Abstract
Tight control of T-cell proliferation and effector function is essential to ensure an effective but appropriate immune response. Here, we reveal that this is controlled by the metalloprotease-mediated cleavage of LAG-3, a negative regulatory protein expressed by all activated T cells. We show that LAG-3 cleavage is mediated by two transmembrane metalloproteases, ADAM10 and ADAM17, with the activity of both modulated by two distinct T-cell receptor (TCR) signaling-dependent mechanisms. ADAM10 mediates constitutive LAG-3 cleavage but increases ∼12-fold following T-cell activation, whereas LAG-3 shedding by ADAM17 is induced by TCR signaling in a PKCθ-dependent manner. LAG-3 must be cleaved from the cell surface to allow for normal T-cell activation as noncleavable LAG-3 mutants prevented proliferation and cytokine production. Lastly, ADAM10 knockdown reduced wild-type but not LAG-3−/− T-cell proliferation. These data demonstrate that LAG-3 must be cleaved to allow efficient T-cell proliferation and cytokine production and establish a novel paradigm in which T-cell expansion and function are regulated by metalloprotease cleavage with LAG-3 as its sole molecular target.
Keywords: ADAM, LAG-3, metalloproteases, shedding, T-cell function
Introduction
Metalloproteases have long been considered viable therapeutic targets for a variety of important human diseases such as cancer, cardiovascular disease, arthritis and multiple sclerosis (Baker et al, 2002; Overall and Kleifeld, 2006). However, many of the clinical trials using broad-range metalloprotease inhibitors have produced disappointing results, in part owing to unexpected side effects. This is complicated by the broad range of molecules targeted by these metalloproteases. Matrix metalloproteases (MMP), membrane-tethered MMPs and the zinc-dependent a disintegrin and metalloproteinases (ADAM), have all been shown to shed proteins from the cell surface (Black and White, 1998; Becherer and Blobel, 2003; Seals and Courtneidge, 2003; Parks et al, 2004; Blobel, 2005). Among these, two members of the ADAM family of metalloproteases, ADAM10 (Kuzbanian) and ADAM17 (TACE), are known to be important cell surface sheddases for a diverse array of transmembrane proteins of immunological importance, such as Notch, EGFR ligands, TNF-α, TNF-α receptor, CD44, CD62L (L-selectin) and CD23 (Black and White, 1998; Becherer and Blobel, 2003; Blobel, 2005; Maretzky et al, 2005; Reiss et al, 2005; Weskamp et al, 2006). For some time, metalloprotease inhibitors have been known to inhibit T-cell proliferation but the target molecule and mechanism that is inhibited remain unknown.
T-cell proliferation and function following antigenic stimulation is a tightly regulated process. Inappropriate or uncontrolled expansion of activated T cells is regulated by activation-induced cell death, downregulation of stimulatory molecules and/or upregulation of inhibitory molecules. Lymphocyte activation gene-3 (LAG-3; CD223) has recently been shown to be a novel inhibitory molecule that is required for maximal regulatory T-cell function, and controls effector T-cell expansion and homeostasis (Huang et al, 2004; Workman et al, 2004; Workman and Vignali, 2005). Importantly, these studies clearly show that LAG-3 has cell-intrinsic regulatory activity, but the physiological importance of this is unclear.
LAG-3 is related to CD4 in chromosomal location, exon organization and structure (Triebel et al, 1990; Bruniquel et al, 1997). They also share the same ligand, MHC class II, although LAG-3 binds with a much higher affinity (Triebel et al, 1990; Bruniquel et al, 1998; Workman et al, 2002a, 2002b). We have shown that binding to MHC class II molecules and a conserved KIEELE motif in the LAG-3 cytoplasmic domain are essential for its function. LAG-3 clearly possesses both cell-intrinsic and cell-extrinsic regulatory activity (Huang et al, 2004; Workman and Vignali, 2005). Ectopic expression of LAG-3 on effector T cells controls their proliferation and cytokine production. It is noteworthy that all activated T cells and NK cells express LAG-3. It is unclear what effect the presence of this negative regulatory pressure might have on their ability to proliferate and function, and if there are any mechanisms present that modulate LAG-3 activity.
We recently observed that LAG-3 is cleaved within the short connecting peptide (CP) between the membrane-proximal D4 domain and the transmembrane domain, resulting in the release of a soluble form of LAG-3 (sLAG-3) (Li et al, 2004). Indeed, significant amounts of sLAG-3 are found in murine sera (∼200 ng/ml), which increases following T-cell stimulation in vivo. LAG-3 is known to have inhibitory activity, yet is expressed by all activated T cells. In this study, we tested our hypothesis that there was a direct link between the ability of metalloproteases to regulate T-cell proliferation and effector function and the possibility that LAG-3 may be the target of metalloprotease activity.
Results
Metalloprotease inhibition reduced wild-type but not LAG-3−/− T-cell proliferation
We first tested whether the metalloprotease inhibitor GM6001, which is stable in long-term cultures (Ethell et al, 2002), can reduce T-cell proliferation. As anticipated, GM6001 significantly reduced the peptide-induced proliferation of wild-type CD4+ OTII T-cell receptor (TCR)-transgenic T cells (Figure 1A). We have previously shown that LAG-3 is cleaved from the cell surface and speculated that this might be mediated by metalloproteases as they have been shown to cleave many cell surface proteins of immunological importance (Black and White, 1998; Becherer and Blobel, 2003; Seals and Courtneidge, 2003; Parks et al, 2004; Blobel, 2005). This was confirmed by our observation that GM6001 blocked the generation sLAG-3 in a dose-dependent manner (Figure 1B). We then asked if GM6001 affected the proliferation of LAG−/− T cells. To our surprise it did not, suggesting that the GM6001-mediated suppression of T-cell proliferation is LAG-3-dependent (Figure 1C and D). Taken together, these data demonstrate that the metalloprotease inhibitor GM6001 can reduce T-cell proliferation and that a candidate molecular target in mediating this effect is LAG-3. These data also raise the possibility that prevention of LAG-3 cleavage may inhibit T-cell proliferation.
Figure 1.
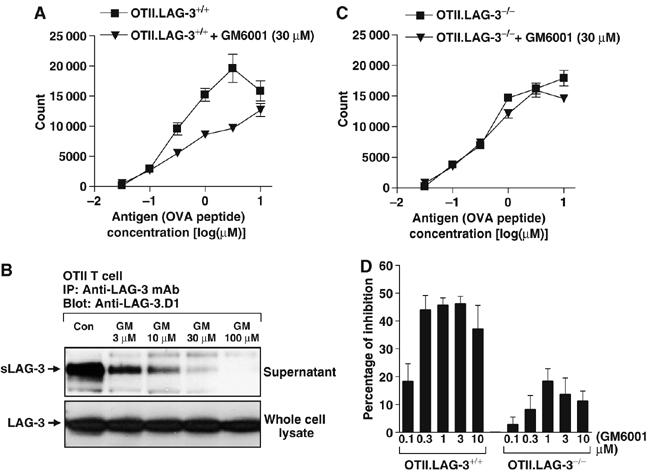
Blocking LAG-3 cleavage by metalloprotease inhibitor GM6001 inhibits T-cell activation. (A, C, D) LAG-3 wild-type OTII TCR-transgenic T cells (A) or LAG-3 knockout OTII TCR-transgenic T cells (C) were MACS purified and activated with OVA326−339 in the presence of irradiated splenocytes for 3 days. Cultures were pulsed with [3H]thymidine for the last 8 h. Data are representative of three independent experiments. The reduced [3H]thymidine incorporation seen with LAG−/− T cells is due to increased activation-induced cell death that often occurs following in vitro stimulation (Workman and Vignali, 2003). (D) Percentage of inhibition on T-cell proliferation by GM6001 treatment was calculated from three individual experiments. (B) Whole splenocytes from OTII TCR transgenic mice were activated with OVA326−339 in the presence of various concentrations of GM6001 (GM) for 3 days. All cells and supernatants were collected and tested as indicated.
ADAM10 mediates constitutive LAG-3 cleavage
To ensure that LAG-3 cleavage was restricted to metalloproteases, we first tested the effect of various protease inhibitors on sLAG-3 production by a LAG-3+ CHO transductant. These included an alternate metalloprotease inhibitor (TAPI-1) and inhibitors of serine proteases (Pefabloc and leupeptin), aspartyl proteases (pepstatin), cysteine proteases (E64), gamma-secretases (DAPT) and proteasome proteases (MG-132 and LLNL). Of these, the only inhibitor that blocked sLAG-3 production was the metalloprotease inhibitor TAPI-1, consistent with our previous results (Figure 2A).
Figure 2.
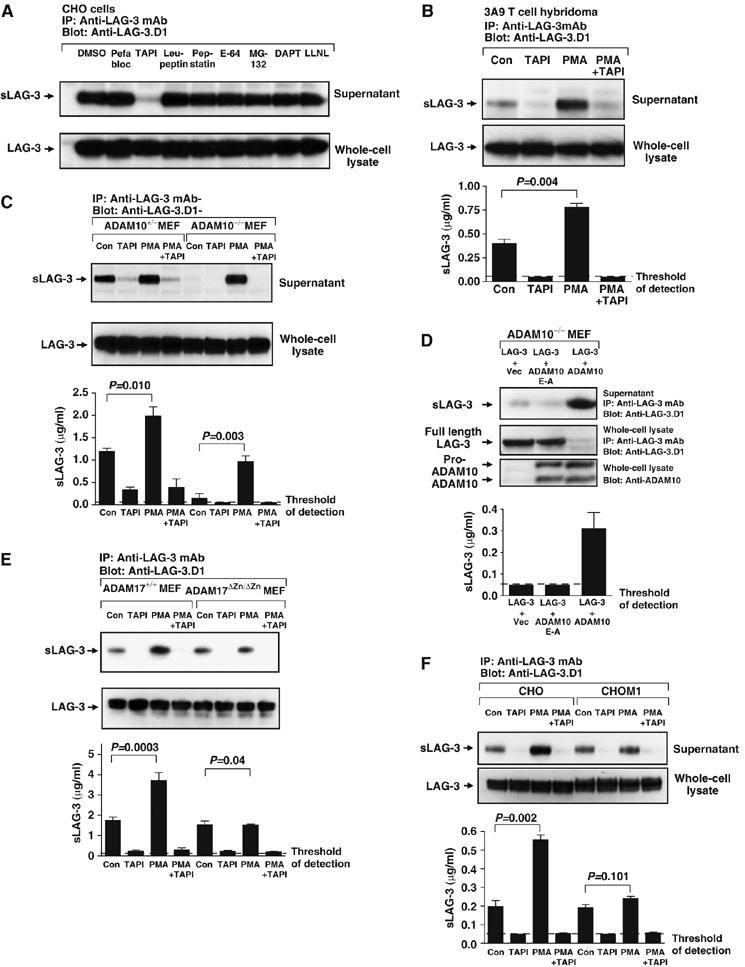
ADAM10 and ADAM17 cleave LAG-3. (A) LAG-3+-transduced CHO cells were cultured in medium for 1 h±various protease inhibitors (0.5 mM Pefabloc, 100 μM TAPI, 10 μM leupeptin, 10 μM pepstatin A, 10 μM E-64, 25 μM MG-132, 1 μM DAPT or 25 μM LLNL) as indicated in the figure. Supernatants were collected and cells were lysed. Both were immunoprecipitated with the anti-LAG-3 mAb and eluted proteins were separated by SDS–PAGE and blotted with anti-LAG-3.D1 antibody. (B, C, E, F) All cells (3A9T cell hybridomas (B), ADAM10−/+ or ADAM10−/− MEFs (C), ADAM17+/+ or ADAM17ΔZnΔ/Zn fibroblasts (E), CHO and CHO.M1 cells (F)) were transduced with LAG-3 retrovirus. Cells were cultured in medium with DMSO (control), 100 μM TAPI, 1 μM PMA or TAPI and PMA for 0.5 h (E) or 1 h (C). Supernatants and lysates were tested as above. (B–F) The concentration of sLAG-3 in cell culture medium was also assessed by ELISA. LAG-3 concentration was calculated using a standard curve generated with affinity-purified sLAG-3. Data are presented as the mean±s.e. of three separate experiments with P-values shown. (D) ADAM10−/− MEFs were cotransfected with LAG-3 in pMIC (LAG-3) and an IRES-GFP vector pIRES (Vec), a dominant negative bADAM10 cDNA in pIREScg (bADAM10E−A) or wild-type bADAM10 in pIREScg. CFP and GFP double-positive cells were sorted and cultured for another 2 days. Cells (1 × 106) were cultured in 12-well plates. Supernatant and lysate were tested as above. ADAM10 expression was confirmed by blotting whole-cell lysate with anti-ADAM10. The concentration of sLAG-3 in cell culture medium was measured by ELISA. Data are the mean±s.e. of three independent experiments.
We focused our attention on the ADAM family of metalloproteases, ADAM10 (Kuzbanian) and ADAM17 (TACE), as they are known to cleave many transmembrane proteins of immunological importance (Black and White, 1998; Becherer and Blobel, 2003; Blobel, 2005). Although the proteolytic activity of ADAM10 is generally constitutive, cleavage by ADAM17 can be induced by PMA (Sahin et al, 2004). Therefore, we first tested whether sLAG-3 production by the LAG-3+ 3A9T cell hybridoma was altered by PMA treatment. Production of sLAG-3 was significantly increased by 1 h PMA treatment (Figure 2B), implicating a role for ADAMs in LAG-3 shedding. This increase was not due to enhanced LAG-3 synthesis, as the total protein in whole-cell lysates was unchanged. It should be noted that sLAG-3 can be generated by multiple transduced or transfected cell types including T cells, CHO and 3T3 cells (Li et al, 2004), suggesting that the sheddase is ubiquitously expressed. Furthermore, shedding does not require LAG-3 ligation, cellular activation or the presence of serum-derived proteases or cofactors (Li et al, 2004) (Supplementary Figure S1A and B).
Initial analysis of serum sLAG-3 concentration in mice lacking ADAM9, 12, 15 and/or 17 suggested that these were not responsible for constitutive LAG-3 cleavage (Supplementary Figure S1C and D). As ADAM10 deficiency results in embryonic lethality (Hartmann et al, 2002), we assessed its role in LAG-3 cleavage using ADAM10−/− and ADAM10+/− MEFs transduced with LAG-3 encoding retrovirus. Strikingly, there was a 90% reduction in sLAG-3 production by ADAM10−/− MEFs compared with heterozygous control cells (Figure 2C). However, when the LAG-3+ ADAM10−/− MEFs were treated with PMA, sLAG-3 was still generated, suggesting that a different protease was responsible for PMA-induced LAG-3 cleavage. It is noteworthy that this increase was comparable to that seen following PMA induction of the ADAM10+/− control MEFs (increase in sLAG-3 production in the presence of PMA over control untreated cells: ADAM10+/−=0.81 μg/ml, ADAM10−/−=0.80 μg/ml) (Figure 2C).
To confirm that ADAM10 was responsible for constitutive LAG-3 cleavage, LAG-3.pMIC was cotransfected into ADAM10−/− MEFs with either bovine ADAM10 (bADAM10) in the GFP containing plasmid pIRES, an enzymatically inactive mutant (bADAM10E−A) or the empty vector control (Vec). Analysis of sLAG-3 production by CFP+/GFP+ MEFs demonstrated that LAG-3 cleavage was restored in the presence of active but not inactive bADAM10 (Figure 2D). In addition, surface LAG-3 expression was drastically reduced in bADAM10-expressing MEFs (Supplementary Figure S1E). Taken together, our data clearly show that ADAM10 is responsible for constitutive LAG-3 cleavage.
ADAM17 mediates PMA-induced LAG-3 cleavage
We established that ADAM17 was the PMA-inducible LAG-3 sheddase with two experiments. First, ADAM17ΔZn/ΔZn and wild-type Ras/Myc-transformed fibroblasts (Reddy et al, 2000) were transduced with retrovirus encoding LAG-3. sLAG-3 production by transduced ADAM17ΔZn/ΔZn fibroblasts was not increased after PMA treatment compared with the wild-type fibroblasts (Figure 2E). Second, we expressed LAG-3 on the ADAM17-deficient CHO-M1 cell line (Li and Fan, 2004; Villanueva de la et al, 2004) and assessed sLAG-3 production following PMA treatment. An ∼2.5-fold increase in sLAG-3 production by ELISA was seen with the wild-type CHO LAG-3 transfectant, whereas PMA treatment had no effect on the sLAG-3 production by the LAG-3+ CHO-M1 cell line (Figure 2F). In both experiments, constitutive LAG-3 cleavage was unaffected. Taken together, these data demonstrate that ADAM17 is responsible for the PMA-induced cleavage of LAG-3. Furthermore, our data show that there are two distinct metalloproteases (ADAM10 and ADAM17) that independently cleave LAG-3.
TCR signaling increases LAG-3 cleavage via two distinct pathways
Our observation that PMA treatment induced LAG-3 cleavage by ADAM17 suggested that this process might be controlled by a protein kinase C (PKC)-dependent signaling pathway. As PKC had been shown to play important roles in T-cell activation, we questioned whether ADAM17-mediated LAG-3 cleavage was regulated by the TCR signaling pathway. MACS-purified CD4+ OTII T cells were activated with OVA326−339 for 2 days. LAG-3 expression was confirmed by flow cytometry and sLAG-3 production was verified by Western blot. As shown above, constitutive sLAG-3 shedding was again increased by PMA treatment and inhibited by TAPI-1 (Figure 3A). Interestingly, TCR crosslinking by anti-CD3ɛ antibody (2C11) also stimulated T cells to produce more sLAG-3, which could be inhibited by TAPI-1 and the tyrosine kinase inhibitor genistein. This increase was not due to induction of LAG-3 synthesis, as the total protein in whole-cell lysates was unchanged.
Figure 3.
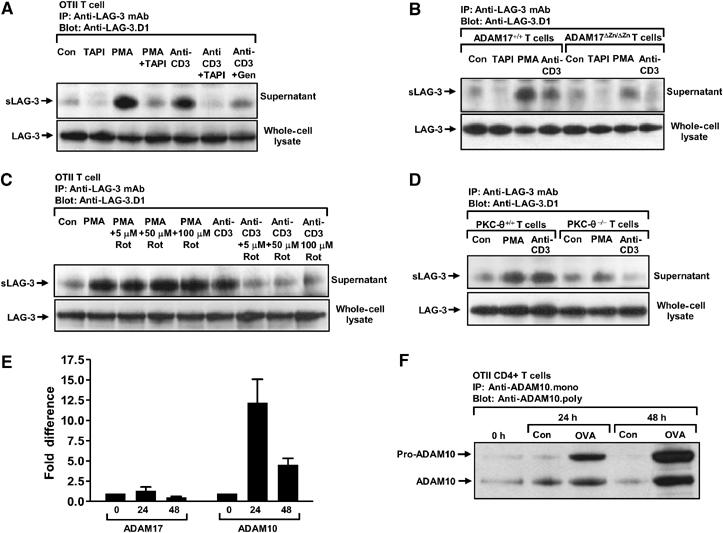
TCR signaling induces ADAM17-mediated LAG-3 cleavage. (A) OTII TCR-transgenic T cells were MACS purified and activated with OVA326−339 in the presence of irradiated LAG-3−/− splenocytes for 3 days. Activated T cells were then treated as indicated (as above plus 2 μg/ml anti-CD3 (2C11), anti-CD3 and TAPI, or anti-CD3 and 50 μg/ml Genistein) for 1 h. (B) Splenocytes from ADAM17ΔZn/ΔZn or ADAM17+/+ littermates were stimulated with 10 μg/ml of SEB for 4 days. Activated splenocytes were then treated and tested as indicated and described above. (C) OTII TCR-transgenic T cells were MACS purified and activated with OVA326−339 in the presence of irradiated LAG-3−/− splenocytes for 3 days. Activated T cells were then treated as indicated (‘Rot' as Rottlerin) for 1 h. (D) Splenocytes from PKCθ+/+ or PKCθ−/− mice were stimulated with 0.25 ng/ml of PMA for 1 day. Activated splenocytes were then treated and tested as indicated and described above. (E, F) CD4+ T cells from OTII TCR transgenic mice were activated with OVA326−339 for 0, 24 or 48 h. ADAM17 and ADAM10 expression was detected by real-time PCR (E) or Western blot (F).
We then asked if TCR-induced LAG-3 cleavage was absent in ADAM17-deficient T cells. As expected, low-level constitutive sLAG-3 shedding was seen with unstimulated wild-type and mutant T cells, whereas sLAG-3 production was increased following PMA and anti-CD3 stimulation of wild-type T cells (Figure 3B). However, CD3 crosslinking induced minimal sLAG-3 production by ADAM17ΔZn/ΔZn T cells. The same observation was also made with T cells from Rag-1−/− mice reconstituted with bone marrow from ADAM17ΔZn/ΔZn mice, eliminating the possibility that this phenotype was due to the development of T cells in an ADAM17-deficient environment (Supplementary Figure S2A). Although we cannot rule out the possibility that the absence of ADAM17 has affected T-cell responsiveness in general and/or the function of ADAM10, these data do suggest that ADAM17 is responsible, at least in part, for the TCR-induced cleavage of LAG-3.
To determine if PKC was required for CD3-induced sLAG-3 production, we stimulated LAG-3+ T cells with anti-CD3 in the presence of rottlerin, a broad-spectrum PKC inhibitor. LAG-3 cleavage was effectively blocked by rottlerin, even at 5 μM (Figure 3C). PKCθ and PKCδ are particularly sensitive to rottlerin, having an ID50 of 5–30 μM (Gschwendt et al, 1993; Villalba et al, 1999). PKCθ is known to be activated by p56lck and recruited to the immunological synapse following TCR ligation (Arendt et al, 2002; Isakov and Altman, 2002). Furthermore, PKCθ−/− T cells are poorly responsive to TCR ligation but respond normally to PMA (Pfeifhofer et al, 2003). Thus, we asked if PKCθ was required for CD3-induced LAG-3 cleavage. While PMA-induced LAG-3 cleavage was intact in PKCθ−/− T cells, CD3 ligation failed to increase LAG-3 shedding (Figure 3D). The simplest explanation for these data is that PKCθ participates directly by phosphorylating ADAM17 and inducing its activation. However, we cannot exclude the possibility that TCR signaling induces ADAM17 activity via a different pathway that indirectly utilizes PKCθ.
Although these data suggest that anti-CD3-induced LAG-3 cleavage is mediated by ADAM17 in a PKCθ-dependent manner, it is also possible that TCR signaling (via PKCθ) increases ADAM17 and/or ADAM10 expression. To assess this, we determined the amount of ADAM10/17 mRNA in resting and activated T cells by real-time PCR. There was essentially no change in ADAM17 mRNA, whereas there was a significant increase in ADAM10 mRNA (∼12-fold) 24 h post T-cell activation (Figure 3E). A substantial increase in pro-ADAM10 and ADAM10 protein was also seen 24 and 48 h post T-cell activation (Figure 3F and Supplementary Figure S2B). It is noteworthy that LAG-3 expression is detectable by flow cytometry in activated T cells and transduced cells lines, suggesting that ADAM expression and activity are limiting. This suggests that changes in ADAM10 expression may significantly affect LAG-3 cleavage. Indeed, this is evident from our MEF overexpression experiments in which high ADAM10 expression by transfection led to the complete cleavage of LAG-3 (Figure 2D). Taken together, these data suggest that there are two pathways by which TCR signaling can induce LAG-3 cleavage: PKCθ-dependent activation of ADAM17 and increased production of constitutively active ADAM10.
What is the physiological function of LAG-3 cleavage?
The physiological function of LAG-3 cleavage is unknown. First, sLAG-3 may have a suppressive ‘cytokine-like' function that blocks CD4 and/or TCR interaction with MHC class II molecules, or compete with membrane-bound LAG-3 to limit its function as a consequence of its high affinity for MHC class II molecules (Huard et al, 1997). Second, it may serve to terminate LAG-3 signaling and thus provide a mechanism for the rapid cessation of LAG-3 regulatory function. Third, ectodomain shedding may be a prerequisite for initiating further intramembrane cleavage (commonly referred to as RIPping) in a manner similar to that required for Notch signaling (McDermott et al, 1999; Brou et al, 2000; Yan et al, 2002; Sahin et al, 2004).
We first asked if sLAG-3 could alter T-cell proliferation. Initial in vitro studies clearly showed that LAG-3+ and LAG-3− T cells were unaffected by the addition of purified sLAG-3 (Supplementary Figures S3A and B). Given that mouse serum contains significant amounts of sLAG-3 (Li et al, 2004), we asked whether this endogenous protein could influence T-cell expansion in vivo. CFSE-labeled CD4+ splenic T cells from LAG-3+/+ and LAG-3−/− Thy1.1+ OTII TCR transgenic mice were adoptively transferred into Thy1.2+ LAG-3−/− or wild-type C57BL/6 mice, stimulated with OVA326–339 and cell division was analyzed 6 days later. While a clear difference in the extent of cell division was seen between the LAG-3−/− and LAG-3+/+ OTII T cells, whereas the presence of sLAG-3 in the serum of recipient mice had no effect on the antigen-induced division of either T-cell population (Supplementary Figure S3C and D). It is possible that the local sLAG-3 concentration in the microenvironment of T-cell activation and/or in the presence of LAG-3+ T cells might be much higher than the serum sLAG-3 concentration. To address this possibility, we generated mice expressing ∼1000-fold higher levels of sLAG-3 than normal serum concentrations by retroviral-mediated stem cell gene transfer (Supplementary Figure S3E) (Workman and Vignali, 2003). These mice then served as recipients for purified, CFSE-labeled, LAG+/+ or LAG−/− Thy1.2+ CD4+ OTII T cells and were treated as above. Despite the presence of substantial quantities of sLAG-3, T-cell proliferation was surprisingly unaffected, and the ability of membrane-associated LAG-3 to control this expansion was also unperturbed (Supplementary Figure S3F and G). Taken together, these results suggest that sLAG-3 has no effect on antigen-driven T-cell activation and proliferation in vitro or in vivo, and does not serve to limit or control LAG-3 function.
Prevention of LAG-3 cleavage blocks T-cell proliferation and cytokine production
We reasoned that if LAG-3 cleavage was required to attenuate its negative regulatory function, a noncleavable version of LAG-3 would be predicted to have enhanced regulatory activity. In contrast, reduced regulatory activity would be observed if cleavage was required to release a ‘functional' sLAG-3 molecule or if LAG-3 RIPping initiated signaling. We had previously shown that LAG-3 cleavage occurs within membrane-proximal CP (Li et al, 2004). To generate noncleavable LAG-3 mutants for functional analysis, we first analyzed the influence of CP length and amino-acid composition on LAG-3 shedding. A series of LAG-3 CP mutants were expressed in a LAG-3/CD4 double loss variant 3A9 T-cell hybridoma by retroviral transduction (Supplementary Figure S4). Constitutive shedding by unstimulated cells was assessed by detection of sLAG-3 using Western blot and ELISA. Analysis of these mutants demonstrated that cleavage requires a long CP (>8 amino acids) and that the protease(s) that mediates this shedding are relatively promiscuous, as indicated by some tolerance for amino-acid substitutions within the CP (Supplementary Figure S4).
Two noncleavable LAG-3 mutants were chosen for functional analysis: LAG-3mCD4CP in which the 20–amino-acid LAG-3 CP has been replaced with the eight-amino-acid CD4 CP, and LAG-3ESCP which has a 12-amino-acid deletion of the LAG-3 CP (Supplementary Figure S4A) (Li et al, 2004). Splenic LAG-3−/− CD4+ OTII T cells were transduced with vector alone (pMIC), wild-type LAG-3 or LAG-3mCD4CP or LAG-3ESCP-encoding retrovirus. Physiological levels of LAG-3 expression, that were comparable to that seen on activated T cells, were ensured by FACS. Uniform CFP and LAG-3 expression was confirmed by Western blot and flow cytometric analysis (Figures 4A and B). No sLAG-3 was detected by Western blot and ELISA in supernatants collected from T cells transduced with either LAG-3mCD4-CP or LAG-3ESCP (Figure 4A and Supplementary Figure S4A) (Li et al, 2004).
Figure 4.
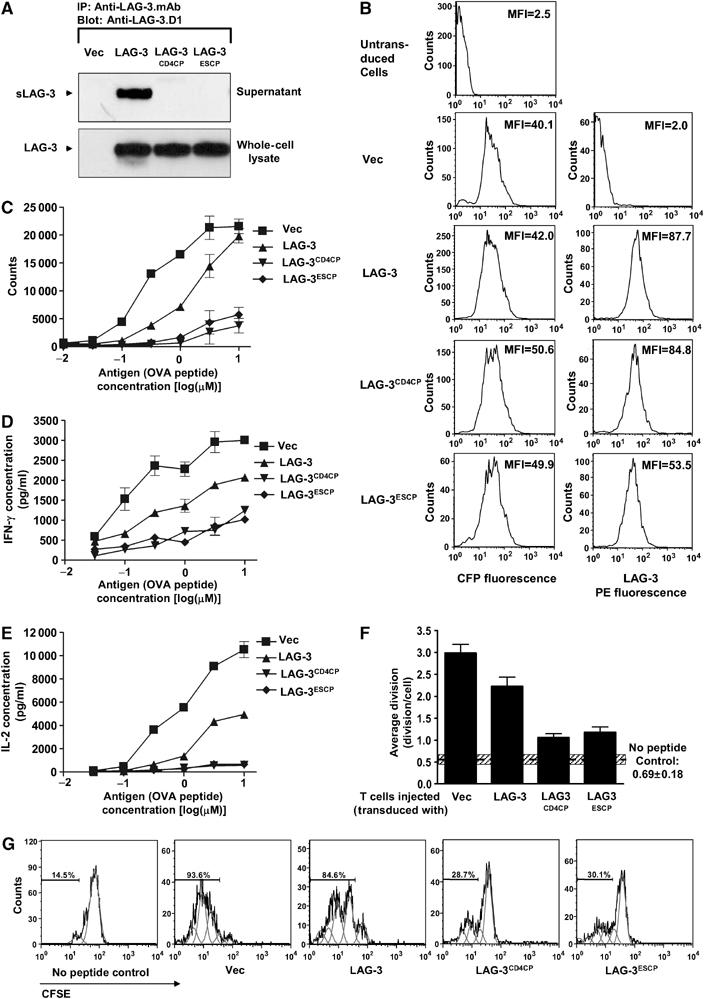
Noncleavable LAG-3 has a more potent inhibitory effect on T-cell activation than wild-type LAG-3. LAG-3−/− Thy1.1+ OTII TCR-transgenic T cells were purified by negative MACS, activated and transduced with empty vector pMIC (Vec), LAG-3 in pMIC (LAG-3), LAG3mCD4CP in pMIC (LAG3mCD4CP) or LAG3ESCP in pMIC (LAG3ESCP). Transduced CD4+ OTII T cells were FACS purified by gating on CFP+ cells. (A) Cells were cultured in medium for 1 h. Both supernatant and whole-cell lysate were immunoprecipitated with anti-LAG-3 mAb. Eluted proteins were separated by SDS–PAGE and blotted with rabbit anti-LAG-3.D1 antisera. (B) Flow cytometric analysis of transductants demonstrating equivalence of CFP and LAG-3 expression. (C–E) Transduced T cells were stimulated with OVA326−339 at the concentrations indicated. Cultures were either pulsed with [3H]thymidine during the last 8 h of a 48 h assay (C) or culture medium collected 24 h after activation for determination of IFN-γ (D) and IL-2 (E) production by ELISA. (F, G) Cells were labeled with CFSE, adoptively transferred into LAG-3−/− mice and cells stimulated in vivo 24 h later with (no peptide control) or without OVA 326–339 peptide. Spleens were removed 6 days later and the percentage of dividing cells was determined by measuring CFSEnegative−low/Thy1.1+/CD4+ T cells. (F) Data are the mean±s.e. of three independent experiments with a total of 8/9 mice per group. Hatched horizontal bar represents the mean±s.e. of the no peptide controls. (G) Representative histograms are also presented with individual divisions displayed using FlowJo.
We first asked if expression of non-cleavable LAG-3 affected T-cell proliferation and cytokine production in vitro. Ectopic expression of LAG-3 significantly reduced T-cell proliferation, but only at lower antigen doses (Figure 4C). In contrast, T cells expressing either of the noncleavable LAG-3 mutants barely proliferated except at the highest antigenic peptide concentration. We also measured interferon-γ (IFN-γ) and interleukin-2 (IL-2) production following peptide stimulation of these transduced T cells. Again, wild-type LAG-3 expression clearly reduced production of both cytokines (Figures 4D and E). However, T cells expressing either version of noncleavable LAG-3 produced substantially less IFN-γ and essentially no IL-2, even at the highest concentration of antigenic peptide.
We then investigated the effect of LAG-3 cleavage on T-cell proliferation in vivo. Retrovirally transduced LAG-3−/− Thy1.1+ OTII T cells were labeled with CFSE, adoptively transferred into Thy1.2+ C57BL/6 mice and then activated with OVA326−339 peptide in vivo. Some background proliferation was seen with the controls (no peptide added) in these experiments owing to the previous in vitro antigen exposure required for retroviral transduction (horizontal hashed bar; Figure 4F). However, peptide treatment in vivo clearly induced substantial T-cell proliferation of the CFP vector control-transduced OTII T cells. Consistent with our in vitro observations, T cells transduced with wild-type LAG-3 proliferated less than the vector control T cells. However, remarkably, very few T cells expressing noncleavable LAG-3 proliferated (Figure 4F and G). In summary, these data show that noncleavable LAG-3 has a potent inhibitory effect on T-cell proliferation and cytokine production that is greater than wild-type LAG-3, suggesting that cell surface cleavage serves as an important negative feedback mechanism to moderate its function.
ADAM10 knockdown inhibits T-cell proliferation in a LAG-3-dependent manner
Given that prolonged and sustained TCR signaling is required to mediate full T-cell activation and function, particularly for naïve T cells, regulation of LAG-3 cell surface expression may be important for modulating immune responses. Indeed, failure to cleave endogenously expressed LAG-3 may lead to poor T-cell proliferation. To test this, we used RNA interference to knock down expression of ADAM10 using an shRNA retroviral vector (pBAN-GFP). LAG-3+/+ and LAG-3−/− CD4+ OTII T cells were transduced with ADAM10-shRNA.pBAN-GFP or the empty pBAN-GFP retrovirus. Real-time PCR analysis demonstrated consistent but moderate ADAM10 knockdown (Figure 5A and B). Importantly, this partial ADAM10 knockdown significantly reduced the peptide-driven proliferation and IFN-γ production of wild-type but not LAG-3−/− OTII T cells (Figure 5C–F). These data demonstrate that even a modest reduction in ADAM10 expression, and thus LAG-3 cleavage, can have a significant effect on T-cell proliferation.
Figure 5.
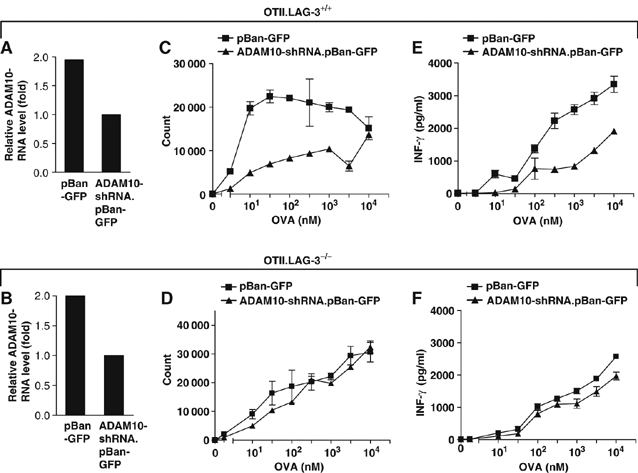
ADAM10 knockdown reduces T-cell proliferation in wild-type but not LAG-3−/− T cells. Thy1.1+ OTII TCR-transgenic T cells from either LAG-3−/− or LAG-3+/+ were purified by negative MACS, activated and transduced with empty vector pBan-GFP or the ADAM10 shRNA cassette in pBan-GFP. Transduced CD4+ OTII T cells were FACS purified by gating on top 50% of GFP+ cells. (A, B) RNAs from retroviral-transduced OTII.LAG-3+/+ T cells (A) or OTII.LAG-3−/− T cells (B) were purified using TRIzol RNA isolation reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's protocol. The relative quantities of ADAM10 mRNA were calculated from standard curves and normalized to 18S mRNA. (C–F) Transduced T cells from either LAG-3−/− (C, E) or LAG-3+/+ (D, F) were stimulated with OVA326−339 at the concentrations indicated. Cultures were either pulsed with [3H]thymidine during the last 8 h of a 72 h assay (C, D) or culture medium collected 48 h after activation for determination of IFN-γ (E, F) production. All figures are representative of three individual experiments.
Discussion
We have previously demonstrated an important effect of LAG-3 on regulatory T cells (extrinsic function) but its effect on conventional effector T cells (intrinsic function) has remained obscure. Our data demonstrate that LAG-3 cleavage is required to control its potent regulatory activity on conventional T cells. An inability to, or failure to, cleave LAG-3 prevents T-cell proliferation but does not appear to result in cell death. Why might this be important? All activated T cells express high levels of LAG-3, yet efficiently proliferate in vivo. The modulation of LAG-3 cleavage, and thus its signaling/function, may be important in facilitating unencumbered expansion. The ability of TCR signaling to modulate both PKCθ-dependent, ADAM17-mediated activation and upregulation of ADAM10 expression provides two direct mechanisms for potentiating LAG-3 cleavage and thus negating its regulatory activity. As activated T cells express high levels of LAG-3, modulation of its negative cell-intrinsic regulatory function may help ‘shape' the contraction phase and perhaps influence the establishment of T-cell memory.
Despite extensive in vitro and in vivo analysis, we found no evidence to support a suppressive ‘cytokine-like' activity for sLAG-3. Indeed, T-cell proliferation and LAG-3 function were unaffected even in the presence of an ∼1000-fold increased concentration of serum sLAG-3 in vivo. Although we cannot completely exclude the possibility that sLAG-3 performs a unique and specific function that has yet to be identified, our data do suggest that sLAG-3 has no ‘global' function and is likely a ‘waste product' of LAG-3 cleavage. Two additional observations are consistent with this notion. First, sLAG-3 appears to be rapidly excreted and/or degraded in vivo, as the half-life of passively transferred, purified sLAG-3 is less than 4 h (Supplementary Figure S5A). Second, whereas dimeric LAG-3:Ig fusion proteins have a high affinity for MHC class II molecules, naturally cleaved sLAG-3 does not specifically bind with MHC class II molecules and no detectable binding of endogenous sLAG-3 on splenic B cells is seen (Supplementary Figure S5B). We and others have shown that LAG-3 is expressed on cells as a weak dimer (Huard et al, 1997; Li et al, 2004). However, gel filtration analysis suggests that purified sLAG-3 is a monomer (data not shown). Thus, based on these data, we propose that only the cell surface LAG-3 dimer or the dimeric LAG-3:Ig fusion protein possesses high affinity for MHC class II molecules. As this is a weak dimer, membrane tethering would be required to maintain this high-affinity form, as this appears to be lost upon cleavage. It is also conceivable that MHC class II binding further stabilizes dimerization but this would still be dependent on membrane tethering. This system provides a unique mechanism for retaining the high-affinity MHC class II interaction required for LAG-3 function while safely rendering sLAG-3 in sera innocuous.
Previous studies have proposed two functions for cell surface metalloprotease-mediated shedding. First, cleavage can serve to activate target proteins. For instance, ADAM10- and ADAM17-mediated cleavage of EGFR ligands is in many cases critical for activation of the EGFR (Peschon et al, 1998; Jackson et al, 2003; Blobel, 2005). Moreover, cleavage of Notch is required for effective signaling (Hartmann et al, 2002). Second, cleavage can serve to inhibit target protein function. Shedding can generate soluble receptors that can act either as scavengers to soak up soluble ligands or competitors to block interaction with membrane-associated ligands, effectively reducing signaling through the intact receptors. One proposed example of this is with the TNF-α receptor (McDermott et al, 1999; Galon et al, 2000). Cleavage defects result in TNF-α receptor accumulation on the cell surface and reduction of the soluble competitor in serum, leading to intensified TNF-α receptor-mediated signaling. Our results may provide an additional twist to the importance of metalloprotease-mediated shedding in immune modulation. Although the shedding of cytokine receptors may generate soluble ligand scavengers, LAG-3 cleavage does not produce a soluble molecule that interferes with LAG-3 function. Thus, the substantially enhanced regulatory activity observed can be attributed purely to increased LAG-3 signaling. Furthermore, this represents the first example of a protein required for dampening T-cell function being controlled by cell surface cleavage.
One intriguing aspect of our data is that the regulatory activity of ectopically expressed noncleavable LAG-3 was enhanced even though its cell surface expression was comparable to wild-type LAG-3. This demonstrates that reduced cleavage enhances the proficiency of signaling rather than simply resulting in increased expression, which may be the case for other receptors. How this might be mediated is unclear, but could be due to prolonged MHC class II ligation. Given that normal LAG-3 expression is highly regulated, it is conceivable that the consequence of preventing cleavage of the endogenous protein could be even greater. Another related issue is the fate of the LAG-3TM-CY fragment (Li et al, 2004) that remains following ADAM10/17 shedding. It is conceivable that it is subjected to a RIPping-like activity by either the γ-secretase complex or a related intramembrane protease system (Urban and Freeman, 2002). A major function of γ-secretase is the clearance of membrane anchors of shed type I membrane proteins (Schenk, 2000; Kopan and Ilagan, 2004). Alternatively, this could serve to ‘activate' the LAG-3 cytoplasmic tail in a Notch-like manner. Given that large ectodomains of 200 amino acids or more appear to prevent γ-secretase cleavage (Struhl and Adachi, 2000), we would argue that RIPping of the LAG-3TM-CY remnant to generate an active signaling fragment is an unlikely scenario as one would predict that the generation of a noncleavable LAG-3 mutant would prevent this and thus lead to a reduction in LAG-3 regulatory activity, which is the antithesis of our observations. Last, the recent observation that ADAM10 can mediate cleavage in trans suggests that antigen-presenting cells may also facilitate T-cell expansion by cleavage of LAG-3 in trans (Janes et al, 2005).
Thus, we would speculate that in normal T cells ADAM10/17 are limiting, and thus very subtle changes in their quantity and/or enzymic activity could have a significant effect on LAG-3 shedding. Given that ADAM10 and ADAM17 mediate the cleavage of many diverse cell surface molecules, constitutive and TCR-induced modulation of their activity could represent a new paradigm for the control of T-cell expansion and function.
Recently, a number of metalloprotease inhibitors have been used in animal models and clinical trials as potential therapies for cancer, multiple sclerosis, arthritis and cardiovascular diseases (Gijbels et al, 1994; Bigg and Rowan, 2001; Hidalgo and Eckhardt, 2001). Interestingly, some studies suggest that administration of metalloprotease inhibitors could prevent inflammation-induced tissue damage (Gijbels et al, 1994; Ramesh and Reeves, 2002). Currently, the protective effects of metalloprotease inhibitors are thought to be due to a reduction of TNF-α function (Gijbels et al, 1994; Ramesh and Reeves, 2002). Our observation that a metalloprotease inhibitor can reduce T-cell proliferation in a LAG-3-dependent manner provides a novel mode of action for these anti-inflammatory agents.
Materials and methods
LAG-3 constructs and cell lines
LAG-3 constructs were produced using recombinant PCR as described previously (Vignali and Vignali, 1999). All LAG-3 constructs were cloned into murine stem cell virus-based retroviral vectors, MSCV-IRES-GFP/CFP (pMIG/pMIC) (Workman et al, 2002a). Details of primers and strategy will be provided on request (nianyu.li@stjude.org). bADAM10 in pIREScg vector was kindly provided by Drs Postina and Fahrenholz. bADAM10E−A was made by substituting amino acid Glu to Ala at position 384 as described before (Lammich et al, 1999). ADAM10−/− and ADAM10+/− MEFs (Hartmann et al, 2002), and ADAM17ΔZn/ΔZn (TaceΔZn/ΔZn-EC-2) and ADAM17+/+ (EC-4) Ras/Myc-transformed fibroblast clones were described elsewhere (Reddy et al, 2000). T-cell hybridomas and fibroblasts were transduced essentially as described (Black et al, 1997). CHO cells and ADAM17-deficient CHO cells (M1) were stably transfected with the LAG-3.pMIG plasmid and pHβAPRII-puro for selection at a DNA ratio 10:1 using Fugene (Roche, Indianapolis, IN) (Arribas and Massague, 1995). Transfectants were selected in 8 μg/ml of puromycin (Clontech, Mountain View, CA) for 7 days and the expression of LAG-3 was confirmed by flow cytometry. All transductants/transfectants were sorted on a MoFlow for uniform GFP expression.
Antibodies and protease inhibitors
The following antibodies were used for immunoprecipitation and or Western blotting: rat anti-LAG-3 mAb (Workman et al, 2002b) (C9B7W specific for the D2 domain; BD-PharMingen, San Diego, CA), rabbit anti-LAG-3.D1 (Li et al, 2004) and rabbit anti-murine ADAM10 (Chemicon International, Temecula, CA). Leupeptin, pepstatin, aprotinin and Pefabloc were obtained from Roche (Indianapolis, IN). E64, TAPI-1, MG132, DAPT and LLNL were obtained from Calbiochem (San Diego, CA).
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously (Li et al, 2004). In brief, whole-cell lysates were generated using 1% NP-40 lysis buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 2 mM Pefabloc, pH 7.4) on ice for 30 min, followed by centrifugation at 15 000 g for 10 min. For immunoprecipitation, whole-cell lysates or culture media were incubated with 5 μg anti-LAG-3 mAb and 30 μl protein G-Sepharose (Amersham Biosciences, Piscataway, NJ) for 3 h at 4°C. Lysates or eluted proteins from immunoprecipitates were resolved by SDS–PAGE (Invitrogen, Carlsbad, CA) and blots probed as detailed. Blots were developed using ECL (Amersham, Piscataway, NJ) and autoradiography.
sLAG-3 ELISA
For sLAG-3 quantification by ELISA, C9B7W (5 μg/ml) mAb was coated on 96-well flat-bottomed-microtiter plates (Dynatech Labs, Franklin, MA) in carbonate buffer (50 mM Na2CO3, pH 10.4) at 37°C for 1 h. The plates were washed three times with PBS–Tween 20 (0.05%) and then blocked with 0.5% FBS in carbonate buffer at 4°C overnight. The plates were washed and the serum or cell culture medium was added. Following 1 h incubation at 37°C, the plates were washed and then probed with rabbit anti-LAG-3.D1 antisera (1:200 dilution, 37°C, 1 h). This was followed by three washes and a 1 h incubation with an HRP-conjugated, anti-rabbit Ig secondary Ab (1:2000 dilution, Amersham, Piscataway, NJ). Plates were developed with TMB substrate solution (Pierce, Rockford, IL) and the reaction was stopped by adding 50 μl of 1 N H2SO4 in each well. Absorbance was measured by a spectrophotometer (Molecular Devices, Sunnyvale, CA). LAG-3 concentration was calculated using a purified sLAG-3 standard curve.
Retroviral transduction of CD4+ T cells and T-cell activation in vitro and in vivo
Splenocytes were stained with biotin-labeled anti-B220, anti-Gr1, anti-Mac1, anti-TER119, anti-CD49b and anti-CD8 antibody (PharMingen, San Diego, CA), incubated with magnetic beads coupled with streptavidin and then negatively sorted on an AutoMACS. Purity of CD4+ T cells was over 80% based on FACS analysis. Purified T cells were activated with 5 μg/ml OVA326−339 peptide in the presence of irradiated whole splenocytes from B6.LAG-3−/− mice. After 2 days activation, T cells were spin transduced (90 min 3000 r.p.m., two times on 2 consecutive days) with supernatant from vector alone (pMIC), LAG-3/CFP, LAG-3.CD4CP/CFP, LAG-3.ESCP/CFP GPE+86 retroviral producer cell lines. Cells were then cultured in the presence of 1 ng/ml of IL-2 for 5 days, sorted on CFP and then allowed to rest for another 3 days.
For the in vitro activation assays, the purified CFP+ T cells (2.5 × 104) were cultured with 5 × 105 irradiated (3000 rad) splenocytes in a 96-well flat-bottomed plate. Antigen (OVA326−339) was added into each well with a 3.3-fold dilution. Cells were cultured for 24 h and pulsed with 1 μCi/well [3 H]thymidine (Du Pont, Wilmington, DE) in the last 7–8 h of culture.
For the in vivo activation assay, purified T cells from either Thy1.1+ OTII TCR or Thy1.1+ OTII TCR LAG-3−/− mice were labeled with 5 μM CFSE for 10 min at 37°C in PBS plus 0.1% BSA at 1 × 107 cells/ml and washed twice. Mice were injected with 5 × 106 cells i.v. and 24 h later with 100 μm of OVA326–339 peptide in 500 μl of PBS i.p. Six days later, splenocytes were counted by Trypan blue exclusion, stained with anti-Thy1.1.PE and anti-CD4.allophycocyanin and analyzed for CFSE levels by flow cytometry. Cell division was calculated by FlowJo (Treestar Inc., Ashland, OR).
Supplementary Material
Supplementary Figure S1A and B1
Acknowledgments
We thank Doug Green for his advice and critical review of the paper. We are grateful to Steve Shaw, Yin Liu and Gottfried Baier for the PKCθ−/− spleens, David Wiest for the pBAN-GFP vector, Rolf Postina and Falk Fahrenholz for the bADAM10 cDNA and Joan Massague and Joaquin Arribas for the CHO-M1 cells. We also wish to thank Richard Cross, Jennifer Hoffrage and Jennifer Smith for FACS, Sue Rowe for cytokine analysis, Mike Nash for AutoMACS, staff in the St Jude Hartwell Center for peptide synthesis, oligo synthesis and DNA sequencing, and Sarah Fitzgerald (Dempsey lab) for the preparation of samples from the ADAM17ΔZn/ΔZn mice. This work was supported by the National Institutes of Health (R01 AI-39480), a Cancer Center Support CORE grant (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC) (to DAAV). PS is supported by the Deutsche Forschungsgemeinschaft SFB45/TPB9. CPB is supported by NIH RO1 GM64750 and EY01571. PJD is supported by NIH DK59778 and DK63363, and a CCFA grant.
References
- Arendt CW, Albrecht B, Soos TJ, Littman DR (2002) Protein kinase C-theta: signaling from the center of the T-cell synapse. Curr Opin Immunol 14: 323–330 [DOI] [PubMed] [Google Scholar]
- Arribas J, Massague J (1995) Transforming growth factor-alpha and beta-amyloid precursor protein share a secretory mechanism. J Cell Biol 128: 433–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Edwards DR, Murphy G (2002) Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115: 3719–3727 [DOI] [PubMed] [Google Scholar]
- Becherer JD, Blobel CP (2003) Biochemical properties and functions of membrane-anchored metalloprotease-disintegrin proteins (ADAMs). Curr Top Dev Biol 54: 101–123 [DOI] [PubMed] [Google Scholar]
- Bigg HF, Rowan AD (2001) The inhibition of metalloproteinases as a therapeutic target in rheumatoid arthritis and osteoarthritis. Curr Opin Pharmacol 1: 314–320 [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385: 729–733 [DOI] [PubMed] [Google Scholar]
- Black RA, White JM (1998) ADAMs: focus on the protease domain. Curr Opin Cell Biol 10: 654–659 [DOI] [PubMed] [Google Scholar]
- Blobel CP (2005) ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol 6: 32–43 [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israel A (2000) A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 5: 207–216 [DOI] [PubMed] [Google Scholar]
- Bruniquel D, Borie N, Hannier S, Triebel F (1998) Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 48: 116–124 [DOI] [PubMed] [Google Scholar]
- Bruniquel D, Borie N, Triebel F (1997) Genomic organization of the human LAG-3/CD4 locus. Immunogenetics 47: 96–98 [DOI] [PubMed] [Google Scholar]
- Ethell DW, Kinloch R, Green DR (2002) Metalloproteinase shedding of Fas ligand regulates beta-amyloid neurotoxicity. Curr Biol 12: 1595–1600 [DOI] [PubMed] [Google Scholar]
- Galon J, Aksentijevich I, McDermott MF, O'Shea JJ, Kastner DL (2000) TNFRSF1A mutations and autoinflammatory syndromes. Curr Opin Immunol 12: 479–486 [DOI] [PubMed] [Google Scholar]
- Gijbels K, Galardy RE, Steinman L (1994) Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest 94: 2177–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M, Kittstein W, Marks F (1993) Protein kinase C forms a complex with and phosphorylates the GTPase activating protein GAP: phosphorylation by PKC is dependent on tyrosine phosphorylation of GAP and/or a GAP-associated protein. Biochem Biophys Res Commun 194: 571–576 [DOI] [PubMed] [Google Scholar]
- Hartmann D, de SB, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena IA, von FK, Saftig P (2002) The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet 11: 2615–2624 [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Eckhardt SG (2001) Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 93: 178–193 [DOI] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA (2004) Role of LAG-3 in regulatory T cells. Immunity 21: 503–513 [DOI] [PubMed] [Google Scholar]
- Huard B, Mastrangeli R, Prigent P, Bruniquel D, Donini S, El-Tayar N, Maigret B, Dreano M, Triebel F (1997) Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc Natl Acad Sci USA 94: 5744–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakov N, Altman A (2002) Protein kinase C(theta) in T cell activation. Annu Rev Immunol 20: 761–794 [DOI] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC (2003) Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J 22: 2704–2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB (2005) Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123: 291–304 [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX (2004) Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol 5: 499–504 [DOI] [PubMed] [Google Scholar]
- Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F (1999) Constitutive and regulated alpha-secretase cleavage of Alzheimer's amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA 96: 3922–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Workman CJ, Martin SM, Vignali DA (2004) Biochemical analysis of the regulatory T cell protein lymphocyte activation gene-3 (LAG-3; CD223). J Immunol 173: 6806–6812 [DOI] [PubMed] [Google Scholar]
- Li X, Fan H (2004) Loss of ectodomain shedding due to mutations in the metalloprotease and cysteine-rich/disintegrin domains of the tumor necrosis factor-alpha converting enzyme (TACE). J Biol Chem 279: 27365–27375 [DOI] [PubMed] [Google Scholar]
- Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, de SB, Hartmann D, Saftig P (2005) ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA 102: 9182–9187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, McCarthy J, Frucht DM, Aringer M, Torosyan Y, Teppo AM, Wilson M, Karaarslan HM, Wan Y, Todd I, Wood G, Schlimgen R, Kumarajeewa TR, Cooper SM, Vella JP, Amos CI, Mulley J, Quane KA, Molloy MG, Ranki A, Powell RJ, Hitman GA, O'Shea JJ, Kastner DL (1999) Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97: 133–144 [DOI] [PubMed] [Google Scholar]
- Overall CM, Kleifeld O (2006) Tumour microenvironment—opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer 6: 227–239 [DOI] [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS (2004) Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 4: 617–629 [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA (1998) An essential role for ectodomain shedding in mammalian development. Science 282: 1281–1284 [DOI] [PubMed] [Google Scholar]
- Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M, Baier G (2003) Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med 197: 1525–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB (2002) TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA (2000) Functional analysis of the domain structure of tumor necrosis factor-alpha converting enzyme. J Biol Chem 275: 14608–14614 [DOI] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de SB, Hartmann D, Saftig P (2005) ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J 24: 742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Weskamp G, Kelly K, Zhou HM, Higashiyama S, Peschon J, Hartmann D, Saftig P, Blobel CP (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J Cell Biol 164: 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk D (2000) Alzheimer's disease. a partner for presenilin. Nature 407: 34–35 [DOI] [PubMed] [Google Scholar]
- Seals DF, Courtneidge SA (2003) The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev 17: 7–30 [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A (2000) Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell 6: 625–636 [DOI] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T (1990) LAG-3, a novel lymphocyte activation gene closely related to CD4. J Exp Med 171: 1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Freeman M (2002) Intramembrane proteolysis controls diverse signalling pathways throughout evolution. Curr Opin Genet Dev 12: 512–518 [DOI] [PubMed] [Google Scholar]
- Vignali DA, Vignali KM (1999) Profound enhancement of T cell activation mediated by the interaction between the TCR and the D3 domain of CD4. J Immunol 162: 1431–1439 [PubMed] [Google Scholar]
- Villalba M, Kasibhatla S, Genestier L, Mahboubi A, Green DR, Altman A (1999) Protein kinase c theta cooperates with calcineurin to induce Fas ligand expression during activation-induced T cell death. J Immunol 163: 5813–5819 [PubMed] [Google Scholar]
- Villanueva de la TT, Bech-Serra JJ, Ruiz-Paz S, Baselga J, Arribas J (2004) Inactivating mutations block the tumor necrosis factor-alpha-converting enzyme in the early secretory pathway. Biochem Biophys Res Commun 314: 1028–1035 [DOI] [PubMed] [Google Scholar]
- Weskamp G, Ford JW, Sturgill J, Martin S, Docherty AJ, Swendeman S, Broadway N, Hartmann D, Saftig P, Umland S, Sehara-Fujisawa A, Black RA, Ludwig A, Becherer JD, Conrad DH, Blobel CP (2006) ADAM10 is a principal ‘sheddase' of the low-affinity immunoglobulin E receptor CD23. Nat Immunol 7: 1293–1298 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA (2004) Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 172: 5450–5455 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Dugger KJ, Vignali DA (2002a) Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. J Immunol 169: 5392–5395 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Rice DS, Dugger KJ, Kurschner C, Vignali DA (2002b) Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3). Eur J Immunol 32: 2255–2263 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA (2003) The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol 33: 970–979 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA (2005) Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol 174: 688–695 [DOI] [PubMed] [Google Scholar]
- Yan Y, Shirakabe K, Werb Z (2002) The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. J Cell Biol 158: 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1A and B1


