or genus leading to classifications such as “apogonid,” or Apogon sp. 1. Voucher specimens are held at the Australian Museum in Sydney.
Olfactory preference tests were conducted in two-channel choice flumes (SI Fig. 3) with steady gravity-driven flow (100 ml/min per channel; ≈1 cm/s) controlled by flow meters. In contrast to the large (2.5-m) shore-based flume used previously to test groups of fish (17), these small (20-cm) flumes were designed to test single fish both on shore and shipboard with minimal volumes of water, because new water needed to be collected regularly from other reefs. As odor stimuli we used water collected at the different test reefs and stored in buckets for at most 3 days; as much as possible, individual tests were done with reef water collected on the same day. Separate analysis (ANOVA, F = 0.015, P = 0.99) showed that age of water did not affect choice behavior. Water temperature was balanced between sides, never exceeding a 0.5°C difference. Regular dye tests ensured that the flumes maintained two distinct parallel-flowing water masses (A and B), which stayed entirely separated up to the downstream mesh screen. Water masses A and B allowed no neutral area in the flume. Single fish were placed into the flume with both water sources (with their inherent odor stimuli) running and given 5 min to acclimate. Fish could swim freely between water masses. Fish position in one or the other water flow was then recorded every 5 s during two 2-min periods separated by a 1-min transition time to switch water sources as a control for possible side bias of the fish. We calculated the difference between the number of observations in which a test fish was in the water mass with stimulus A or B, and we tested whether that difference was significantly different from zero using a Wilcoxon signed-rank test (two-tailed) in the program JMP (55). A random distribution across water masses (zero difference) is expected if a fish does not express a preference for one of the odor stimuli or is unable to detect a difference between them. The choices made by a species or family were based on the behavior of individuals that showed great differences ranging from no preference at all to strong preference for the home reef or, less frequently, for the other reef. The mean response favored the home reef. Fish that did not swim during the acclimation period (<1%) were eliminated. Most choice tests were done in a field laboratory on One Tree Island; at F reef we successfully conducted shipboard tests using the same flumes and water conditions.
Supplementary Material
Acknowledgments
We thank Mark O'Callaghan, David Welsh, and Felicity Smith for assistance with sample collection; Scott Burgess for help with model runs; Robert Cowen for critical reading of the manuscript; and Andreas Bally for help with the figures. This work was funded by National Science Foundation Grant OCE-0452885 (to G.G. and J.A.), National Geographic Society Grant 7236-02, an Australian Research Council grant (to M.J.K.), and a National Science Foundation Graduate Research Fellowship (to V.M.-S.).
Abbreviations
- OTI
One Tree Island
- S
Sykes
- H
Heron
- L
Lamont
- F
Fitzroy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606777104/DC1.
References
- 1.Dobzhansky T. Am Nat. 1940;74:312–321. [Google Scholar]
- 2.Beltman JB, Haccou P. Theor Popul Biol. 2005;67:189–202. doi: 10.1016/j.tpb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Behrmann-Godel J, Gerlach G, Eckmann R. Behav Ecol Sociobiol. 2006;59:461–468. [Google Scholar]
- 4.Lobel PS, Robinson AR. Deep Sea Res. 1986;33:483–500. [Google Scholar]
- 5.Sponaugle S, Cowen RK, Shanks A, Morgan SG, Leis JM, Pineda JS, Boehlert GW, Kingsford MJ, Lindeman KC, Grimes C, Munro JL. Bull Mar Sci. 2002;70:341–375. [Google Scholar]
- 6.Jones GP, Planes S, Thorrold SR. Curr Biol. 2005;15:1314–1318. doi: 10.1016/j.cub.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MS, Hellberg ME. Science. 2003;299:107–109. doi: 10.1126/science.1079365. [DOI] [PubMed] [Google Scholar]
- 8.Cowen RK. In: Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem. Sale PF, editor. New York: Academic; 2002. pp. 149–170. [Google Scholar]
- 9.Paris CB, Cowen RK. Limnol Oceanogr. 2004;49:1964–1979. [Google Scholar]
- 10.Burgess SC, Kingsford MJ, Black KP. Mar Ecol Prog Ser. 2006 in press. [Google Scholar]
- 11.Armsworth PR. J Theor Biol. 2001;210:81–91. doi: 10.1006/jtbi.2001.2299. [DOI] [PubMed] [Google Scholar]
- 12.Cowen RK, Paris CB, Srinivasan A. Science. 2006;311:522–527. doi: 10.1126/science.1122039. [DOI] [PubMed] [Google Scholar]
- 13.Fisher R, Leis JM, Clark DL, Wilson SK. Mar Biol. 2005;147:1201–1212. [Google Scholar]
- 14.Leis JM, Carson-Ewart BM, Cato DH. Mar Ecol Prog Ser. 2002;232:259–268. [Google Scholar]
- 15.Simpson SD, Meekan M, Montgomery J, McCauley R, Jeffs A. Science. 2005;308:221–221. doi: 10.1126/science.1107406. [DOI] [PubMed] [Google Scholar]
- 16.Tolimieri N, Jeffs A, Montgomery JC. Mar Ecol Prog Ser. 2000;207:219–224. [Google Scholar]
- 17.Atema J, Kingsford MJ, Gerlach G. Mar Ecol Progr Ser. 2002;241:151–160. [Google Scholar]
- 18.Robertson DR. Z Tierpsychol. 1973;32:319–324. doi: 10.1111/j.1439-0310.1973.tb01108.x. [DOI] [PubMed] [Google Scholar]
- 19.Brothers EB, Williams DM, Sale PF. Mar Biol. 1983;76:319–324. [Google Scholar]
- 20.Wellington GM, Victor BC. Mar Biol. 1989;101:557–567. [Google Scholar]
- 21.Miller-Sims V, Atema J, Kingsford MJ, Gerlach G. Mol Ecol Notes. 2005;5:424–426. doi: 10.1111/j.1365-294X.2008.03986.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller-Sims V, Atema J, Kingsford MJ, Gerlach G. Mol Ecol Notes. 2004;4:336–338. doi: 10.1111/j.1365-294X.2008.03986.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Sims V, Delaney M, Atema J, Kingsford MJ, Gerlach G. Mol Ecol Notes. 2005;5:841–843. [Google Scholar]
- 24.Kingsford MJ. Mar Biol. 2001;138:853–867. [Google Scholar]
- 25.Mann DA, Casper BM, Boyle KS, Tricas TC. Mar Ecol Progr Ser. in press. [Google Scholar]
- 26.Finn MD, Kingsford MJ. Mar Freshw Res. 1996;47:423–432. [Google Scholar]
- 27.Bay LK, Buechler K, Gagliano M, Caley MJ. J Fish Biol. 2006;68:1206–1214. [Google Scholar]
- 28.Doherty PJ, Planes S, Mather P. Ecology. 1995;76:2373–2391. [Google Scholar]
- 29.Bernardi G. Evolution (Lawrence, Kan) 2000;54:226–237. [Google Scholar]
- 30.Dittman AH, Quinn TP. J Exp Biol. 1996;199:83–91. doi: 10.1242/jeb.199.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Hasler AD. Science. 1975;1975:1–7. [Google Scholar]
- 32.Arvedlund M, McCormick MI, Fautin DG, Bildsoee M. Mar Ecol Prog Ser. 1999;188:207–218. [Google Scholar]
- 33.Hansen A, Zeiske E. J Comp Neurol. 1993;333:289–300. doi: 10.1002/cne.903330213. [DOI] [PubMed] [Google Scholar]
- 34.Arvedlund M, Bundgaard I, Nielsen LE. Environ Biol Fish. 2000;58:203–213. [Google Scholar]
- 35.Lara MR. Williamsburg, VA: College of William and Mary; 1999. Ph.D. thesis. [Google Scholar]
- 36.Doving KB, Stabell OB, Ostlund-Nilsson S, Fisher R. Chem Senses. 2006;31:265–272. doi: 10.1093/chemse/bjj028. [DOI] [PubMed] [Google Scholar]
- 37.Gerlach G, Lysiak N. Anim Behav. 2006;71:1371–1377. [Google Scholar]
- 38.Olsen KH, Grahn M, Lohm J, Langefors A. Anim Behav. 1998;56:319–327. doi: 10.1006/anbe.1998.0837. [DOI] [PubMed] [Google Scholar]
- 39.Kingsford MJ, Leis JM, Shanks A, Lindeman KC, Morgan SG, Pineda J. Bull Mar Sci. 2002;70:309–340. [Google Scholar]
- 40.Walker MM, Diebel CE, Haugh CV, Pankhurst PM, Montgomery JC, Green CR. Nature. 1997;390:371–376. doi: 10.1038/37057. [DOI] [PubMed] [Google Scholar]
- 41.Lohmann KJ, Hester JT, Lohmann CMF. Ethol Ecol Evol. 1999;11:1–23. [Google Scholar]
- 42.Simpson SD, Meekan MG, McCauley RD, Jeffs S. Mar Ecol Progr Ser. 2004;276:263–268. [Google Scholar]
- 43.Tolimieri N, Haine O, Jeffs A, McCauley R, Montgomery J. Coral Reefs. 2004;23:184–191. [Google Scholar]
- 44.Myrberg AA, Jr, Fuiman LA. In: Coral Reef Fishes. Sale PF, editor. Amsterdam: Academic; 2002. pp. 123–148. [Google Scholar]
- 45.Montgomery JC, Baker CF, Carton AG. Nature. 1997;389:960–963. [Google Scholar]
- 46.Baker CF, Montgomery JC. Polar Biol. 1999;21:305–309. [Google Scholar]
- 47.Black KP, Moran PJ, Hammond LS. Mar Ecol Prog Ser. 1991;74:1–11. [Google Scholar]
- 48.Black KP, Moran PJ, Hammond LS. Mar Ecol Progr Ser. 1991;74:1–11. [Google Scholar]
- 49.Jenkins GP, Black KP, Hamer PA. Mar Ecol Progr Ser. 2000;199:231–242. [Google Scholar]
- 50.Belkhir K, Borsa P, Goudet J, Chikhi L, Bonhomme F. Genetix v. 3.0, Logiciel Sous Windows Pour la Génétique des Populations. Montpellier, France: Université Montpellier 2; 1997. [Google Scholar]
- 51.van Oosterhout C, Hutchinson WF, Derek PMW, Shipley P. Mol Ecol Notes. 2004;4:535–538. [Google Scholar]
- 52.Schneider S, Kuffer JM, Roessle D, Excoffier L. ARLEQUIN: A Software for Population Genetic Data Analysis. Geneva, Switzerland: University of Geneva; 1997. [Google Scholar]
- 53.Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A. J Hered. 2004;95:536–539. doi: 10.1093/jhered/esh074. [DOI] [PubMed] [Google Scholar]
- 54.Rannala B, Mountain JL. Proc Natl Acad Sci USA. 1997;94:9197–9201. doi: 10.1073/pnas.94.17.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.SAS Institute. JMP Statistics and Graphics Guide. Cary, NC: SAS Institute; 1995. Version 3.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.