Abstract
OBJECTIVE
To review the evidence on safe and effective prescribing of opioids for chronic non-malignant pain.
QUALITY OF EVIDENCE
MEDLINE was searched using the terms “opioid effectiveness” and “adverse effects.” There is strong evidence that opioids are effective for both nociceptive and neuropathic pain, but limited evidence that they are effective for pain disorder. There is little information on their effectiveness at high doses or on the adverse effects of high doses.
MAIN MESSAGE
Opioids should be initiated after an adequate trial of acetaminophen or nonsteroidal anti-inflammatory drugs for nociceptive pain and of tricyclic antidepressants or anticonvulsants for neuropathic pain. Patients should be asked to sign treatment agreements and to give informed consent to treatment. Patients should experience a graded analgesic response with each dose increase. Titrate doses of immediate-release opioids slowly upward until pain reduction is achieved, and then switch patients to controlled-release opioids. Most patients with chronic non-malignant pain can be managed with<300 mg/d of morphine (or equivalent).
CONCLUSION
Opioids are safe and effective for managing chronic pain.
Abstract
OBJECTIF
Examiner les données scientifiques concernant la prescription efficace et en toute sûreté d’opiacés pour la douleur chronique d’origine non cancéreuse.
QUALITÉ DES PREUVES
Une recension a été effectuée dans MEDLINE à l’aide des termes en anglais «efficacité des opiacés» et «effets indésirables». De nombreuses données convaincantes démontrent l’efficacité des opiacées pour la douleur nociceptive et neuropathique, mais elles se font plus rares en ce qui a trait à leur efficacité pour les troubles de la douleur. Les renseignements sont limités quant à leur efficacité à doses élevées ou aux effets indésirables de telles doses.
PRINCIPAL MESSAGE
On devrait administrer des opiacés après un essai adéquat de l’acétaminophène ou des anti-inflammatoires non stéroïdiens pour la douleur nociceptive et d’antidépresseurs tricycliques ou d’anticonvulsifs pour la douleur neuropathique. Il faut demander aux patients de signer une entente de traitement et de donner leur consentement éclairé à la thérapie. Les patients devraient ressentir une réponse analgésique progressive avec chaque augmentation de la dose. Augmenter lentement la dose d’opiacés à effet immédiat jusqu’à ce que la douleur soit réduite, puis passer ensuite à des opiacés à effet contrôlé. La plupart des patients souffrant de douleur chronique d’origine non cancéreuse peuvent être traités avec <300 mg/j de morphine (ou l’équivalent).
CONCLUSION
Les opiacés sont sécuritaires et efficaces pour prendre en charge la douleur chronique.
EDITOR’S KEY POINTS.
Despite a rapid increase in the number of opioids prescribed in recent years, there is evidence that some chronic pain is undertreated due to physicians’ concerns about the safety of opioid therapy and the risk of patients’ abusing opioids.
Opioids are effective, however, for chronic non-malignant pain and can be safely prescribed. Asking patients to sign treatment contracts is the first step.
Start with intermediate-acting opioids and titrate the dose slowly until a reasonable reduction in pain (ie, 50%) is achieved, then switch to controlled-release preparations. In most cases, doses of <300 mg/d of morphine (or equivalent) will suffice.
POINTS DE REPÈRE DU RÉDACTEUR.
En dépit de la hausse rapide du nombre d’opiacés prescrits au cours des dernières années, des données scientifiques indiquent que certaines douleurs chroniques demeurent sans traitement en raison de l’inquiétude du médecin entourant l’innocuité de la thérapie aux opiacés et le risque d’un usage abusif par les patients.
Par ailleurs, les opiacés sont efficaces pour les douleurs d’origine non cancéreuse et peuvent être prescrits en toute sûreté. La première étape est de demander aux patients de signer un contrat de traitement.
Commencer avec des opiacés à action intermédiaire et augmenter lentement la dose jusqu’à ce que la douleur soit raisonnablement réduite (p. ex. 50%), puis, passer à des préparations à action contrôlée. Dans la plupart des cas, des doses de <300 mg/j de morphine (ou l’équivalent) suffiront.
While opioid prescribing has increased dramatically in recent years,1 there is evidence that chronic pain remains undertreated.2 Surveys have found that physicians are uncertain about the indications for opioid use and concerned about the risk of addiction, and that their charting of opioid management is inadequate.3-5 Yet opioids are important for managing chronic pain, and primary care physicians should be knowledgeable about their use.
Quality of evidence
MEDLINE was searched using the terms “opioid effectiveness” and “adverse effects.” Level I evidence indicates that opioids are effective for both nociceptive and neuropathic pain, but there is little evidence that they are effective for pain disorder. There is little information on their effectiveness at high doses or on the adverse effects of high doses. Level II evidence shows that, at stable moderate doses, opioids have minimal effects on cognitive function and cause little long-term toxicity in organs.
Characteristics of opioids
Opioids attach to endogenous u-receptors in the central nervous system. Their duration of analgesic action is generally 3 to 6 hours. Oral sustained-release preparations and fentanyl patches have a duration of action of 12 hours and 72 hours, respectively. Opioids are effective for chronic nociceptive and neuropathic pain (level I evidence).6-10 Subjective pain ratings have been shown to decrease by 20% to 50% (with great variation in individual responses). Some trials have documented improvement in functional status and pain-related disability also.9 A recent systematic review concluded that strong opioids are more effective than nonsteroidal anti-inflammatory drugs (NSAIDs) or tricyclic antidepressants (TCAs) for pain relief, but not for improving functional outcomes.11 Quality of life might improve with optimal dosing, less frequent dosing intervals, and aggressive management of gastrointestinal side effects.12,13
Indications
The indications listed below are based on the results of systematic reviews of randomized controlled trials.14
Somatic pain.
Opioids are useful for chronic musculoskeletal pain that has not responded adequately to acetaminophen or NSAIDs.
Neuropathic pain.
Randomized trials have demonstrated that opioids are at least as effective as TCAs for neuropathic pain and have fewer side effects.15,16 Higher doses of opioids are often needed, however, for neuropathic pain than for somatic pain, and even at high doses, some patients do not respond.17 A combination of morphine and gabapentin reduces neuropathic pain more effectively and at lower doses than either drug alone does.18
Fibromyalgia.
Two controlled trials using a weak opioid have demonstrated that opioids reduce the pain of fibromyalgia.11 Functional outcomes did not improve in these trials. A high proportion of fibromyalgia patients have concurrent mood and anxiety disorders,19,20 and antidepressant therapy has had promising results for both mood and pain in fibromyalgia.21-23 An exercise program and low doses of amitriptyline are recommended first-line treatments.24,25
Other types of pain.
Opioids are sometimes used for recurrent, severe visceral pain, such as that associated with pancreatitis. They are not indicated for irritable bowel syndrome or for tension headaches. Opioids stronger than codeine should be reserved for patients with severe migraine headaches who do not respond to first-line treatments.
Side effects
Opioids cause little long-term organ toxicity and are safer as a class than NSAIDs.26-28 Opioids frequently cause fatigue, constipation, and nausea. Regular use of opioid-acetaminophen combinations can lead to rebound headaches in migraine patients.29,30 Chronic opioid therapy can cause long-term hyperalgesia.31 Erectile dysfunction is common with high-dose opioid therapy.32
Opioids given at stable moderate doses appear to have few effects on cognitive functioning. A single oral dose of 15 mg/d of morphine causes less cognitive impairment than 1 mg of lorazepam does.33 A recent systematic review concluded that stable doses of opioids did not impair driving performance.34 Existing studies have not examined the effect on cognitive functioning or driving performance of poorly controlled pain and anxiety, high doses of opioids, or combination pharmacotherapy (opioids and TCAs, anticonvulsants, or benzodiazepines).
Overdose of opioids is characterized by a decreased level of consciousness, decreased respiratory rate, bradycardia, and miosis. Long-acting opioids, particularly methadone, can have a gradual, insidious onset of symptoms. Risk increases with parenteral use, dose and potency of the opioid, and patients’ underlying tolerance. Other risk factors include older age, renal insufficiency, respiratory disease, and use of sedating medications. Risk of overdose and accidents can be reduced by slow titration of low-potency opioids and by avoiding sedating drugs, particularly benzodiazepines and alcohol. During initial titration or after a dosage increase, patients should be advised to contact their physicians at the first sign of decreased alertness and to limit their driving for several days. Fentanyl patches should be used only for patients who are already fully tolerant to a potent opioid, such as morphine.
Opioids and pain disorders
The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), diagnostic criteria for pain disorders include pain causing distress or impaired functioning and the presence of psychological factors that have an important role in onset or maintenance of pain. Pain disorders might be associated with medical conditions, but might also occur when underlying medical disease cannot be found. Patients with pain disorders have severe pain, even though the original injury might have occurred months or years before. The pain is often accompanied by depression, anxiety, and severe functional disability.35,36 Patients often have poor coping skills and have negative responses to pain, such as catastrophizing.37 Patients with pain disorders have minimal response to multiple trials of medications and procedures.38
There is little evidence that opioids are effective in pain disorders. Overreliance on opioid therapy can cause side effects and contribute to patients’ belief that they have serious organic illnesses. Cohort studies and one controlled trial have demonstrated that patients with severe pain despite opioid therapy show improvements in pain, mood, and activity if their opioids are tapered and they attend comprehensive pain programs.39-43 While family physicians have limited access to comprehensive pain clinics, they can incorporate some elements of a comprehensive approach by counseling patients on stress reduction, exercise, and engagement in work and social activities. Population studies suggest that patients taking chronic opioid therapy have a substantially higher prevalence of mood and anxiety disorders than is seen in the general population.44
Management of opioid therapy
Before starting opioid therapy, patients should understand that the goal of treatment is not the complete elimination of pain but a 25% to 50% reduction in its intensity and improvement in mood and functioning. A complete assessment and baseline investigations should focus on respiratory, hepatic, and renal function; mental status; activity level; social supports; and substance use. Records should be obtained from previous care providers. Useful office instruments for assessing pain are available from the National Pain Education Council at http://www.npecweb.org.
Treatment agreements.
There is evidence that treatment agreements improve compliance.45 Agreements should state that patients will not receive opioids from other sources, will not receive early refills, and will comply with scheduled visits and urine drug screens, and that physicians might cease to prescribe opioids if the agreement is broken (Figure 1).
Figure 1.
Opioid (narcotic) medication treatment agreement
Follow-up and documentation.
Patients should be seen frequently at the beginning of treatment. At each visit, physicians should document the “4 As”:
Analgesia,
Activities of daily living,
Adverse effects, and
Aberrant drug taking.
Analgesic effectiveness can be measured using a 10-point scale at rest and with activity. Physicians should record the amount of opioid dispensed and reasons for changes in dose or medication.
Choice of opioid.
Physicians can choose among a variety of options.
First-line opioids:
Codeine is usually the initial choice because it is the least potent opioid. If immediate-release codeine is effective, it can be converted to an equivalent dose of controlled-release codeine.10 Up to 10% of white people lack the enzyme that converts codeine into its active metabolite morphine,46 however, so stronger opioids might be required.
Second-line opioids:
Tramadol, recently available in Canada as Tramacet (tramadol and acetaminophen), is a weak opioid that blocks uptake of norepinephrine and serotonin47 and is effective for treating neuropathic48 and musculoskeletal49,50 pain (level I evidence). Its analgesic efficacy is comparable to that of codeine (level I evidence).50-52 For musculoskeletal pain, it enhances the analgesic effectiveness of acetaminophen and NSAIDS.49,53,54 The most common adverse effects are headaches, nausea, and dizziness. Tramadol causes less constipation and carries a lower risk of respiratory depression than other opioids,55 but it can cause seizures when taken in overdose or in combination with serotonergic medications.56,57 Because of its safety profile, several guidelines have suggested that tramadol be a second-line medication in the World Health Organization’s ladder of analgesics for nociceptive pain, after acetaminophen and NSAIDS.58
Overall, there is no evidence that one opioid is superior to another, although recommendations can be made for specific populations. Oxycodone and hydromorphone might be less likely than morphine to cause sedation in elderly patients.59,60 The active metabolites of morphine can accumulate to toxic levels in patients with renal dysfunction.61
Oral meperidine has poor bioavailability. Oxycodone and hydromorphone should be used with caution in patients with current or past history of addiction.62,63 Parenteral opioids should not be used for long-term pain management because of the risk of overdose and addiction.
Third-line opioids:
Fentanyl patches should be used only for patients who are taking the equivalent of 90 to 100 mg of morphine daily and who are fully tolerant to this dose. Methadone is the treatment of first choice for patients with both chronic pain and opioid dependence.64 Methadone might also be useful for severe neuropathic pain because it antagonizes N-methyl D-aspartate receptors,65,66 but methadone titration can be dangerous,67 and 1 controlled trial demonstrated that methadone was no more effective than morphine as a first-line treatment for nociceptive cancer pain.68 Therefore, for patients who are not addicted, methadone should be reserved for those with severe organic pain (particularly neuropathic pain) when all other opioids have failed.
Patients’ opioids should be switched to controlled-release preparations once a stable dose has been reached. Controlled-release preparations taken twice daily provide analgesia equivalent to immediate-release preparations taken 4 times daily69 and superior analgesia to immediate-release opioids used as needed.70 Some patients require a slightly higher dose of controlled-release medication than of immediate-release medication.71 If patients report that pain relief does not last a full 12 hours, their doses should be increased rather than switched to 3 times daily.
Dose titration.
Opioids elicit a graded response, with greatest incremental benefit at lower doses and a gradual leveling out of response at higher doses. Continued dose increases are futile in patients who receive only minimal analgesic benefit from several dose increases. Titration can be facilitated by using a simple 10-point pain-rating scale. With each dose increase, patients should experience a decline in pain intensity and a longer duration of action. Titration should be slower and have closer follow-up in patients who are older; are taking sedating drugs; or have respiratory, renal, or hepatic dysfunction. An optimal dose will provide good analgesia (ie, 50% reduction in pain intensity) and improvements in mood and functioning.
Pain relief versus functioning.
Physicians should be alert for inconsistencies in patients’ reports of analgesic benefit and functional status. If, for example, patients’ pain has decreased to 3/10 from 9/10 on the pain scale but they remain in bed all day, physicians should assume that the pain does not respond to opioid therapy or that patients’ dysfunction is due to other causes.
Schedules and breakthrough pain.
Opioids should be taken on a schedule, with the total daily breakthrough dose no more than one third of the total daily scheduled dose. If possible, the same opioid should be used for both scheduled and breakthrough doses. Scheduled and breakthrough doses might need adjustment during periods of increased rest or activity.
Switching and combining opioids.
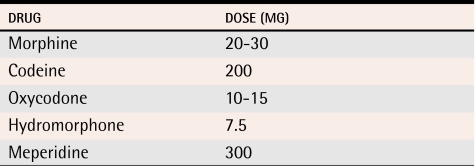
Opioids could be switched because they are ineffective or because they produce intolerable side effects.72 Because of incomplete cross-tolerance, an initial dose of a new opioid should be equivalent to 50% or less of the dose of the original opioid (Table 173). Opioids should be discontinued if patients’ pain remains unresponsive after a trial of 3 or 4 different opioids. There is no clear evidence to support combining different types of opioids.
Table 1.
Oral opioid analgesic equivalence
Adapted from Canadian Pharmacists Association.73
Usual maximum dose.
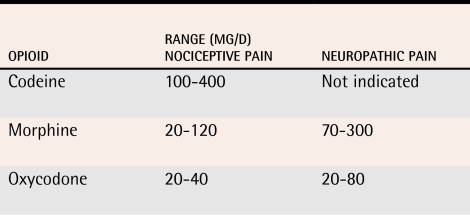
Some recommend that opioids be “dosed to effect.” There is little evidence on the effectiveness and safety of opioid doses above 300 mg/d of oral morphine, however, and the College of Physicians and Surgeons of Ontario’s task force stated that it was “unusual” for patients to require more than this for chronic non-malignant pain.14 The daily opioid doses used in controlled trials for both malignant and non-malignant pain were generally well below this level (Table 2).70,71,74,75 High doses of opioids in at-risk patients could have adverse effects on cognition.76-79
Table 2.
Range of daily opioid doses used in controlled trials on chronic non-malignant pain
Conclusion
Opioids are safe and effective treatment for nociceptive and neuropathic pain. Evidence supports maintaining treatment with sustained-release preparations at doses lower than 300 mg/d of morphine or equivalent. Doses should be carefully titrated for high-risk patients, such as the elderly and those taking benzodiazepines.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Biographies
Dr Kahan is Medical Director of the Addiction Medical Service at St Joseph’s Health Centre and Head of the Alcohol Clinic at the Centre for Addiction and Mental Health in Toronto, Ont.
Dr Srivastava is a staff physician at St Joseph’s Health Centre and a clinical researcher at the Centre for Addiction and Mental Health.
Dr Wilson is Chief of the Department of Family Medicine at St Joseph’s Health Centre.
Dr Mailis-Gagnon is Medical Director of the Comprehensive Pain Program at Toronto Western Hospital.
Ms Midmer is a research scholar in the Department of Family and Community Medicine at the University of Toronto.
Footnotes
Competing interests: None declared
References
- 1.Bell JR. Australian trends in opioid prescribing for chronic non-cancer pain, 1986-1996. Med J Aust. 1997;167(1):26–29. doi: 10.5694/j.1326-5377.1997.tb138759.x. [DOI] [PubMed] [Google Scholar]
- 2.Moulin DE, Clark AJ, Speechley M, Morley-Forster PK. Chronic pain in Canada—prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag. 2002;7(4):179–184. doi: 10.1155/2002/323085. [DOI] [PubMed] [Google Scholar]
- 3.Bendtsen P, Hensing G, Ebeling C, Schedin A. What are the qualities of dilemmas experienced when prescribing opioids in general practice? Pain. 1999;82(1):89–96. doi: 10.1016/S0304-3959(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 4.Deehan A, Taylor C, Strang J. The general practitioner, the drug misuser, and the alcohol misuser: major differences in general practitioner activity, therapeutic commitment, and ‘shared care’ proposals. Br J Gen Pract. 1997;47(424):705–709. [PMC free article] [PubMed] [Google Scholar]
- 5.Clark JD. Chronic pain prevalence and analgesic prescribing in a general medical population. J Pain Symptom Manage. 2002;23(2):131–137. doi: 10.1016/s0885-3924(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 6.Watson CP, Babul N. Efficacy of oxycodone in neuropathic pain: a randomized trial in postherpetic neuralgia. Neurology. 1998;50(6):1837–1841. doi: 10.1212/wnl.50.6.1837. [DOI] [PubMed] [Google Scholar]
- 7.Watson CP, Moulin D, Watt-Watson J, Gordon A, Eisenhoffer J. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003;105(1-2):71–78. doi: 10.1016/s0304-3959(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 8.Moulin DE, Iezzi A, Amireh R, Sharpe WK, Boyd D, Merskey H. Randomised trial of oral morphine for chronic non-cancer pain. Lancet. 1996;347(8995):143–147. doi: 10.1016/s0140-6736(96)90339-6. [DOI] [PubMed] [Google Scholar]
- 9.Arkinstall W, Sandler A, Goughnour B, Babul N, Harsanyi Z, Darke AC. Efficacy of controlled-release codeine in chronic non-malignant pain: a randomized, placebo-controlled clinical trial. Pain. 1995;62(2):169–178. doi: 10.1016/0304-3959(94)00262-D. [DOI] [PubMed] [Google Scholar]
- 10.Peloso PM, Bellamy N, Bensen W, Thomson GT, Harsanyi Z, Babul N, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol. 2000;27(3):764–771. [PubMed] [Google Scholar]
- 11.Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E. Opioids for chronic non-cancer pain: a meta-analysis of effectiveness and side effects. CMAJ. 2006;174(11):1589–1594. doi: 10.1503/cmaj.051528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazarus H, Fitzmartin RD, Goldenheim PD. A multi-investigator clinical evaluation of oral controlled-release morphine (MS Contin tablets) administered to cancer patients. Hosp J. 1990;6(4):1–15. doi: 10.1080/0742-969x.1990.11882680. [DOI] [PubMed] [Google Scholar]
- 13.McCarberg BH, Barkin RL. Long-acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther. 2001;8(3):181–186. doi: 10.1097/00045391-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Tunks E, Mailis-Gagnon A, Moulin D, Moore D, Wilson L, Kahan M. Evidence-based recommendations for the medical management of chronic non-malignant pain. Toronto, Ont: College of Physicians and Surgeons of Ontario; 2000. [Google Scholar]
- 15.Collins SL, Moore RA, McQuay HJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: a quantitative systematic review. J Pain Symptom Manage. 2000;20(6):449–458. doi: 10.1016/s0885-3924(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 16.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159(16):1931–1937. doi: 10.1001/archinte.159.16.1931. [DOI] [PubMed] [Google Scholar]
- 17.Portenoy RK, Foley KM, Inturrisi CE. The nature of opioid responsiveness and its implications for neuropathic pain: new hypotheses derived from studies of opioid infusions. Pain. 1990;43(3):273–286. doi: 10.1016/0304-3959(90)90025-9. [DOI] [PubMed] [Google Scholar]
- 18.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352(13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 19.Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66(6):837–844. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- 20.Rao SG, Clauw DJ. The management of fibromyalgia. Drugs Today (Barc) 2004;40(6):539–554. doi: 10.1358/dot.2004.40.6.850485. [DOI] [PubMed] [Google Scholar]
- 21.Samborski W, Lezanska-Szpera M, Rybakowski JK. Effects of antidepressant mirtazapine on fibromyalgia symptoms. Rocz Akad Med Bialymst. 2004;49:265–269. [PubMed] [Google Scholar]
- 22.Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 23.Finset A, Wigers SH, Gotestam KG. Depressed mood impedes pain treatment response in patients with fibromyalgia. J Rheumatol. 2004;31(5):976–980. [PubMed] [Google Scholar]
- 24.Lautenschlager J. Present state of medication therapy in fibromyalgia syndrome. Scand J Rheumatol Suppl. 2000;113:32–36. doi: 10.1080/030097400446616. [DOI] [PubMed] [Google Scholar]
- 25.Gowans SE, deHueck A, Voss S, Silaj A, Abbey SE, Reynolds WJ. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. 2001;45(6):519–529. doi: 10.1002/1529-0131(200112)45:6<519::aid-art377>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 26.Langman MJ. Ulcer complications associated with anti-inflammatory drug use. What is the extent of the disease burden? Pharmacoepidemiol Drug Saf. 2001;10(1):13–19. doi: 10.1002/pds.561. [DOI] [PubMed] [Google Scholar]
- 27.Schug SA, Zech D, Grond S. Adverse effects of systemic opioid analgesics. Drug Saf. 1992;7(3):200–213. doi: 10.2165/00002018-199207030-00005. [DOI] [PubMed] [Google Scholar]
- 28.Marmor M, Penn A, Widmer K, Levin RI, Maslansky R. Coronary artery disease and opioid use. Am J Cardiol. 2004;93(10):1295–1297. doi: 10.1016/j.amjcard.2004.01.072. [DOI] [PubMed] [Google Scholar]
- 29.Warner JS. The outcome of treating patients with suspected rebound headache. Headache. 2001;41(7):685–692. doi: 10.1046/j.1526-4610.2001.041007685.x. [DOI] [PubMed] [Google Scholar]
- 30.Zed PJ, Loewen PS, Robinson G. Medication-induced headache: overview and systematic review of therapeutic approaches. Ann Pharmacother. 1999;33(1):61–72. doi: 10.1345/aph.18184. [DOI] [PubMed] [Google Scholar]
- 31.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63(2):139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 32.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3(5):377–384. doi: 10.1054/jpai.2002.126790. [DOI] [PubMed] [Google Scholar]
- 33.Hanks GW, O’Neill WM, Simpson P, Wesnes K. The cognitive and psychomotor effects of opioid analgesics. II. A randomized controlled trial of single doses of morphine, lorazepam and placebo in healthy subjects. Eur J Clin Pharmacol. 1995;48(6):455–460. doi: 10.1007/BF00194334. [DOI] [PubMed] [Google Scholar]
- 34.Fishbain DA, Cutler RB, Rosomoff HL, Rosomoff RS. Are opioid-dependent/tolerant patients impaired in driving-related skills? A structured evidence-based review. J Pain Symptom Manage. 2003;25(6):559–577. doi: 10.1016/s0885-3924(03)00176-3. [DOI] [PubMed] [Google Scholar]
- 35.Benjamin S, Morris S, McBeth J, Macfarlane GJ, Silman AJ. The association between chronic widespread pain and mental disorder: a population-based study. Arthritis Rheum. 2000;43(3):561–567. doi: 10.1002/1529-0131(200003)43:3<561::AID-ANR12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 36.Nakao M, Yamanaka G, Kuboki T. Major depression and somatic symptoms in a mind/body medicine clinic. Psychopathology. 2001;34(5):230–235. doi: 10.1159/000049315. [DOI] [PubMed] [Google Scholar]
- 37.Petrak F, Hardt J, Kappis B, Nickel R, Tiber Egle U. Determinants of health-related quality of life in patients with persistent somatoform pain disorder. Eur J Pain. 2003;7(5):463–471. doi: 10.1016/S1090-3801(03)00014-4. [DOI] [PubMed] [Google Scholar]
- 38.Tylee A, Gandhi P. The importance of somatic symptoms in depression in primary care. Prim Care Companion J Clin Psychiatry. 2005;7(4):167–176. doi: 10.4088/pcc.v07n0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Covington E, Kotz M. Pain reduction with opioid elimination. Pain Med. 2002;3(2):183. [Google Scholar]
- 40.Lang E, Liebig K, Kastner S, Neundorfer B, Heuschmann P. Multidisciplinary rehabilitation versus usual care for chronic low back pain in the community: effects on quality of life. Spine J. 2003;3(4):270–276. doi: 10.1016/s1529-9430(03)00028-7. [DOI] [PubMed] [Google Scholar]
- 41.Nissen LM, Tett SE, Cramond T, Williams B, Smith MT. Opioid analgesic prescribing and use—an audit of analgesic prescribing by general practitioners and The Multidisciplinary Pain Centre at Royal Brisbane Hospital. Br J Clin Pharmacol. 2001;52(6):693–698. doi: 10.1046/j.1365-2125.2001.01502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olason M. Outcome of an interdisciplinary pain management program in a rehabilitation clinic. Work. 2004;22(1):9–15. [PubMed] [Google Scholar]
- 43.Rome JD, Townsend CO, Bruce BK, Sletten CD, Luedtke CA, Hodgson JE. Chronic noncancer pain rehabilitation with opioid withdrawal: comparison of treatment outcomes based on opioid use status at admission. Mayo Clin Proc. 2004;79(6):759–768. doi: 10.4065/79.6.759. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan M, Edlund M, Steffick D, Unutzer J. Regular use of prescribed opioids: association with common psychiatric disorders in a population-based sample. J Pain. 2005;6(3 Suppl 1):53. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Fishman SM, Mahajan G, Jung SW, Wilsey BL. The trilateral opioid contract. Bridging the pain clinic and the primary care physician through the opioid contract. J Pain Symptom Manage. 2002;24(3):335–344. doi: 10.1016/s0885-3924(02)00486-4. [DOI] [PubMed] [Google Scholar]
- 46.Tyndale RF, Droll KP, Sellers EM. Genetically deficient CYP2D6 metabolism provides protection against oral opiate dependence. Pharmacogenetics. 1997;7(5):375–379. doi: 10.1097/00008571-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 47.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260(1):275–285. [PubMed] [Google Scholar]
- 48.Duhmke RM, Cornblath DD, Hollingshead JR. Tramadol for neuropathic pain. Cochrane Database Syst Rev. 2004. CD003726. [DOI] [PubMed]
- 49.Emkey R, Rosenthal N, Wu SC, Jordan D, Kamin M. Efficacy and safety of tramadol/acetaminophen tablets (Ultracet) as add-on therapy for osteoarthritis pain in subjects receiving a COX-2 nonsteroidal antiinflammatory drug: a multicenter, randomized, double-blind, placebo-controlled trial. J Rheumatol. 2004;31(1):150–156. [PubMed] [Google Scholar]
- 50.Jung YS, Kim DK, Kim MK, Kim HJ, Cha IH, Lee EW. Onset of analgesia and analgesic efficacy of tramadol/acetaminophen and codeine/acetaminophen/ibuprofen in acute postoperative pain: a single-center, single-dose, randomized, active-controlled, parallel-group study in a dental surgery pain model. Clin Ther. 2004;26(7):1037–1045. doi: 10.1016/s0149-2918(04)90175-0. [DOI] [PubMed] [Google Scholar]
- 51.Moore RA, McQuay HJ. Single-patient data meta-analysis of 3453 postoperative patients: oral tramadol versus placebo, codeine and combination analgesics. Pain. 1997;69(3):287–294. doi: 10.1016/S0304-3959(96)03291-5. [DOI] [PubMed] [Google Scholar]
- 52.Savoia G, Loreto M, Scibelli G. [Systemic review of trials on the use of tramadol in the treatment of acute and chronic pain]. Minerva Anestesiol. 2000;66(10):713–731. [PubMed] [Google Scholar]
- 53.Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002;23(2):121–130. doi: 10.1016/s0885-3924(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 54.Schnitzer TJ, Kamin M, Olson WH. Tramadol allows reduction of naproxen dose among patients with naproxen-responsive osteoarthritis pain: a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 1999;42(7):1370–1377. doi: 10.1002/1529-0131(199907)42:7<1370::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 55.Vickers MD, O’Flaherty D, Szekely SM, Read M, Yoshizumi J. Tramadol: pain relief by an opioid without depression of respiration. Anaesthesia. 1992;47(4):291–296. doi: 10.1111/j.1365-2044.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 56.Ripple MG, Pestaner JP, Levine BS, Smialek JE. Lethal combination of tramadol and multiple drugs affecting serotonin. Am J Forensic Med Pathol. 2000;21(4):370–374. doi: 10.1097/00000433-200012000-00015. [DOI] [PubMed] [Google Scholar]
- 57.Marquardt KA, Alsop JA, Albertson TE. Tramadol exposures reported to statewide poison control system. Ann Pharmacother. 2005;39(6):1039–1044. doi: 10.1345/aph.1E577. [DOI] [PubMed] [Google Scholar]
- 58.Schnitzer TJ; American College of Rheumatology. Update of ACR guidelines for osteoarthritis: role of the coxibs. J Pain Symptom Manage. 2002;23(4 Suppl):24–30. doi: 10.1016/s0885-3924(02)00372-x. [DOI] [PubMed] [Google Scholar]
- 59.Payne R. Factors influencing quality of life in cancer patients: the role of transdermal fentanyl in the management of pain. Semin Oncol. 1998;25(3 Suppl 7):47–53. [PubMed] [Google Scholar]
- 60.Allan L, Hays H, Jensen NH, de Waroux BL, Bolt M, Donald R, et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ. 2001;322(7295):1154–1158. doi: 10.1136/bmj.322.7295.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osborne R, Joel S, Grebenik K. The pharmacokinetics of morphine and morphine glucuronides in kidney failure. Clin Pharmacol Ther. 1993;54:158–167. doi: 10.1038/clpt.1993.127. [DOI] [PubMed] [Google Scholar]
- 62.Novak S, Nemeth WC, Lawson KA. Trends in medical use and abuse of sustained-release opioid analgesics: a revisit. Pain Med. 2004;5(1):59–65. doi: 10.1111/j.1526-4637.2004.04001.x. [DOI] [PubMed] [Google Scholar]
- 63.Zacny JP, Gutierrez S. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non–drug-abusing volunteers. Psychopharmacology (Berl) 2003;170(3):242–254. doi: 10.1007/s00213-003-1540-9. [DOI] [PubMed] [Google Scholar]
- 64.Gourlay D. Methadone for pain guidelines. Toronto, Ont: College of Physicians and Surgeons of Ontario; 2005. [Google Scholar]
- 65.Morley JS, Bridson J, Nash TP, Miles JB, White S, Makin MK. Low-dose methadone has an analgesic effect in neuropathic pain: a double-blind randomized controlled crossover trial. Palliat Med. 2003;17(7):576–587. doi: 10.1191/0269216303pm815oa. [DOI] [PubMed] [Google Scholar]
- 66.Davis AM, Inturrisi CE. d-Methadone blocks morphine tolerance and N-methyl-D-aspartate–induced hyperalgesia. J Pharmacol Exp Ther. 1999;289(2):1048–1053. [PubMed] [Google Scholar]
- 67.Caplehorn JR. Deaths in the first two weeks of maintenance treatment in NSW in 1994: identifying cases of iatrogenic methadone toxicity. Drug Alcohol Rev. 1998;17:9–17. doi: 10.1080/09595239800187551. [DOI] [PubMed] [Google Scholar]
- 68.Bruera E, Palmer JL, Bosnjak S, Rico MA, Moyano G, Sweeney C, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: a randomized, double-blind study. J Clin Oncol. 2004;22(1):185–192. doi: 10.1200/JCO.2004.03.172. [DOI] [PubMed] [Google Scholar]
- 69.Hale ME, Fleischmann R, Salzman R, Wild J, Iwan T, Swanton RE, et al. Efficacy and safety of controlled-release versus immediate-release oxycodone: randomized, double-blind evaluation in patients with chronic back pain. Clin J Pain. 1999;15(3):179–183. doi: 10.1097/00002508-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Kaiko RF, Grandy RP, Oshlack B, Pav J, Horodniak J, Thomas G, et al. The United States experience with oral controlled-release morphine (MS Contin tablets). Parts I and II. Review of nine dose titration studies and clinical pharmacology of 15-mg, 30-mg, 60-mg, and 100-mg tablet strengths in normal subjects. Cancer. 1989;63(11 Suppl):2348–2354. doi: 10.1002/1097-0142(19890601)63:11<2348::aid-cncr2820631146>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 71.Chary S, Goughnour BR, Moulin DE, Thorpe WR, Harsanyi Z, Darke AC. The dose-response relationship of controlled-release codeine (Codeine Contin) in chronic cancer pain. J Pain Symptom Manage. 1994;9(6):363–371. doi: 10.1016/0885-3924(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 72.Kloke M, Rapp M, Bosse B, Kloke O. Toxicity and/or insufficient analgesia by opioid therapy: risk factors and the impact of changing the opioid. A retrospective analysis of 273 patients observed at a single center. Support Care Cancer. 2000;8(6):479–486. doi: 10.1007/s005200000153. [DOI] [PubMed] [Google Scholar]
- 73.Canadian Pharmacists Association. Compendium of pharmaceuticals and specialties. Ottawa, Ont: Canadian Pharmacists Association; 2004. [Google Scholar]
- 74.Brooks I, De Jager R, Blumenreich M, George E, Savarese JJ. Principles of cancer pain management. Use of long-acting oral morphine. J Fam Pract. 1989;28(3):275–280. [PubMed] [Google Scholar]
- 75.Parris WC, Johnson BW, Jr, Croghan MK, Moore MR, Kojasteh J, Reder RF, et al. The use of controlled-release oxycodone for the treatment of chronic cancer pain: a randomized, double-blind study. J Pain Symptom Manage. 1998;16(4):205–211. doi: 10.1016/s0885-3924(98)00064-5. [DOI] [PubMed] [Google Scholar]
- 76.Guo Z, Wills P, Viitanen M, Fastbom J, Winblad B. Cognitive impairment, drug use, and the risk of hip fracture in persons over 75 years old: a community-based prospective study. Am J Epidemiol. 1998;148(9):887–892. doi: 10.1093/oxfordjournals.aje.a009714. [DOI] [PubMed] [Google Scholar]
- 77.Ensrud KE, Blackwell T, Mangione CM, Bowman PJ, Bauer DC, Schwartz A, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163(8):949–957. doi: 10.1001/archinte.163.8.949. [DOI] [PubMed] [Google Scholar]
- 78.Davis PE, Liddiard H, McMillan TM. Neuropsychological deficits and opiate abuse. Drug Alcohol Depend. 2002;67(1):105–108. doi: 10.1016/s0376-8716(02)00012-1. [DOI] [PubMed] [Google Scholar]
- 79.Darke S, Sims J, McDonald S, Wickes W. Cognitive impairment among methadone maintenance patients. Addiction. 2000;95(5):687–695. doi: 10.1046/j.1360-0443.2000.9556874.x. [DOI] [PubMed] [Google Scholar]