Abstract
The peptide hormone glucagon-like peptide (GLP)-1 has important actions resulting in glucose lowering along with weight loss in patients with type 2 diabetes. As a peptide hormone, GLP-1 has to be administered by injection. Only a few small-molecule agonists to peptide hormone receptors have been described and none in the B family of the G protein coupled receptors to which the GLP-1 receptor belongs. We have discovered a series of small molecules known as ago-allosteric modulators selective for the human GLP-1 receptor. These compounds act as both allosteric activators of the receptor and independent agonists. Potency of GLP-1 was not changed by the allosteric agonists, but affinity of GLP-1 for the receptor was increased. The most potent compound identified stimulates glucose-dependent insulin release from normal mouse islets but, importantly, not from GLP-1 receptor knockout mice. Also, the compound stimulates insulin release from perfused rat pancreas in a manner additive with GLP-1 itself. These compounds may lead to the identification or design of orally active GLP-1 agonists.
Keywords: ago-allosteric modulator, allosteric, G protein-coupled receptor, screening, cAMP
Type 2 diabetes and the underlying obesity, also called diabesity, is rapidly becoming a worldwide epidemic, sometimes even referred to as a pandemic. Current drugs have limited efficacy and do not address the most important problems, the declining β-cell function and the associated obesity. Glucagon-like peptide (GLP)-1 is a 30-aa peptide hormone synthesized in l-cells of the small intestine (1). GLP-1, one of the incretins, is a natural postprandial hormone released in response to nutrient intake and acts to stimulate insulin secretion. GLP-1 has attracted much interest as a future treatment for type 2 diabetes because it has multiple antidiabetic actions and, at the same time, lowers body weight; it has been shown to be highly efficacious in clinical studies (2, 3). GLP-1 has several actions. Functional effects in the pancreas include glucose-dependent release of insulin as well as an up-regulation of insulin biosynthesis, the glucokinase enzyme, and the glucose transporters. Other effects include (i) growth, proliferation, and antiapoptosis of pancreatic β-cells and neogenesis from ductal precursor cells; (ii) glucose-dependent lowering of glucagon secretion, leading to lower hepatic glucose output; (iii) inhibition of gastric acid secretion and gastric emptying, the latter causing a reduction in postprandial plasma glucose excursions; and (iv) inhibition of appetite and lowering of food intake leading to decreased body weight. New data also show GLP-1 to be both neuro- and cardioprotective (4, 5). Because type 2 diabetes is characterized by a progressive decline in β-cell mass and function (6–8), increased glucagon secretion (9), and often is accompanied by severe obesity, GLP-1 seems ideal for its treatment. The two main limitations for GLP-1 are a relatively narrow therapeutic window, with nausea being the dose limiting parameter, and a very short half-life of the native peptide (10, 11). The action of GLP-1 is terminated partly via degradation by the almost ubiquitous enzyme dipeptidyl peptidase-IV (DPP-IV), which cleaves the molecule into inactive forms, and partly via rapid renal clearance and degradation (12). Current efforts aim to identify GLP-1 analogs with more suitable pharmacokinetic properties than the native peptide. Exenatide, a GLP-1 analog originally isolated from the saliva of the Gila monster, recently was approved by the Food and Drug Administration as a twice-daily treatment regimen (13). Liraglutide [N-ε(γ-l-glutamoyl(N-α-hexadecanoyl)-Lys26,Arg34-GLP-1(7–37)] is a long-acting analog in phase 3 clinical development as a once-daily treatment regimen (14, 15). However, as peptides, GLP-1 and analogs thereof have to be administered by injection.
The GLP-1 receptor belongs to the glucagon-secretin B family of the G protein-coupled receptors (GPCRs) (16). The B family is characterized by a rather large N-terminal extracellular domain. The most closely related receptors to GLP-1 are the GLP-2, glucagon, and GIP receptors with homologies ranging ≈40%, highest for the glucagon and the GLP-2 receptor (42%). The glucagon and GLP-1 peptides have some homology as well, 47%, as well as some overlap in binding sites. Glucagon binds to the GLP-1 receptor with low affinity, whereas GLP-1 does not bind to the glucagon receptor (17). In recent years, small-molecule agonists have been described, even for receptors for larger hormones like insulin, TPO, and EPO (18–20), but none of these receptors belong to the GPCR superfamily. Within the GPCRs, small-molecule agonists have been described, e.g., for the arginine vasopressin V-2 receptor, the somatostatin receptor, the bradykinin receptor, the cholecystokinin receptor, the angiotensin II receptor, and the growth hormone secretagogue receptor (21–26). However, none of these GPCRs belong to the B family, and the natural ligands are all either fairly small or have a defined secondary structure. To date, no small-molecule agonists have been described in the B family. Small-molecule antagonists have been described for two members of this family, the glucagon receptor and the CRF receptor (27–29). The GLP-1 peptide and several of the other peptide hormone ligands in this receptor family do not have a well defined secondary structure. Today, most small-molecule ligands have been identified in binding assays. This approach has not proven useful within this class of receptors, at least for the identification of agonists (28). We have used a functional screening assay to identify nonpeptide agonists for the human GLP-1 receptor.
Results
Screening for Small-Molecule Agonists.
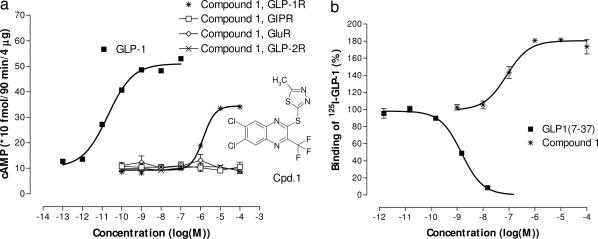
We first screened 500,000 discrete small molecules in a competition-binding assay. We found some hits, but they were not agonists and they were unspecific. The lack of hits by using the binding assay motivated us to change our strategy and perform a second functional screen. The complexity and costs of the functional assay forced us to select a chemically diverse explorative subset of 250,000 compounds for this screen. This screen, followed by structural modifications, led to the discovery of substituted quinoxalines that acted as hGLP-1 receptor agonists in several biochemical and cellular assays. 2-(2′-methyl)thiadiazolylsulfanyl-3-trifluoromethyl-6,7-dichloroquinoxaline (Fig. 1a, compound 1) did not activate the closely related GLP-2, glucagon, and GIP receptors. In radioligand-binding experiments, the compound did not, as expected, displace GLP-1. However, the binding of the peptide to the receptor was augmented by compound 1 in a concentration-related manner (Fig. 1b).
Fig. 1.
Structure of compound 1 and effects on the cloned human GLP-1 receptor and the closely related GIP, GLP-2, and glucagon receptors, all expressed in BHK cells. (a) cAMP functional assay by using either the cloned human GLP-1, GLP-2, glucagon, or GIP receptors. The EC50 value for GLP-1 and compound 1 was 23 pM and 1.4 μM, respectively. (b) Binding assay for the cloned human GLP-1 receptor. Both assays were carried out by using plasma membranes prepared from BHK cells expressing the different cloned human receptors. Data are from one of three to five identical experiments, in each experiment all concentrations were tested in triplicate.
Identification of More Potent Agonists.
Single compound and combinatorial medicinal chemistry was applied in an effort to convert compound 1 to a more potent agonist [synthesis in supporting information (SI) Schemes 1–3 and SI Text]. However, more potent compounds often had a bell-shaped dose–response curve, as shown for compound 2 in Fig. 2a. For compound 2 and several other compounds with similar bell-shaped dose–response curves, we determined by mass spectroscopy whether the compounds were actually in solution at the highest concentrations in the assay, which they all were (data not shown). Compound 2 was the most potent agonist we obtained, the EC50 value was 101 ± 21 nM (mean ± SEM, n = 7).
Fig. 2.
Structure of compound 2 and mechanistic functional data (cAMP) by using the cloned human GLP-1 receptor. (a) Activation of the GLP-1 receptor by GLP-1 and compound 2. (b) Antagonism of forskolin-induced cAMP by using the cloned human glucagon receptor. (c) Dose–response curves of GLP-1 and compound 2 and GLP-1 receptor antagonist exendin (9–39) added to fixed half-maximal concentrations of either GLP-1 or compound 2, respectively. (d) Potentiation of GLP-1 activity by compound 2. Dose–response curves for GLP-1 in the absence or presence of three different fixed concentrations of compound 2. For a and b, data are from one of three identical experiments with samples in triplicate. For c and d, data from 3 identical experiments were pooled and normalized.
We identified several compounds with EC50 values in the 2–300 nM range. However, all had bell-shaped dose–response curves. These compounds inhibited glucagon and forskolin-induced cAMP production at the closely related glucagon receptor when present in the high concentrations that corresponded to the downhill side of the bell-shaped dose–response curve (Fig. 2b). Importantly, they remained selective agonists for the GLP-1 receptor. During the optimization of this quinoxaline series, most compounds had bell-shaped dose–response curves, but proper selection of the substituents resulted in compounds that had normal sigmoid dose–response curves (SI Fig. 6).
Molecular Mechanism of Optimized Agonist.
The compounds were not antagonized by the selective GLP-1 receptor antagonist, exendin (9–39). Exendin (9–39) is a fragment of a close analog of GLP-1 and must be expected to bind at the orthosteric agonist-binding site. Shown in Fig. 2c, exendin (9–39) inhibited the cAMP formation by GLP-1, but not compound 2.
The ability of the agonist to activate the GLP-1 receptor as measured by cAMP accumulation seemed closely correlated with its ability to augment the binding of [125I]GLP-1 to the receptor. The mechanism behind the phenomenon was investigated further in a saturation-binding experiment measuring the affinity and number of binding sites for GLP-1 in the absence or presence of compound 2, shown in Fig. 3a. From the transformed plot in Fig. 3b, it is evident that particularly the slope of the curves, representing the affinity constant Kd, is different (from 0.510 ± 0.090 to 0.190 ± 0.020 nM in the absence and presence of compound 2, respectively, mean ± SD). The intersection with the x axis, which represents the number of binding sites, only changed marginally (5.0 ± 1.1 and 7.3 ± 0.9 pM, respectively). The augmented [125I]GLP-1 binding in Fig. 1b thus represents an apparent increase of GLP-1 affinity mediated by compound 2.
Fig. 3.
Saturation plot and Scatchard analysis for GLP-1 radioligand binding to the cloned human GLP-1 receptor, in the absence or presence of compound 2. (a) Saturation plot with GLP-1 + 100 nM compound 2 and GLP-1 alone. (b) Scatchard plot of data from a. 125I-GLP-1 (7–36)amide (80 kBq/pmol) was dissolved in buffer and added in amounts ranging from 600,000 cpm down to ≈10,000 cpm per well. Data are from one of two identical experiments with samples in triplicate.
In functional experiments, we investigated whether we could detect an increased potency of GLP-1 in the presence of compound 2. Fig. 2d shows GLP-1 dose–response curves in the presence of increasing concentrations of compound 2. We did not find an increased potency (EC50 was 8.0, 13.0, 9.6, and 18.0 pM for GLP-1 alone and in the presence of 0, 10, 30, and 100 nM compound 2, respectively). The difference in actual affinity and potency of GLP-1 is rather large (Kd, 510 pM; EC50, 8 pM) in this cloned human GLP-1 receptor cell line. The cell line has many spare receptors because of overexpression of the receptor, which may explain why we cannot detect an increased potency in the presence of compound 2. From Fig. 2d, it also may be concluded that compound 2 did not increase the efficacy of GLP-1, but only potentiated GLP-1-induced receptor activation.
Optimized Small-Molecule Agonists Specifically Stimulate Insulin Secretion.
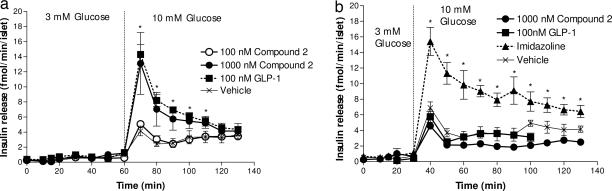
We continued to characterize compound 2 with respect to its specificity for the GLP-1 receptor by using islets isolated from normal and GLP-1 receptor knockout mice. We measured insulin release in perifusion experiments with islets isolated from CD1 wild-type and CD1 GLP-1 receptor knockout mice by using low- or high-glucose concentrations. Using islets from CD1 wild-type mice, GLP-1 and compound 2 at 100 nM and 1,000 nM, respectively, potentiated glucose (10 mM) induced insulin release equally well and with a similar time course, a 3-fold enhancement compared with 10 mM glucose alone (Fig. 4a). Compound 2 at 100 nM had no effect compared with glucose alone. Neither GLP-1 nor compound 2 influenced insulin release at 3 mM glucose, demonstrating that compound 2, like GLP-1, is strongly glucose-dependent in its potentiation of insulin secretion. To confirm that the glucose-dependent potentiation of insulin secretion of compound 2 is mediated through the GLP-1 receptor, similar perifusion studies were carried out in islets isolated from CD1 GLP-1 receptor knockout mice. We first investigated whether the lack of GLP-1 receptor in any way impaired the insulin secretion pathway and kinetics in these mice. The insulin secretion and kinetics induced by 10 mM glucose appeared normal compared with islets from wild-type CD1 mice (Fig. 4b). Likewise, these islets showed a normal insulin response when stimulated with a GLP-1 receptor independent insulin secretagoue, an imidazoline compound (30). However, neither GLP-1 nor compound 2 was able to potentiate glucose-mediated insulin secretion in the CD1 GLP-1 receptor knockout mice, further supporting that the effect of compound 2 is specifically mediated through the GLP-1 receptor.
Fig. 4.
Insulin secretion from islets isolated from normal and GLP-1 receptor knockout mice. (a) Islets from CD1 wild-type mice. After a preperifusion the concentration of glucose was 3 mM from 0–60 min and 10 mM after 60 min. At 10 min, 100, and 1,000 nM compound 2, 100 nM GLP-1 or glucose alone was added and removed again at 70 min. Data are shown as mean ± SEM for three groups of 30 islets. Asterisks indicate a significant difference between GLP-1 and the 1,000 nM compound 2 vs. glucose alone. (b) Islets from CD1 GLP-1 receptor knockout mice. After the preperifusion, the concentration of glucose was 3 mM from 0 to 30 min and 10 mM after 30 min. At 10 min, 1,000 nM compound 2 and 100 nM GLP-1, 10 μM imidazoline control compound NNC77-0074, or vehicle were added and removed again at 60 min. Groups of islets were perifused the day after isolation. Before perifusate collection was started, all groups of islets were perifused with 3 mM glucose for 30 min to establish stable basal rates of release. Data are shown as mean ± SEM for three groups of 30 islets. Asterisks indicate a significant difference between imidazoline vs. all other groups.
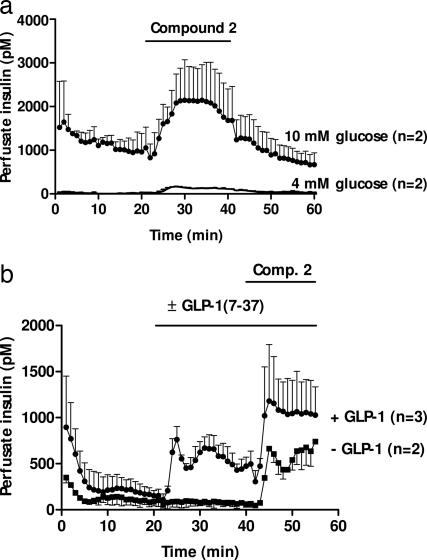
Also under ex vivo experimental conditions, in the rat-perfused pancreas, compound 2 potentiated glucose-induced insulin release (Fig. 5a). Thus, a profound stimulation of insulin secretion was observed at 10 mM glucose, whereas a much smaller stimulation of insulin secretion was observed at 4 mM glucose. Similar historic in-house perfused pancreas data comparing the response to GLP-1 at 5 and 10 mM glucose demonstrate the same qualitative phenomenon (unpublished observations). We found that compound 2 and GLP-1 had additive effects on insulin release in the same order of magnitude (Fig. 5b).
Fig. 5.
Insulin release from perfused rat pancreas. (a) compound 2 (10 μM) was administered at t = 20–40 min with 10 mM glucose or with 4 mM glucose. (b) compound 2 (10 μM) was administered at t = 40–55 min, with 7 mM glucose and with 20 pM GLP-1 or without GLP-1. Data are presented as mean ± SD of two to three experiments.
Discussion
Since the discovery that morphine and related alkaloids are agonists for the opioid receptors, researchers have sought other nonpeptide agonists for peptide GPCRs by using two diverse approaches. It originally was proposed that the message-containing amino acid side chains and spatial orientation of the peptides could be mimicked by small molecules, assuming that the peptide and the small-molecule agonists would occupy the same binding site in the receptor. This approach has proven unsuccessful, at least no small-molecule agonists have appeared that seem to bind to the same binding site as the peptide hormone. Many of the identified small-molecule agonists were discovered through random or directed functional high-throughput screening of large chemical libraries, which also would identify agonists that do not necessarily bind to the peptide-binding site, the orthosteric site. Peptides are thought to bind to the extracellular portion of the receptor, whereas small molecules may bind more deeply in the transmembrane region (31, 32). Several GPCR agonists that affect the receptor response to the natural ligands via a noncompetitive site or allosteric site have during recent years been found for receptor families A and C, but so far not for family B.
We now have identified a series of small-molecule agonists for a receptor, where the endogenous ligand is a peptide that does not have a well defined secondary structure. The receptor belongs to the B family of the GPCR superfamily, where no small-molecules agonists have been identified so far. The compounds were identified through a functional screen and did not compete for binding with the peptide ligand. The compounds later were discovered to increase binding of the radiolabeled peptide ligand isotope and, thus, could theoretically have been identified in a binding screen if it had been set up to look for increased tracer binding. The lack of competition with GLP-1 would be consistent with the existence of an allosteric-binding site for the compounds on the GLP-1 receptor, as also described for other small-molecule ligands of GPCRs (33, 34), especially the muscarinic and metabotropic glutamate receptors (35, 36). However, the compounds described here are also agonists themselves. Recently, a review was published discussing these different new types of ago-allosteric modulators and other types of allostery (37). According to the classification suggested in this review, these compounds are to be called ago-allosteric modulators. However, they do not completely fit into the categories described, which are positive, neutral, and negative ago-allosteric modulators. They all increase Emax, but the difference lies in whether they increase, decrease, or do not affect agonist potency. Examples of positive allosteric modulators are Zn++ on the MC1 and MC4 receptors, CGP7930 on the GABAB receptor, and L-692,429 on the ghrelin receptor (38–40). Our compound 2 causes only a very small increase in Emax at the highest concentration tested, so perhaps a different category exists, which are positive ago-allosteric modulators, but with unaltered Emax.
The most potent compounds we identified all had bell-shaped dose–response curves. This inhibition, or apparently antagonistic effect, was unspecific as shown by using forskolin stimulation on the cloned human glucagon receptor. The unspecific nature of this antagonist effect was confirmed with a series of related compounds by using carbachol-induced IP3 accumulation on the muscarin M1 receptor (data not shown). A similar bell-shaped dose–response curve also was described for a G-CSF small-molecule mimetic (41). However, we were able to identify structurally related compounds with no inhibition at higher concentrations.
We have shown that compound 2 is an ago-allosteric modulator. It does not interact with the peptide hormone-binding site, the orthostatic site, because a specific peptide-derived antagonist, exendin (9–39), could not antagonize compound 2. Our saturation-binding experiment showed that the addition of compound 2 caused an apparent increase in the affinity of the peptide ligand for its receptor. Studies involving dynamic fluorescence techniques have demonstrated the existence of several agonist-induced conformations of GPCRs (42), and the existence of multiple receptor conformations now seems generally accepted (43). Our saturation binding data provide pharmacological support for the existence of at least two distinct agonist induced conformations for the GLP-1 receptor, with different affinity for GLP-1. Much emphasis recently has been put on receptor dimerization, both homo- and heterodimerization, and its role in activation and inhibition of GPCRs (44). It is being hypothesized that an allosteric activator could be acting via stimulation of receptor dimerization (45). It is possible that compound 2 could be stimulating homodimerization.
Finally, we have shown that compound 2 specifically releases insulin from wild-type mouse islets, but not islets isolated from GLP-1 receptor knockout mice. Importantly, control experiments showed the islets from knockout mice to release insulin from the control substance imidazoline, acting via a different mechanism on insulin secretion. Also, the insulin secretion was shown to be glucose-dependent in a fashion similar to GLP-1, in both perifused mouse islets, and the perfused rat pancreas.
In conclusion, we have demonstrated that small-molecule agonists for a receptor from the GPCR B family can be identified and that these molecules act both as ago-allosteric modulators, being both agonists and allosteric modulators. Our findings have implications for future searches for small-molecule peptide receptor agonists, where traditional screening by receptor binding assays have not seemed to result in the discovery of small-molecule agonists for the GLP-1 receptor. Even though our studies have not yet resulted in the identification of potent drug-like structures, they represent an important tool toward identifying orally active GLP-1 receptor agonists.
Methods
Receptor Functional Assays.
Plasma membranes from BHK cells expressing the GLP-1, glucagon, GIP, and GLP-2 receptors were prepared as described for the GLP-1 receptor (14). The functional receptor assay was carried out by measuring cAMP as a response to stimulation by GLP-1 or small-molecule activators. Incubations were carried out in 96-well microtiter plates in a total volume of 140 μl and with the following final concentrations: 50 mM Tris·HCl/1 mM EGTA/1.5 mM MgSO4/1.7 mM ATP/20 mM GTP/2 mM 3-isobutyl-1-methylxanthine (IBMX) 0.01 wt/vol % Tween 20, pH 7.4. Compounds were dissolved and diluted in DMSO and added in 10 μl (resulting DMSO 7.1%); this DMSO concentration does not influence assay (see SI Fig. 7). Peptides were dissolved and diluted in buffer, except for GIP, which was dissolved in acetic acid and diluted to pH 7.4. Plasma membrane was added to each well, and the mixture was incubated for 90 min at room temperature in the dark with shaking. cAMP was measured by a scintillation proximity assay (RPA 542; GE Healthcare, Little Chalfont, U.K.). Prism GraphPad software (GraphPad, San Diego, CA) was used for all curve fitting. Sigmoidal dose–response fitting was used. Compounds with unspecific inhibition at high concentrations were plotted point-to-point without curve fitting.
Receptor Binding Assay.
Binding curve experiments were carried out in 96-well microtiter plates (MADV N65; Millipore). The buffer used was 25 mM Hepes/0.1% BSA, pH 7.4. GLP-1 was dissolved and diluted in buffer. Compounds were dissolved and diluted in 100% DMSO (resulting DMSO 4.4%). 125I-GLP-1 (7–36)amide (80 kBq/pmol) was dissolved in buffer and added at 50,000 cpm per well. Nonspecific binding was determined with 1 μM GLP-1. Buffer (165 μl) with or without GLP-1 was added to each well, followed by 10 μl of compound 1/25 μl of plasma membrane/25 μl of tracer. The plates were incubated for 1 h at 37°C. The bound tracer and the unbound tracer were separated by vacuum filtration (Millipore vacuum manifold). The plates were washed once.
The saturation binding experiments were carried out in the same assay system. Different dilutions of tracer (starting from 600,000 cpm per well) were made in the assay buffer. Nonspecific binding was determined with 1 μM GLP-1. Buffer (165 μl) with or without GLP-1 were added to each well, followed by 10 μl of compound 2/25 μl of plasma membrane/25 μl of tracer. The plates were incubated for 1 h at 37°C. The bound tracer and the unbound tracer were separated by vacuum filtration (Millipore vacuum manifold). The plates were washed once.
Prism GraphPad software was used for all curve fitting. The binding curves were fitted as one-site competition, and the saturation data were fitted as two-site binding.
Insulin Secretion Assays.
Mouse islet isolation and assay were performed as previously described (46). In brief, the islets were isolated from adult CD1 wild-type mice or CD1 GLP-1 receptor knockout mice by collagenase digestion and kept in tissue culture overnight in 5 ml of RPMI medium 1640 supplemented with 10% newborn calf serum. The perifusion buffer was 20 mM Hepes (pH 7.4)/5 mM NaHCO3/4.7 mM KCl/2.6 mM CaCl2/2 mM glutamine/1.2 mM KH2PO4/4.7 mM MgSO4 (pH 7.4) supplemented with 100 units/ml penicillin/100 μg/ml streptomycin/2 g/liter human serum albumin and different concentrations of d-glucose. Peptides were dissolved and diluted in buffer. Compounds were dissolved in DMSO and then diluted in buffer (final DMSO concentration was 0.1% or less). In each experiment, 30 islets were added in buffer to the top of a column consisting of 300 ml of Bio-Gel P-2 beads (Bio-Rad Laboratories, Richmond, CA). The islets were perifused at 37°C and at a flow rate of 0.30 ml/min in a Brandel perifusion equipment with Superfusion 2000 computer control (Brandel, Gaithersburg, MD).
The rat pancreas was isolated from male SD rats and perfused at 37°C in a custom made Plexiglas chamber by using a modification of procedures described in ref. 47. The perfusate consisted of an oxygenated Krebs Ringer solution with 0.25% BSA/4% Dextran T-70/2 mM calcium/≈4 or 10 mM glucose. Peptides were dissolved and diluted in buffer. Compounds were dissolved in DMSO and diluted in buffer (final DMSO concentration was 0.25%). The system used two peristaltic pumps (Gilson Minipuls 3) used in parallel with flows merging before the pancreas. Pump 1 administered oxygenated buffer containing no BSA and no glucose and provided 71% of the total flow of 1.9 ml/min, whereas pump 2 administered buffer containing BSA and glucose. Fractions of the effluent were collected every minute.
Insulin released was determined by using a guinea pig antiserum, mono-125I-[TyrA14] human insulin (Novo Nordisk) as a tracer, and rat insulin (Novo Nordisk) as the standard.
Supporting Information.
Additional data can be found in SI Schemes 1–3, SI Figs. 6 and 7, and SI Text.
Supplementary Material
Acknowledgments
We thank Karin Hamborg Albrechtsen, Tina Olsen, and Marianne Bojsen Jappe for their excellent technical assistance; Christine Thomas, James Lakis, and Shelley Aytes for their scientific assistance; Nigel Birdshall, Hector BeltrandelRio, Hans Schambye, and the Department of Molecular Pharmacology at Novo Nordisk for the valuable and constructive comments to the manuscript; and Johan Selmer, Niels Fiil, Behrendt Lundt and Alex Polinsky for their enthusiastic support.
Abbreviations
- GLP
glucagon-like peptide
- GPCR
G protein-coupled receptor.
Footnotes
Conflict of interest statement: All of the authors (except J.J.H. and D.K.) are employees of pharmaceutical companies, which have a financial interest in this article. All authors except J.J.H. also have shares or stocks. J.J.H. is a consultant for Novo Nordisk.
This article is a PNAS direct submission.
See Commentary on page 689.
This article contains supporting information online at www.pnas.org/cgi/content/full/0605701104/DC1.
References
- 1.Kieffer TJ, Habener JF. Endocr Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 2.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 3.Zander M, Madsbad S, Madsen JL, Holst JJ. Lancet. 2002;359:824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 4.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, et al. Nat Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 5.Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Endocrinology. 2003;144:2242–2252. doi: 10.1210/en.2003-0007. [DOI] [PubMed] [Google Scholar]
- 6.United Kingdom Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Bonadonna RC, Ferrannini E. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- 9.Unger RH, Orci L. Lancet. 1975;1:14–16. doi: 10.1016/s0140-6736(75)92375-2. [DOI] [PubMed] [Google Scholar]
- 10.Vilsboll T, Agerso H, Krarup T, Holst JJ. J Clin Endocrinol Metab. 2003;88:220–224. doi: 10.1210/jc.2002-021053. [DOI] [PubMed] [Google Scholar]
- 11.Larsen J, Hylleberg B, Ng K, Damsbo P. Diabetes Care. 2001;24:1416–1421. doi: 10.2337/diacare.24.8.1416. [DOI] [PubMed] [Google Scholar]
- 12.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Diabetes. 1995;44:1126–1131. doi: 10.2337/diab.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 14.Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thogersen H, Wilken M, Agerso H. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 15.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. Diabetes. 2004;53:1187–1194. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 16.Thorens B. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runge S, Wulff BS, Madsen K, Brauner-Osborne H, Knudsen LB. Br J Pharmacol. 2003;138:787–794. doi: 10.1038/sj.bjp.0705120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, Salituro G, Szalkowski D, Li Z, Zhang Y, Royo I, Vilella D, Diez MT, Pelaez F, Ruby C, et al. Science. 1999;284:974–977. doi: 10.1126/science.284.5416.974. [DOI] [PubMed] [Google Scholar]
- 19.Naranda T, Wong K, Kaufman RI, Goldstein A, Olsson L. Proc Natl Acad Sci USA. 1999;96:7569–7574. doi: 10.1073/pnas.96.13.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy KJ, Darcy MG, Delorme E, Dillon SB, Eppley DF, Erickson-Miller C, Giampa L, Hopson CB, Huang Y, Keenan RM, et al. J Med Chem. 2001;44:3730–3745. doi: 10.1021/jm010283l. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S, Yamamura Y, Itoh S, Hirano T, Tsujimae K, Aoyama M, Kondo K, Ogawa H, Shinohara T, Kan K, et al. Br J Pharmacol. 2000;129:1700–1706. doi: 10.1038/sj.bjp.0703221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ankersen M, Crider M, Liu S, Ho B, Andersen HS, Stidsen C. J Am Chem Soc. 1998;120:1368–1373. [Google Scholar]
- 23.Aramori I, Zenkoh J, Morikawa N, Asano M, Hatori C, Sawai H, Kayakiri H, Satoh S, Inoue T, Abe Y, et al. Mol Pharmacol. 1997;52:16–20. doi: 10.1124/mol.52.1.16. [DOI] [PubMed] [Google Scholar]
- 24.Perlman S, Schambye HT, Rivero RA, Greenlee WJ, Hjorth SA, Schwartz TW. J Biol Chem. 1995;270:1493–1496. doi: 10.1074/jbc.270.4.1493. [DOI] [PubMed] [Google Scholar]
- 25.Aquino CJ, Armour DR, Berman JM, Birkemo LS, Carr RAE, Croom DK, Dezube M, Dougherty RW, Ervin GN, Grizzle MK, et al. J Med Chem. 1996;39:562–569. doi: 10.1021/jm950626d. [DOI] [PubMed] [Google Scholar]
- 26.Smith RG, Cheng K, Schoen WR, Pong SS, Hickey G, Jacks T, Butler B, Chan WW, Chaung LY, Judith F, et al. Science. 1993;260:1640–1643. doi: 10.1126/science.8503009. [DOI] [PubMed] [Google Scholar]
- 27.Ling A, Hong Y, Gonzalez J, Gregor V, Polinsky A, Kuki A, Shi S, Teston K, Murphy D, Porter J, et al. J Med Chem. 2001;44:3141–3149. doi: 10.1021/jm000547o. [DOI] [PubMed] [Google Scholar]
- 28.Cascieri MA, Koch GE, Ber E, Sadowski SJ, Louizides D, de Laszlo SE, Hacker C, Hagmann WK, MacCoss M, Chicchi GG, et al. J Biol Chem. 1999;274:8694–8697. doi: 10.1074/jbc.274.13.8694. [DOI] [PubMed] [Google Scholar]
- 29.Hoare SRJ, Brown BT, Santos MA, Malany S, Betz SF, Grigoriadis DE. Biochem Pharmacol. 2006;72:244–255. doi: 10.1016/j.bcp.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Hoy M, Olsen HL, Andersen HS, Bokvist K, Buschard K, Hansen J, Jacobsen P, Petersen JS, Rorsman P, Gromada J. Eur J Pharmacol. 2003;466:213–221. doi: 10.1016/s0014-2999(03)01537-1. [DOI] [PubMed] [Google Scholar]
- 31.Cascieri MA, Koch GE, Ber E, Sadowski SJ, Louizides D, de Laszlo SE, Hacker C, Hagmann WK, MacCoss M, Chicchi GG, et al. J Biol Chem. 1999;274:8694–8697. doi: 10.1074/jbc.274.13.8694. [DOI] [PubMed] [Google Scholar]
- 32.Schambye HT, Hjorth SA, Bergsma DJ, Sathe G, Schwartz TW. Proc Natl Acad Sci USA. 1994;91:7046–7050. doi: 10.1073/pnas.91.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rees S, Morrow D, Kenakin T. Recept Channels. 2002;8:261–268. [PubMed] [Google Scholar]
- 34.Birdsall NJM, Browning C, Hern J, Lazareno S. Med Chem Res. 2004;13:52–62. [Google Scholar]
- 35.Jakubik J, Bacakova L, Lisa V, El-Fakahany EE, Tucek S. Proc Natl Acad Sci USA. 1996;93:8705–8709. doi: 10.1073/pnas.93.16.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagano A, Ruegg D, Litschig S, Stoehr N, Stierlin C, Heinrich M, Floersheim P, Prezeau L, Carroll F, Pin JP, et al. J Biol Chem. 2000;275:33750–33758. doi: 10.1074/jbc.M006230200. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz TW, Holst B. J Recept Signal Transduct Res. 2006;26:107–128. doi: 10.1080/10799890600567570. [DOI] [PubMed] [Google Scholar]
- 38.Holst B, Elling CE, Schwartz TW. J Biol Chem. 2002;277:47662–47670. doi: 10.1074/jbc.M202103200. [DOI] [PubMed] [Google Scholar]
- 39.Holst B, Brandt E, Bach A, Heding A, Schwartz TW. Mol Eendocrinol. 2005;19:2400–2411. doi: 10.1210/me.2005-0059. [DOI] [PubMed] [Google Scholar]
- 40.Binet V, Brajon C, Le Corre L, Acher F, Pin JP, Prezeau L. J Biol Chem. 2004;279:29085–29091. doi: 10.1074/jbc.M400930200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian SS, Lamb P, King AG, Miller SG, Kessler L, Luengo JI, Averill L, Johnson RK, Gleason JG, Pelus LM, et al. Science. 1998;281:257–259. doi: 10.1126/science.281.5374.257. [DOI] [PubMed] [Google Scholar]
- 42.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. J Biol Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 43.Kenakin T. Trends Pharmacol Sci. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Bai M. Cell Signal. 2004;16:175–186. doi: 10.1016/s0898-6568(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 45.Jensen AA, Spalding TA. Eur J Pharm Sci. 2004;21:407–420. doi: 10.1016/j.ejps.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Fuhlendorff J, Rorsman P, Kofod H, Brand CL, Rolin B, MacKay P, Shymko R, Carr RD. Diabetes. 1998;47:345–351. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- 47.Sturis J, Pugh WL, Tang JP, Ostrega DM, Polonsky JS, Polonsky KS. Am J Physiol. 1994;267:E250–E259. doi: 10.1152/ajpendo.1994.267.2.E250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.