Abstract
Ventricular tachycardia is a common and lethal complication after myocardial infarction. Here, we show that focal gene transfer of KCNH2-G628S to the infarct scar border eliminated all ventricular arrhythmias in a porcine model. No proarrhythmia or other negative effects were discernable. Our results demonstrate the potential viability of gene therapy for ablation of ventricular arrhythmias.
Cardiac arrest remains the leading cause of death in the developed world, with over 400,000 deaths per year in the US.1 Approximately two-thirds of cardiac arrest victims have a prior myocardial infarction, and cardiac arrest results from arrhythmias originating at the infarct scar border.1,2 Current therapy for at-risk patients is defibrillator implantation, but recent problems with defibrillators motivate our search for alternatives.3,4 We hypothesized that gene transfer could eliminate post-infarct ventricular arrhythmias.
First, we developed an animal model with consistently inducible post-infarct ventricular tachycardia (VT). In this model, we evaluated gene transfer efficacy, and we tested gene transfer-mediated elimination of VT using KCNH2-G628S, a dominant negative potassium channel mutation.5 We found that balloon occlusion of the mid-left anterior descending coronary artery reproducibly created the substrate for post-infarct VT (Supplementary Methods). In a baseline study, we infarcted seven pigs and evaluated the animals with echocardiography (Supplementary Table 1) and electrophysiology (EP) study (Supplementary Table 2). Three and four weeks post-infarct, sustained monomorphic VT could be repeatedly and consistently induced in all animals (Fig. 1a). Intracardiac electrogram analysis pinpointed VT origination to the anterior septum in all animals (Fig. 1b). Histology of that area revealed surviving myocardium interwoven with fibrotic scar (Fig. 1c), confirming the existence of pathways for conduction of VT.
Figure 1.
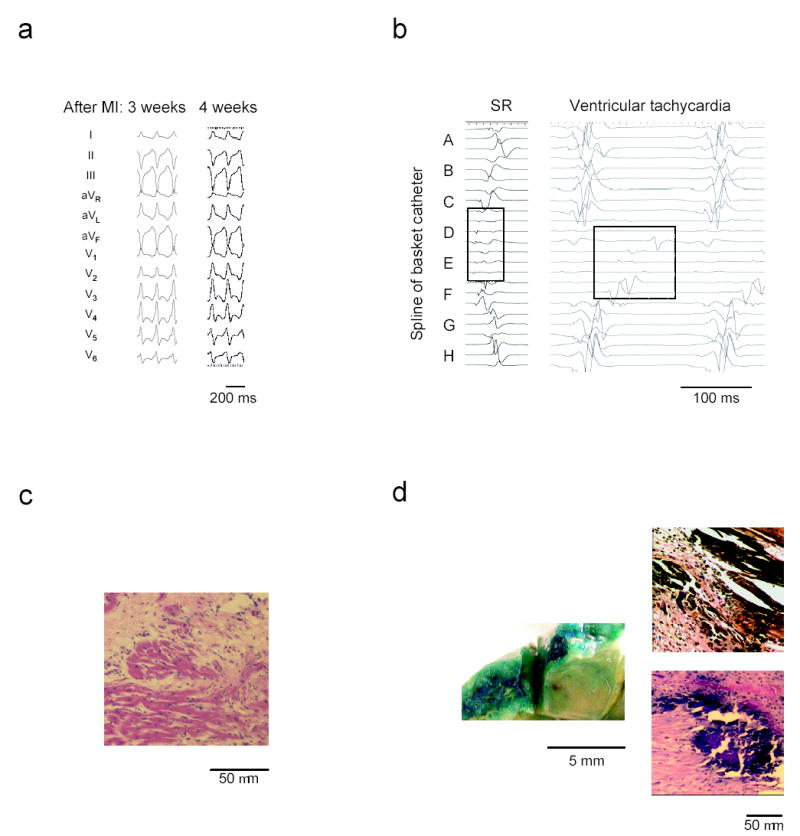
Ventricular tachycardia after myocardial infarction in a porcine model. (a) ECG example showing identical VT morphology and cycle length from one week to the next in a representative animal. (b) Intracardiac electrograms during sinus rhythm (SR) show low amplitude, fractionated signals in the anterior septal region (splines D and E). The ventricular tachycardia recording demonstrates slow, progressive activation of this region through diastole. (c) Hematoxylin-Eosin stained microsection of the infarct border zone reveals surviving strands of myocardium surrounded by fibrotic scar in the anterior septum. (d) X-gal staining to identify lacZ gene transfer. (left) Gross tissue shows intense blue staining indicative of lacZ expression in the target area at the anterior septal border zone. (right) Microscopic sections taken from the target region exhibit blue, lacZ positive myocytes.
We assessed borderzone-targeted gene transfer three weeks post-infarct in five pigs by perfusing lacZ-encoding adenovirus. Reporter gene expression occurred in 45 ± 7% of cardiac myocytes inside the target region (Fig. 1d). No myocytes outside the target region expressed the reporter gene. Control animals did not have any myocyte staining, verifying the specificity of the positive results in the lacZ animals.
In 15 post-infarct animals with reproducibly inducible VT, we evaluated gene transfer effects on the arrhythmia. Five animals receiving KCNH2-G628S (G628S animals) were compared to five lacZ-infected control animals and five animals without gene transfer (uninfected controls). At repeat EP study one week after gene transfer, all G628S animals had complete elimination of ventricular arrhythmia inducibility, and all control animals continued to have reproducibly inducible VT (Fig. 2a, Supplementary Fig. 1).
Figure 2.
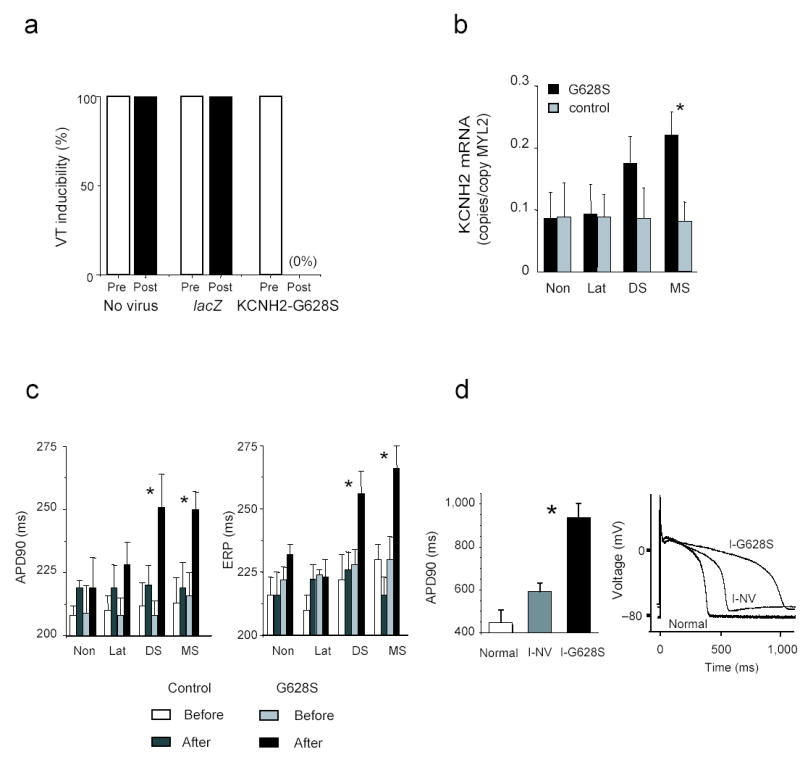
KCNH2-G628S gene transfer in the MI-VT model. (a) Prior to gene transfer, VT was repeatedly inducible in all animals. After gene transfer, no arrhythmias could be induced in the KCNH2-G628S infected animals. All control animals remained inducible. (b) RT-PCR results show increased ERG mRNA in mid and distal septum of G628S animals. Data is normalized to MCL-2v expression (copies of ERG per copy of MLC2v). Non—non-infarcted basal lateral wall. MS—mid-anterior septal infarct border. DS—distal anterior septal infarct border. Lat—anterior lateral infarct border. (c) Measurement of action potential duration to 90% repolarization (APD90) and effective refractory period (ERP) from the indicated regions. Prolongation of APD90 and ERP is isolated to the septal border of the infarct. (d) Patch clamp data of action potentials from uninfarcted anterior septal cells (Normal) compared to infarct-no virus (I-NV) or infarct-KCNH2-G628S (I-G628S) infected cells from the anterior septal infarct border zone. The left panel shows summary data from the indicated groups. The right panel shows representative tracings. * p < 0.05.
We verified gene expression by measuring total ERG mRNA. The G628S animals had higher levels of ERG within the target region compared to controls (copies ERG/copy MLC2v: mid-septum G628S 0.22±0.04, controls 0.08±0.03, p=0.04; distal septum G628S 0.17±0.04, controls 0.09±0.05, p=0.17). Importantly, ERG expression outside the target region was not increased in the G628S animals relative to controls (lateral borderzone G628S 0.09±0.05, controls 0.09±0.04; non-infarct G628S 0.09±0.04, controls 0.09±0.05, p=NS), confirming the above histological data that gene transfer was isolated to the target (Fig. 2b, Supplementary Fig. 2).
We evaluated functional effects of gene transfer using intracardiac electrogram, monophasic action potential and patch clamp analysis. Sinus rhythm electrograms in all animals continued to show low amplitude, fractionated electrical activation inside the gene transfer zone, suggesting that gene transfer did not disrupt conduction at sinus heart rates. In the G628S animals, monophasic action potential duration (APD) and effective refractory period (ERP) lengthened in the anterior septum but not in other regions of the heart. lacZ and uninfected control animals had no changes in APD or ERP for any cardiac region (Fig. 2c). Patch clamping of isolated cardiac myocytes corroborated the in vivo data (Fig. 2d); APD was significantly longer in the G628S-expressing cells (G628S: 937 ± 117 ms, control anterior septal borderzone 592 ± 69 ms, normal anterior septum 448 ± 104 ms, p < 001). The APDs were prolonged in isolated myocytes compared to in vivo recordings, a difference likely caused by faster stimulation frequencies in vivo and by electrotonic interactions between coupled G628S-expressing and non-expressing cells in vivo.6 The implications of these interactions require further investigation. Overall, these data suggest that VT elimination resulted from increased refractoriness of G628S-expressing cells preventing electrical activation at frequencies sufficient to sustain VT.
To compare gene transfer to conventional pharmacology, we administered the KCNH2-blocking drug dofetilide to a group of three post-infarct animals with reproducibly inducible VT. Dofetilide caused global QT prolongation and global ERP prolongation in all animals (Supplementary Fig. 3,Supplementary Table 2). The increased refractoriness had the functional effect of eliminating 1:1 conduction during ventricular pacing at the 250 ms cycle length. In spite of these changes, all animals continued to have inducible VT, but the cycle length was slower than pre-dofetilide. Since dofetilide and G628S affect the same potassium channel, better efficacy of G628S may come from the greater channel blockade afforded by very localized delivery of the gene therapeutic.
We assessed several measures of safety. We implanted ICDs to monitor for sustained arrhythmias, and none were detected. We evaluated premature ventricular contraction (PVC) frequency during daily ECG evaluation and found that PVCs were exceedingly rare in all animals (a single PVC in 2 of 10 controls, a single PVC in 1 of 5 G628S animals). We attempted to elicit triggered arrhythmias with burst ventricular pacing, but none were observed. We looked for afterdepolarizations both in vivo and in vitro but saw none. On 12 lead ECG, global QTc, anterior precordial QTc and QT dispersion did not change with gene transfer (Supplementary Table 2). In general, there were no echocardiographic changes (Supplementary Table 1), and there were no deaths post-gene transfer in this study. There were no observable differences between groups in animal behavior, appetite or weight.
Previous arrhythmia gene transfer reports demonstrated viability for limited indications (heart rate control in atrial fibrillation, pacemaker activity, ventricular repolarization shortening), but none of these therapies eliminated the target arryhthmia.7–10 Our study is the first to show that gene transfer can eradicate cardiac tachyarrhythmias in a clinically relevant disease model. In this proof of principle study, we used adenoviral vectors where gene expression peaked one week after gene transfer.11 Ultimately long-term efficacy and rigorous safety testing is required before translation to humans can be contemplated. Such testing will most likely use AAV or lentivirus vectors because they are capable of inducing long-term gene expression.12,13 If proven safe and efficacious, the initial use of this technology will probably be to replace drugs as adjunct therapy for ICD patients, with the eventual goal of eliminating the need for ICD implantation altogether. These data present hope for a completely new strategy to treat ventricular arrhythmias.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institutes of Health. ICD equipment was donated by St. Jude, Inc. Basket catheters and electrogram mapping system were donated by Boston Scientific Corp.
Footnotes
Author Information TS designed the study, carried out experiments, performed analyses and assisted in writing the paper, ADM assisted with performing and troubleshooting the experiments, KK assisted with design of the model and performing experiments, JKD conceived and designed the study, performed analyses and took the primary role in writing the paper. The authors declare no competing financial interests.
References
- 1.American Heart Association, 2001. Heart and Stroke Statistical Update. American Heart Association; Dallas, TX: 2002. [Google Scholar]
- 2.Rothman S, et al. Circulation. 1997;96:3499–3508. doi: 10.1161/01.cir.96.10.3499. [DOI] [PubMed] [Google Scholar]
- 3.Gould P, Krahn A Canadian Heart Rhythm Society Working Group on Device Advisories. JAMA. 2006;295:1907–1911. doi: 10.1001/jama.295.16.1907. [DOI] [PubMed] [Google Scholar]
- 4.Maisel W, et al. JAMA. 2006;295:1901–1906. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]
- 5.Sanguinetti MC, Curran ME, Spector PS, Keating MT. Proc Natl Acad Sci USA. 1996;93:2208–2212. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesh M, Pring M, Spear J. Circ Res. 1989;65:1426–1440. doi: 10.1161/01.res.65.5.1426. [DOI] [PubMed] [Google Scholar]
- 7.Donahue JK, et al. Nat Med. 2000;6:1395–1398. doi: 10.1038/82214. [DOI] [PubMed] [Google Scholar]
- 8.Miake J, Marban E, Nuss H. Nature. 2002;419:132–133. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 9.Qu J, et al. Circulation. 2003;107:1106–1109. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 10.Brunner M, et al. Am J Physiol Heart Circ Physiol. 2003;285:H194–H203. doi: 10.1152/ajpheart.00971.2002. [DOI] [PubMed] [Google Scholar]
- 11.Quinones M, et al. Circulation. 1996;94:1394–1401. doi: 10.1161/01.cir.94.6.1394. [DOI] [PubMed] [Google Scholar]
- 12.Snyder R, et al. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 13.Kafri T, et al. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


