Abstract
This study was carried out to determine the effect of 2-aminoethoxydiphenyl borate (2APB), a common activator of transient receptor potential vanilloid (TRPV) types 1, 2 and 3 channels, on cardiorespiratory reflexes, pulmonary C-fiber afferents and isolated pulmonary capsaicin-sensitive neurons. In anesthetized, spontaneously breathing rats, intravenous bolus injection of 2APB elicited the pulmonary chemoreflex responses, characterized by apnea, bradycardia and hypotension. After perineural treatment of both cervical vagi with capsaicin to block the conduction of C fibers, 2APB no longer evoked any of these reflex responses. In open-chest and artificially ventilated rats, 2APB evoked an abrupt and intense discharge in vagal pulmonary C fibers in a dose-dependent manner. The stimulation of C fibers by 2APB was attenuated but not abolished by capsazepine, a selective antagonist of the TRPV1, that completely blocked the response to capsaicin in these C-fiber afferents. In isolated pulmonary capsaicin-sensitive neurons, 2APB concentration-dependently evoked an inward current which is partially inhibited by capsazepine but almost completely abolished by ruthenium red, an effective blocker of all TRPV channels. In conclusion, 2APB evokes a consistent and distinct stimulatory effect on pulmonary C fibers in vivo, and on isolated pulmonary capsaicin-sensitive neurons in vitro. These results establish the functional evidence demonstrating that TRPV1, V2 and V3 channels are expressed on these sensory neurons and their terminals.
Keywords: pulmonary chemoreflex, vagal C-fiber, pulmonary sensory neuron, TRP channel
INTRODUCTION
The transient receptor potential (TRP) superfamily is a novel and rapidly expanding group of ion channel proteins. Mammalian homologues of the TRP channel gene encode a family of >20 related ion channels that are further divided into TRPC (canonical), TRPV (vanilloid) and TRPM (melastatin) subfamilies (12, 32, 33). These channels are widely distributed in a variety of mammalian organisms, tissues and cells types, and sense local changes in stimuli ranging from light, olfaction, temperature, pH, osmolarity, to mechanical, chemical and metabolic stress (31, 43). Currently the TRPV subfamily has six members. Like members in other TRP subfamilies, they are all cation channels; however, they vary substantially in their selectivity and mode of activation (32, 33). Except the temperature as the common stimulus of thermosensitive TRPV channels, namely TRPV1-4, most TRPV channels can be activated by nonthermal stimuli (11, 16, 20, 40); for example, TRPV1 can be activated by capsaicin, protons or endocannabinoids (8, 10, 21, 22, 27); TRPV4 can be activated by 4α-phorbol 12,13-didecanoate and several archidonic acid metabolites (11, 35, 44, 45). 2-Aminoethoxydiphenyl borate (2APB), which has been used extensively to investigate the effects of inositol 1,4,5-trisphosphate (IP3) -induced Ca2+ release and Ca2+ influx in a variety of cell types (15, 30, 46), was recently found to be a common and potent activator of three TRPV channels: TRPV1, V2 and V3, but not TRPV4, V5 and V6 (20).
Nonmylinated (C-) fibers represent approximately 75% of the afferent fibers in the vagal branches innervating the entire respiratory tract (2, 14). Cell bodies of these afferent fibers reside in two adjacent but distinct anatomic structures, the nodose and intracranial jugular ganglia. The afferent activity arising from these C-fiber endings plays an important role in regulating the respiratory and cardiovascular functions under both normal and abnormal physiologic conditions (14, 26). Capsaicin, a pungent active ingredient of hot pepper, is the chemical agent most commonly used in the studies of the physiological properties and functions of these afferents because of its specificity and potency in stimulating these afferents (18, 19). Bolus injection of capsaicin into the pulmonary circulation stimulates pulmonary C-fiber afferents and elicits pulmonary chemoreflex responses (apnea, bradycardia and hypotension) which are mediated through the TRPV1 activation (14, 26). Indeed, expression of the TRPV1 and other members in the TRPV family in the vagal sensory (nodose and jugular) ganglia has been recently demonstrated (10, 17, 48). However, whether they are expressed by the neurons innervating the lungs is not known. Furthermore, physiological roles of these TRPV channels, except the TRPV1, in regulating the function of pulmonary afferent nervous system has been hampered due to a lack of selective activator and blocker of these channels. In light of the ability of 2APB in activating these TRPV channels (11, 20), this study was therefore carried out: 1) to characterize the cardiorespiratory reflex responses to intravenous (i.v.) injection of 2APB in spontaneously breathing rats and to evaluate the role of vagal C-fiber afferents in eliciting these responses; 2) to determine the contribution of TRPV1 to the response of single-unit pulmonary C fibers to 2APB in artificially ventilated rats; and 3) to investigate the response of isolated pulmonary capsaicin-sensitive neurons to 2APB, and to differentiate the relative roles of TRPV1 and other TRPV channels in these responses.
MATERIALS AND METHODS
Animal preparation
The procedures described below were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and also were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Sprague-Dawley rats (355-440 g) were initially anesthetized with an intraperitional injection of α-chloralose (100 mg/kg; Sigma Chemical, St. Louis, MO, USA) and urethane (500 mg/kg; Sigma) dissolved in a borax solution (2 %; Sigma); supplemental doses of α-chloralose (∼10 mg/kg/h) and urethane (∼50 mg/kg/h) were i.v. injected to maintain abolition of pain reflexes elicited by paw-pinch. One femoral artery was cannulated for recording the arterial blood pressure (ABP) with a pressure transducer (Statham P23AA). For i.v. administration of pharmacological agents, the left jugular vein was cannulated and a catheter was advanced until its tip was positioned just above the right atrium. The volume of each bolus injection was 0.1 ml, which was first injected into the catheter (dead space, ∼0.2 ml) and then flushed into the circulation by an injection of 0.3 ml saline. A short tracheal cannula was inserted after a tracheotomy, and tracheal pressure (Ptr) was measured (Validyne MP 45-28) via a side-port of the trachea cannula. Body temperature was maintained at ∼36 °C by means of a heating pad placed under the animal lying in a supine position. At the end of the experiment, the animal was killed by an i.v. injection of KCl (200 mg/kg).
Measurements of respiratory and cardiovascular responses
Rats breathed spontaneously via the tracheal cannula. Respiratory flow was measured with a heated pneumotachograph and a differential pressure transducer (Validyne MP45-14), and integrated to give tidal volume (VT). Respiratory frequency (f), inspiratory and expiratory durations (TE), VT, minute ventilation (V̇E), heart rate (HR) and ABP were analyzed (Biocybernetics TS-100) on a breath-by-breath basis by an on-line computer.
Recording of pulmonary C-fiber activity
Pulmonary C-fiber activities were recorded in anesthetized, open-chest, artificially ventilated rats, as described in details previously (18, 27). Briefly, the expiratory outlet of the respirator was placed under 3-cmH2O pressure to maintain a near-normal functional residual capacity. VT and f were set at 8-10 ml/kg and 50 breaths/min, respectively. Both cervical vagus nerves were separated from carotid arteries and then sectioned to eliminate any possible influence of vagal-mediated reflex bronchoconstriction on afferent responses. The vagus nerve was ligated just above the diaphragm to eliminate afferent signals arising from lower visceral organs. The caudal end of the cut right vagus nerve was placed on a small dissecting platform and immersed in a pool of mineral oil. A thin filament was teased away from the desheathed nerve trunk and placed on a platinum-iridium hook electrode. Action potentials were amplified (Grass P511K), monitored by an audio monitor (Grass AM8RS) and displayed on an oscilloscope (Tektronix 2211). The thin filament was further split until the afferent activity arising from a single unit was electrically isolated. Our criteria for identifying pulmonary C fibers were as follows: 1) an abrupt and intense response to right-atrial injection of capsaicin (1 μg/kg) within 2 s after the injection; 2) a weak response to lung inflation (Ptr = 30 cmH2O); and 3) the general locations of the receptors identified by their responses to gentle probing of the lungs with a blunt-ended glass rod. The signals of the fiber activity (FA), Ptr and ABP were recorded on a VCR format data recorder (Vetter 4000A) and were analyzed by an online data acquisition system (Biocybernetics TS-100) in 0.5-s intervals. Increase in FA (ΔFA) was calculated as the difference between peak FA (2-s average) and baseline FA (10-s average). A C-fiber was considered to be activated when ΔFA exceeded 1 impulse/s.
Labeling pulmonary vagal sensory neurons with DiI
Sensory neurons innervating the lungs and airways were identified by retrograde labeling from the lungs by using the fluorescent tracer, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI). Young adult Sprague-Dawley rats (150-200 g) were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg) and intubated with a polyethylene catheter (PE 150) with its tip positioned in the trachea above the thoracic inlet. With the head tilted up at ∼30°, DiI was initially dissolved and sonicated in ethanol, diluted in saline (1% ethanol v/v), and then instilled into the lungs (0.15 mg/ml; 0.2 ml × 2). After 7-11 days, an interval previously determined to be sufficient for the dye to diffuse to the cell body, the nodose and jugular ganglia were extracted.
Isolation and culture of nodose and jugular ganglion neurons
The animals were anesthetized with halothane inhalation and decapitated. The head was immediately immersed in ice-cold Hank's balanced salt solution. Nodose and jugular ganglia were extracted under a dissecting microscope and placed in ice-cold Dulbecco's minimal essential medium/F12 (DMEM/F12) solution. Each ganglion was desheathed, cut into ∼10 pieces, placed in 0.125% type IV collagenase, and incubated for 1 h in 5% CO2 in air at 37 °C. The ganglion suspension was centrifuged (150 × g, 5 min) and supernatant-aspirated. The cell pellet was resuspended in 0.05% trypsin and 0.53 mM EDTA in Hanks' balanced salt solution for 5 min and centrifuged (150 × g, 5 min); the pellet was then resuspended in a modified DMEM/F-12 solution (DMEM/F-12 supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μM MEM nonessential amino acids) and gently triturated with a small bore fire-polished Pasteur pipette. The dispersed cell suspension was centrifuged (500 × g, 8 min) through a layer of 15% bovine serum albumin to separate the cells from the myelin debris. The pellets were resuspended in the modified DMEM/F-12 solution supplemented with 50 ng/ml 2.5S nerve growth factor, plated onto poly-l-lysine-coated glass coverslips, and then incubated overnight (5% CO2 in air at 37 °C).
Whole cell perforated patch clamp recording
The methods were described previously (20). Briefly, the coverslip containing the attached cells was placed in the center of a small (0.2 ml) recording chamber that was perfused by gravity feed (VC-6 perfusion valve controller; Warner Instruments, Hamden, CT) with extracellular solution (ECS), and ECS containing capsazepine or ruthenium red at 2 ml/min, whereas the chemical stimulants (2APB and capsaicin) were applied by a pressure-driven drug delivery system (ALA-VM8; ALA Scientific Instruments, Westbury, NY), with its tip positioned to ensure that the cell was fully within the stream of the injectate. The ECS consisted of (in mM) 136 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 glucose, 10 Hepes, pH at 7.4. The intracellular solution contained (in mM) 92 potassium gluconate, 40 KCl, 8 NaCl, 1 CaCl2, 0.5 MgCl2, 10 EGTA, 10 Hepes, pH at 7.2. Recordings were made in the whole cell perforated patch configuration (50 μg/ml gramicidin) using Axopatch 200B/pCLAMP 9.0 (Axon Instruments, Union City, CA). The experiments were performed at room temperature (22 °C). The data were acquired at 5 kHz and filtered at 2 kHz. Series resistance was compensated at ∼80%. The resting membrane potential was held at −70 mV.
Experimental design and protocol
Six series of experiments were carried out. Study Series 1. The ventilatory and cardiovascular responses elicited by injections of increasing doses of 2APB (1, 2 and 3 mg/kg) and its vehicle (for the 3 mg/kg dose) were determined; to avoid any accumulated effect, at least 10 min elapsed between 2 injections in this and subsequent protocols. Study Series 2. The role of vagal C-fiber afferents in 2APB-induced cardiorespiratory responses was evaluated. The responses to injections of capsaicin (1 μg/kg) and 2APB (3 mg/kg) were repeated at 2 and 15 min, respectively, after a bilateral perineural capsaicin treatment (PNCT), which produces a selective and reversible blockade of the C-fiber conduction in the vagus nerves (27). Briefly, cotton strips soaked in capsaicin solution (250 μg/ml) were wrapped around a 2-3 mm segment of the isolated cervical vagi for 20 min and then removed. The responses to capsaicin and 2APB were again tested at 60 and 75 min, respectively, after PNCT. Study Series 3. Single-unit pulmonary C-fiber responses to increasing doses of 2APB (1, 2 and 3 mg/kg) and its vehicle (for the 3 mg/kg dose) were determined in 26 C-fibers from 15 rats. Study Series 4. This series was carried out to determine the role of the TRPV1 receptor in the 2APB-induced stimulation of pulmonary C fibers. The 2APB- (3 mg/kg) and capsaicin- (1 μg/kg) induced afferent responses were studied and compared before and after a constant i.v. infusion of capsazepine (0.3 mg/kg/min; 5 min), a selective TRPV1 receptor antagonist (4, 41), or its vehicle. When responses to 2APB and capsaicin were studied in the same fibers (n = 8), the sequence of the injections of these two chemicals was alternated to achieve a balanced design. Study Series 5. The responses to increasing concentrations of 2APB (30, 100 and 300 μM) or its vehicle (for the 300 μM concentration) was determined in isolated pulmonary capsaicin-sensitive neurons. Study Series 6. This series was carried out to differentiate the relative roles of TRPV1 and other TRPV channels in 2APB-induced inward current in pulmonary capsaicin-sensitive neurons. Responses to 2APB (300 μM) and capsaicin (0.3 or 1 μM) were studied before and after a 5-min pretreatment with capsazepine (10 μM) or ruthenium red (5 μM); the latter is an inorganic dye and a non-specific blocker of Ca2+ channels, and is also known for its non-selective but effective blocking effect on all the TRPV channels (13, 16). The sequence of applications of 2APB and capsaicin was alternated when they were studied in the same neurons (n = 10).
Materials
A stock solution of 2APB (100 mM; Tocris, Ellisville, MO, USA) was prepared in dimethyl sulfoxide (DMSO, Sigma), and that of capsaicin (1 mM; Sigma) in 1% Tween 80, 1 % ethanol and 98 % saline (study series 1-4) or ECS (study series 5 and 6). Stock solutions of capsazepine (Sigma) and ruthenium red (Sigma) were both prepared in DMSO at the concentration of 30 mM. Solutions of these chemical agents at desired concentrations were then prepared daily by dilution with saline on the basis of the animal's body weight in study series 1-4, or diluted with ECS in study series 5 and 6.
Statistical analysis
Data were analyzed with a one-way or two-way ANOVA. When the ANOVA showed a significant interaction, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). A P value < 0.05 was considered significant. Data are means ± SE.
RESULTS
2APB-induced pulmonary chemoreflex responses
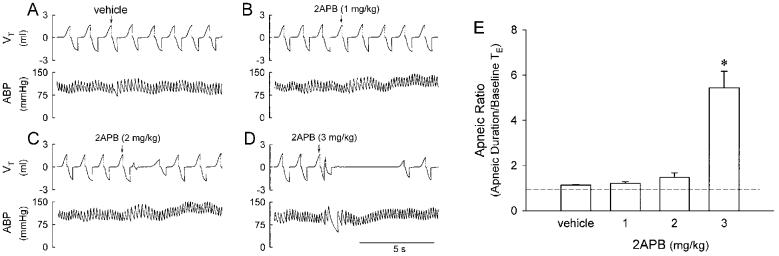
Bolus injections of 2APB at the dose of 1 and 2 mg/kg only produced very mild ventilatory and cardiovascular inhibitions. A higher dose (3 mg/kg) of 2APB consistently elicited apnea, bradycardia and hypotension (Fig. 1). Both respiratory and cardiovascular responses returned to baseline within 10 s. The reproducibility of the cardiorespiratory responses to this high dose of 2APB was established in four rats and no tachyphylaxis was observed when at least 10 min elapsed between two injections. The apneic response induced by 2APB at the dose of 3 mg/kg was clearly shown by a prolonged TE, reaching 545 ± 73 % of the baseline TE (Fig. 1E). In contrast, the injection of vehicle did not cause any significant change in respiration.
Fig. 1.
Pulmonary chemoreflex responses to intravenously injection of 2APB in anesthetized rats. A, B, C and D: experimental records illustrating the responses to intravenous injections of vehicle, and 1, 2 and 3 mg/kg 2APB, respectively, in an anesthetized rat breathing spontaneously. Injectate (0.1 ml) was first slowly injected into the catheter (dead space, 0.2 ml) and then flushed (at arrow), as a bolus with saline (0.3 ml). Rat body weight, 390 g. VT, tidal volume; ABP, arterial blood pressure. E: effect of increasing doses of 2APB on apneic ratio (apneic duration/baseline TE). Apneic duration, longest expiratory duration within 3 s after the injection. Baseline TE, average expiratory duration over 10 control breaths. Dashed line, apneic ratio of 100% level (no apnea). * Significantly different (P < 0.05) from the response to vehicle. Data of each group are means ± SE of 12 rats.
Effect of PNCT on capsaicin- and 2APB-induced pulmonary chemoreflex responses
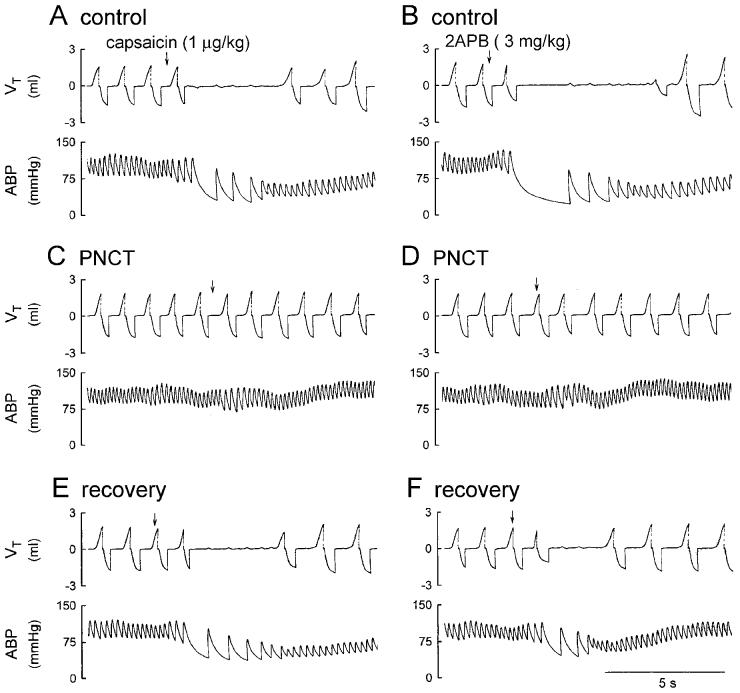
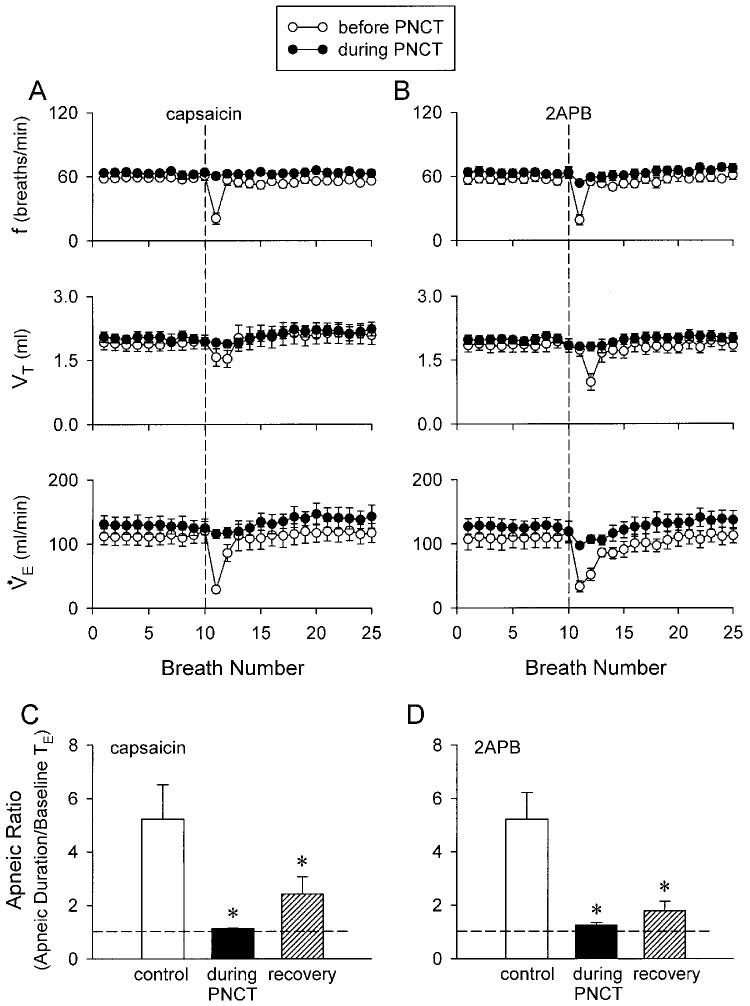
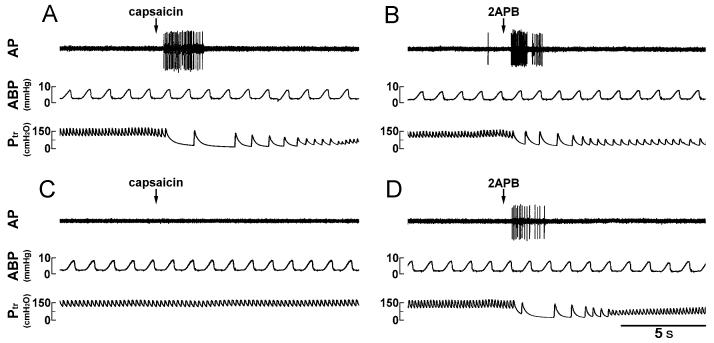
In control condition, i.v. injection of 2APB (3 mg/kg) induced ventilatory and cardiovascular inhibition that was comparable to the degree and pattern of that caused by 1 μg/kg capsaicin (e.g., Fig. 2, A and B). PNCT of both cervical vagi caused only slight but not significant increases in baseline f, VT, HR and ABP. However, it completely abolished the chemoreflexes (apnea, bradycardia and hypotension) induced by capsaicin (1 μg/kg), verifying its effectiveness in blocking the conduction of vagal C-fiber afferents (Figs. 2 and 3). In the same animals, the 2APB (3 mg/kg) -induced respiratory and cardiovascular inhibitions were also abolished after the treatment (Figs. 2 and 3). The responses to both capsaicin and 2APB returned toward the control in ∼60 min; however, the recovery of chemoreflex response to 2APB was consistently slower and less complete, as compared to that to capsaicin (e.g., Fig. 2, E and F; Fig 3, C and D).
Fig. 2.
Experimental records illustrating the effect of perineural capsaicin treatment (PNCT) of both cervical vagi on cardiorespiratory responses to injections of capsaicin and 2APB in an anesthetized rat. A, C and E: responses to intravenous injections of capsaicin (1 μg/kg) before, 5 min and 60 min after PNCT of both vagi, respectively. B, D and F: responses to injections of 2APB (3 mg/kg) before, 15 min and 75 min after PNCT, respectively. Rat body weight, 365 g.
Fig. 3.
Effect of perineural capsaicin treatment (PNCT) of both cervical vagi on ventilatory responses to intravenous injections of capsaicin and 2APB in anesthetized rats. A and B: ventilatory responses to capsaicin (1 μg/kg) and 2APB (3 mg/kg), repectively, before (open circle), and 5 (capsaicin) or 15 (2APB) min after (filled circle) PNCT of both vagi. f, respiratory frequency; V̇E , minute ventilation. The vertical dashed line depicts time of injection. C and D: apneic responses to capsaicin and 2APB, respectively. Control (open bars), before PNCT; PNCT (solid bars), 5 (capsaicin) or 15 (2APB) min after PNCT; recovery (hatched bars), 60 (capsaicin) or 75 (2APB) min after PNCT. For calculation of apneic ratio, see legend of Fig. 1. Dashed line, apneic ratio of 100% level (no apnea). * Significantly different (P < 0.05) from the corresponding control. Data of each group are means ± SE of 6 rats.
Dose-dependence of the stimulating effect of 2APB on pulmonary C fibers
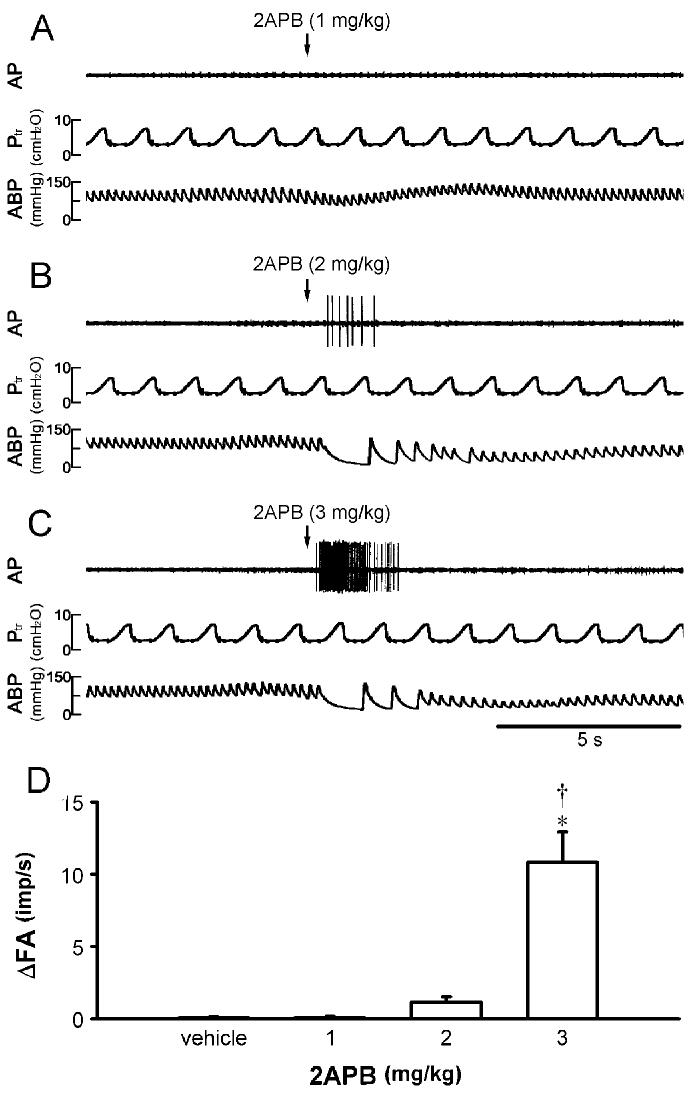
2APB stimulated pulmonary C-fiber afferents in a dose-dependent manner (Fig. 4). Injections of 2APB at the dose of 2 mg/kg activated 13 out of 26 C fibers tested but the average response (ΔFA, 1.13 ± 0.38 impulses/s; n = 26) was not significantly different from that to either 1 mg/kg (0.10 ± 0.08 impulses/s; n = 26) or vehicle (0.09 ± 0.07 impulses/s; n = 10). A higher dose of 2APB (3 mg/kg) activated all the C fibers tested (ΔFA, 10.82 ± 2.12 impulses/s; n = 26); most of the fibers started to discharge within 1 s, and the activity reached a peak in ∼2 s and returned to baseline within 4 s after the injection. In majority of the animals, this high dose of 2APB (3 mg/kg) also elicited a cardiovascular depression; the magnitudes of these responses were comparable to that by 1 μg/kg capsaicin (e.g., Fig. 5, A and B).
Fig. 4.
Effect of intravenous injection of 2APB on single pulmonary C-fiber afferents in anesthetized and open-chest rats. A, B and C: experimental records illustrating the responses to injections (arrows) of 1, 2 and 3 mg/kg 2APB, respectively. Receptor location, right accessory lobe; rat body weight, 430 g. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure. D: effect of increasing dose of 2APB on the response of pulmonary C fibers (n = 26). ΔFA, difference between peak FA (2-s average) after the injection and baseline FA (10-s average); imp, impulses. * Significantly different (P < 0.05) from the response to vehicle. †Significantly different (P < 0.05) from the response to 2 mg/kg 2APB. Data are means ± SE.
Fig. 5.
Experimental records illustrating the responses of a pulmonary C fiber arising from the right middle lobe to capsaicin or 2APB before and after pretreatment with capsazepine in an anesthetized and open-chest rat. A and C: responses to intravenous injections of capsaicin (1 μg/kg) before and 2 min after intravenously infusion of capsazepine (0.3 mg/kg/min; 5 min), respectively. B and D: responses to intravenous injections of 2APB (3 mg/kg) before and 2 min after pretreatment with the same dose of capsazepine, respectively. Rat body weight, 405 g.
Effect of capsazepine on capsaicin- and 2APB-induced pulmonary C-fiber activation and cardiovascular responses
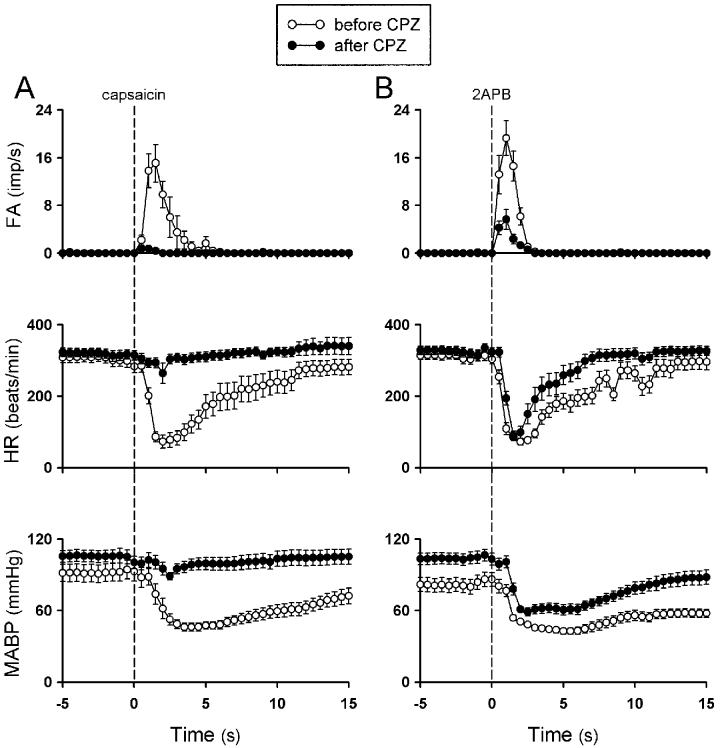
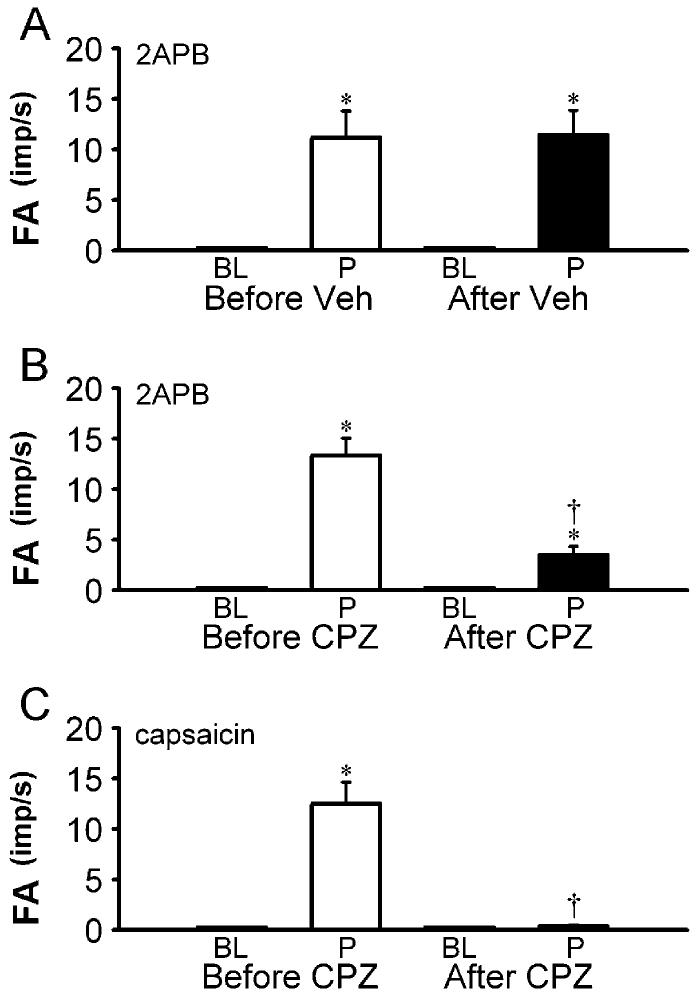
After the pretreatment with capsazepine, there was no change in the baseline FA of pulmonary C fibers (before capsazepine, 0.01 ± 0.01 impulses/s, after capsazepine, 0.01 ± 0.01 impulses/s; P > 0.05; n = 20). However, the C-fiber discharges induced by injection of 1 μg/kg capsaicin was almost completely abolished by the treatment (ΔFA: before capsazepine, 12.4 ± 2.12 impulses/s; after capsazepine, 0.35 ± 0.13 impulses/s; P < 0.05; n = 11); so did the capsaicin-induced cardiovascular depressor responses (Figs. 5, 6 and 7); the response to the same dose of capsaicin returned to the control level ∼20 min after capsazepine treatment (10.2 ± 2.25 impulses/s). In sharp contrast, capsazepine treatment only blocked ∼74% of the ΔFA induced by 2APB (before capsazepine, 13.4 ± 1.7 impulses/s, after capsazepine, 3.5 ± 0.83 impulses/s; P < 0.05, n = 17), whereas its blocking effect on 2APB-induced cardiovascular depressor responses were substantially less (Figs. 5 , 6 and 7). In a separate group of sixteen C fibers, treatment with capsazepine vehicle did not have any detectable effect on the responses induced by either capsaicin (not shown) or 2APB (Fig. 7A).
Fig. 6.
Effect of pretreatment with capsazepine on averaged pulmonary C-fiber and cardiovascular responses to capsaicin and 2APB in anesthetized and open-chest rats. A and B: response to intravenous injections of capsaicin (1 μg/kg) and 2APB (3 mg/kg), respectively, before and 2 min after intravenously infusion of capsazepine (0.3 mg/kg/min; 5 min). Open circle, before capsazepine; filled circle, after capsazepine; FA, fiber activity; HR, heart rate; imp, impulses. Data are means ± SE of 11 and 17 C fibers in A and B, respectively. Note that capsazepine only slightly shortened but did not prevent the bradycardia induced by 2APB (panel B).
Fig. 7.
Effect of pretreatment with capsazepine on pulmonary C-fiber responses to capsaicin and 2APB in anesthetized and open-chest rats. A: responses to intravenously injections of 2APB (3 mg/kg) before and 2 min after pretreatment with the vehicle of capsazepine. B and C: responses to intravenously injections of 2APB (3 mg/kg) and capsaicin (1 μg/kg), respectively, before and 2 min after pretreatment with capsazepine (0.3 mg/kg/min; 5 min). BL, baseline fiber activity averaged over 20-s; P, peak fiber activity averaged over 2-s; Veh, vehicle; CPZ, capsazepine; imp, impulses. * Significantly different (P < 0.05) from the corresponding baseline response. † Significantly different (P < 0.05) from the corresponding response before treatment with CPZ or vehicle. Data are means ± SE of 16, 17 and 11 in A, B and C, respectively.
2APB-induced whole-cell inward currents in isolated rat pulmonary capsaicin-sensitive neurons
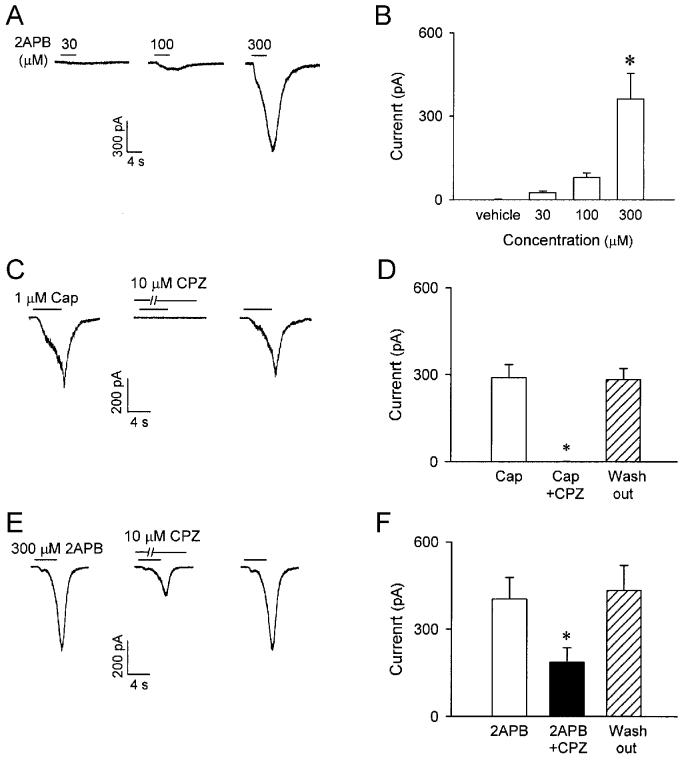
Because of the potency and selectivity of capsaicin in activating the pulmonary C fibers (18), we consider “capsaicin-sensitive neurons” as “C neurons” in this study. To identify the sensory neurons that give rise to pulmonary C fibers for whole-cell perforated patch-clamp recording, neurons isolated from nodose and jugular ganglia were selected based upon the following criteria: 1) labeled with DiI as indicated by fluorescence intensity; 2) smooth surface and spherical shape without visible processes; 3) whole-cell capacitance <30 pF; and 4) responding to capsaicin (1 μM). Application of 2APB in the range of 30-300 μM (4-8 s) evoked incremental whole-cell inward currents in a concentration-dependent manner (Fig. 8, A and B). 300 μM 2APB stimulated all the capsaicin-sensitive pulmonary nodose and jugular ganglion neurons held at −70 mV; the inward currents evoked by this high concentration of 2APB and 0.3-1 μM capsaicin followed a very similar pattern and reached a comparable amplitude in 13 out of 24 pulmonary sensory neurons tested, and a clear disparity displayed in the remaining neurons.
Fig. 8.
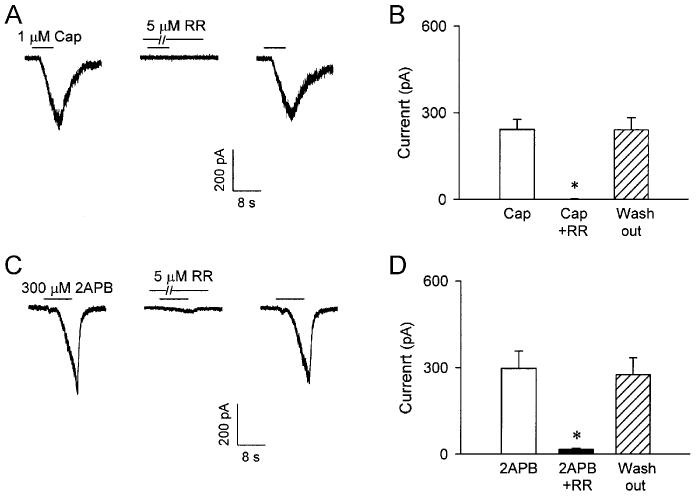
2APB-induced whole-cell inward current and effect of capsazepine on the currents induced by capsaicin and 2APB in isolated rat pulmonary capsaicin-sensitive neurons. A: experimental records illustrating that a small pulmonary nodose ganglia neuron (18.5 pF) was activated by application of three concentrations of 2APB (30, 100 and 300 μM; 4 s, added horizontal bars). B: group data showing the concentration-dependence of the whole-cell inward currents induced by 2APB (4-8 s). * Significantly different (P < 0.05) from the response to 2APB vehicle. Data are means ± SE of 14 neurons tested for each group, except the group of 300 μM (n = 24). C and E: experimental records illustrating the effect of pretreatment with capsazepine (10 μM; 5 min), a selective TRPV1 antagonist, on the inward currents induced by capsaicin (1 μM; 5 s) and 2APB (300 μM; 4 s), respectively. D and F: group data for responses to capsaicin (0.3-1 μM; 4-8 s) and 2APB (300 μM; 4-8 s), respectively, before (open bars), during (closed bars) and ∼60 min after (hatched bars) capsezepine (10 μM; 5 min). Cap, capsaicin; CPZ, capsazepine. * Significantly different (P < 0.05) from the response before capsazepine treatment. Data are means ± SE of 8 and 9 pulmonary capsaicin-sensitive neurons in D and F, respectively.
Effect of capsazepine and ruthenium red on capsaicin- and 2APB-induced whole-cell inward currents in isolated pulmonary capsaicin-sensitive neurons
Pretreatment with capsazepine (10 μM; 5 min) completely abolished capsaicin (0.3-1 μM; 4-8 s) –induced inward current (Fig. 8, C and D; before capsazepine, 289.5 ± 44.7 pA; after capsazepine, 0.8 ± 0.4 pA; P < 0.05; n = 8), but only inhibited ∼60 % of the currents induced by 2APB (300 μM; 4-8 s) (Fig. 8, E and F; before capsazepine, 403.1 ± 74.2 pA; after capsazepine, 186.6 ± 49.8 pA; P < 0.05; n = 9); both capsaicin- and 2APB-induced inward currents returned to the control level after ∼60 min wash out. In contrast, pretreatment with ruthenium red (5 μM; 5 min) almost completely abolished the inward currents induced by both capsaicin (before ruthenium red, 241.7 ± 34.9 pA; after ruthenium red, 0.7 ± 0.3 pA; P < 0.05; n = 6) and 2APB (before ruthenium red, 293.1 ± 62.3 pA; after ruthenium red, 16.1 ± 4.9 pA; P < 0.05; n = 7); the antagonizing effects of ruthenium red were also reversed after ∼60 min of recovery (Fig. 9). Pretreatment with the vehicles of capsazepine and ruthenium red did not have any detectable effect on the inward currents induced by either capsaicin or 2APB (n = 4).
Fig. 9.
Effect of ruthenium red on the whole-cell inward currents induced by capsaicin and 2APB in isolated rat pulmonary capsaicin-sensitive neurons. A and C: experimental records illustrating the effect of pretreatment with ruthenium red (5 μM; 5 min), a non-selective TRPV channels blocker, on the inward currents induced by capsaicin (1 μM; 6 s) and 2APB (300 μM; 8 s), respectively. B and D: group data for responses to capsaicin (0.3-1 μM; 4-8 s) and 2APB (300 μM; 4-8 s), respectively, before (open bars), during (closed bars) and ∼60 min after (hatched bars) ruthenium red (5 μM; 5 min). Cap, capsaicin; RR, ruthenium red. * Significantly different (P < 0.05) from the response before ruthenium red treatment. Data are means ± SE of 6 and 7 pulmonary capsaicin-sensitive neurons in B and D, respectively.
DISCUSSION
In the present study, we have demonstrated that right atrium injection of 2APB evoked the classic “pulmonary chemoreflex” responses, characterized by apnea, bradycardia and hypotension. These reflex responses were completely abolished by PNCT of both cervical vagi, suggesting the involvement of vagal C-fibers. The single-fiber recording experiments have confirmed that 2APB (1, 2 and 3 mg/kg) stimulated the individual pulmonary C fibers in a dose-dependent manner. Results obtained from the capsazepine experiment indicated that the stimulatory effect of 2APB resulted, in part, from the activation of TRPV1. Our patch-clamp studies in isolated pulmonary capsaicin-sensitive neurons have further demonstrated that 2APB caused a stimulatory effect on these neurons. This stimulating effect was probably mediated through the activation of TRPV1, V2 and V3 channels because it was attenuated but not totally abolished by capsazepine, a selective TRPV1 antagonist (4, 41). In contrast, it was completely abrogated by ruthenium red, a non-selective TRPV channels blocker (13, 16).
2APB was originally reported as a membrane permeable antagonist of IP3 receptors (30), and has been used extensively as an inhibitor of the store-operated Ca2+ influx and IP3-medicated Ca2+ release (5, 15). Recent studies have revealed a novel effect of 2APB on TRPV channels (11, 20). In HEK293 cells transfected with mouse TRPVs, Chung and colleagues (11) reported that 2APB, at the concentration of 100 μM, generated a robust stimulatory effect on TRPV3, little effect on TRPV1, and no activation of TRPV2. In contrast, Hu and coworkers (20) demonstrated that 2APB at higher concentration (500 μM) is a common and potent activator of three TRPV channels, TRPV1-3. Using the fluo4-based Ca2+ assay, they further determined that the EC50 values of 2APB were 114 ± 8, 129 ± 13 and 34 ± 12 μM for TRPV1, V2 and V3, respectively, in HEK293 cells expressing these TRPVs. As the first cloned member of TRPV subfamily, TRPV1 is a Ca2+ permeable cation channel activated not only by vanilloids such as capsaicin, but also by endogenous lipids (endocannabinoids, eicosanoids), protons (pH < ∼5.9) and temperature (>43°C) (8, 10, 21, 22, 27). The expression of TRPV1 was found exclusively in small- to medium-diameter neurons within dorsal root, trigeminal and nodose sensory ganglia (8, 10, 25, 41). Both TRPV2 and TRPV3 are insensitive to capsaicin and show a steep dependence of temperature (43). TRPV2 seems to be expressed preferentially in medium- to large-diameter sensory neurons and is activated by hypo-osmolarity (34), growth-factor (23) and noxious heat (>52°C) (9). Expression of TRPV3 has been found not only in sensory neurons (e.g., dorsal root, trigeminal and superior cervical ganglia) but also in skin, tongue and other tissues (47). TRPV3 is activated by innocuous temperatures (>34-38°C) (36, 38, 47), and by the only known chemical stimulus, 2APB (11, 20). In a recent study using RT-PCR, Zhang and colleagues (48) have demonstrated the presence of temperature-sensitive TRP channel transcripts (TRPVs, TRPN1 and TRPM8) in nodose ganglia gastrointestinal neurons. The expression of TRPV1 and TRPV2 in nodose ganglia was further confirmed by their immunohistochemical staining studies. Whether these TRPV channels are also expressed in the cell bodies of isolated pulmonary neurons and the pulmonary C-fiber sensory endings is not known. Based on the results obtained in the present study and the literature described above, we suggest that activation of TRPV1, V2 and V3 is probably involved in the stimulation of these sensory neurons by 2APB. Our results can not completely rule out other potential excitatory effect of 2APB on these neurons. For example, in addition to its inhibitory effect, 2APB has been shown to activate or potentiate store-operated Ca2+ channels and a nonselective cation channel of unknown function (6, 29, 37). However, this possible involvement is less likely in our study because the 2APB effect was compleltely abolished by ruthenium red.
Immunohistochemical studies have illustrated the presence of C-fiber sensory endings in the mucosa of all size of airways in various species including humans (1, 3, 24). These nerve endings display extensive axon arborization that either extends into the space between epithelial cells or forms network-like plexus immediately beneath the basement membrane of epithelium (1, 3, 24). The superficial locations of these nerve endings suggest an important role of these afferents in regulating the airway responses to inhaled irritants such as sulfur dioxide, ammonia, cigarette smoke, acid aerosol, etc. Furthermore, pulmonary C-fiber afferents are generally known to possess polymodel sensitivity; in addition to chemical irritants (e.g., acid, nicotine, capsaicin), these afferents can be also activated by certain endogenously released autacoids (e.g., prostaglandin, bradykinin, anandamide, etc) as well as mechanical stimuli such as hyperinflation of the lungs (14, 18, 26, 27, 42). The overall electrophysiological properties and signal transduction processes of pulmonary sensory neurons are regulated by the functional expression of various receptor proteins. Indeed, a number of ligand- (e.g., TRPV1, 5-HT3, P2X3, etc) and voltage-gated ion channels (e.g., tetrodotoxin-resistant Na+ channel, Ca2+ dependent K+ channels, etc) have been identified on the sensory terminals of C fibers as well as in the isolated C neurons innervating airways and lungs (7, 25). Using 2APB as a chemical tool and based upon the comparison of the antagonistic effects between capsazepine and ruthenium red, we have now for the first time demonstrated the functional expressions of TRPV2 and/or TRPV3 channels in pulmonary C-fiber terminals as well as in their cells bodies. Considering the fact that the TRPV channels are the primary sensors of a diverse range of external physical (e.g., heat, cold, mechanical stress) and chemical (e.g., pH, pheromones, osmolarity) stimuli (12, 43), a better understanding of the roles of these channels in regulating the function of airway sensory nerves is certainly very important.
Our present study has provided direct evidence of a distinct stimulatory effect of 2APB on pulmonary C-fiber afferents. Stimulation of these sensory afferents is known to elicit other important defence reflex responses, in addition to the pulmonary chemoreflexes. These reflex responses, such as bronchoconstriction, mucus hypersecretion, airway irritation and coughing, are mediated through the central nervous system and/or autonomic nervous system (14, 26). Moreover, sensory neuropeptides such as tachykinins (e.g. substance P, neurokinin A) and calcitonin gene-related peptide synthesized in the cell bodies of pulmonary C fibers in various species including humans are released locally from the sensory terminals upon stimulation (28, 39). These peptides are known to act on a number of effector cells (e.g. airway smooth muscles, cholinergic ganglia, inflammatory cells, mucous glands) and produce potent local effects such as bronchoconstriction, plasma extravasation and oedema of airway mucosa (39). Although these reflexes and local responses resulting from the C-fiber activation were not measured in this study, 2APB can, presumably, evoke these airway responses to varying degrees of intensity, depending on the dose administered.
In the present study, we intended to compare the responses induced by 2APB and capsaicin in the same pulmonary C fibers. Despite the large difference in the doses applied between these two chemicals (3 mg/kg vs 1 μg/kg), more than half of the C fibers (16 out of 26 fibers tested) displayed an interesting resemblance between the afferent responses to 2APB and capsaicin. However, certain disparity is evident in the remaining fibers; for example, a greater stimulatory effect of 2APB than capsaicin was found in six fibers, whereas the opposite was found in others. This observation also holds true in the responses of isolated pulmonary sensory neurons to these two chemicals. Although, the exact underlying mechanism is not known, we suspect that different expressions and distributions of various TRPV channel proteins among pulmonary C-fiber terminals should, at least in part, account for their different sensitivities to capsaicin and 2APB. This explanation is supported by the fact that the difference in the chemoreflex responses to these two chemicals at the same doses was much less (Fig. 3, C and D) because these reflex responses reflect integrated activities of the entire population of pulmonary C fibers. In addition, we have demonstrated that PNCT completely abolished the cardiorespiratory responses to both capsaicin and 2APB (Figs. 2 and 3) indicating that these responses were indeed mediated through pulmonary C fibers. In sharp contrast to the responses to capsaicin, the cardiovascular depressor responses (bradycardia and hypotension) induced by 2APB persisted even after the pretreatment with capsazepine (e.g., Fig. 6B). This observation lends additional support to our conclusion that the pulmonary chemoreflex responses elicited by 2APB did not result exclusively from activation of the TRPV1 channel.
In summary, this study using 2APB as a chemical tool to activate TRPV1, V2 and V3 channels has suggested the functional expression of these channels in pulmonary C neurons. This conclusion is further supported by our observation that the 2APB-evoked response is only partially blocked by capsazepine but completely abrogated by ruthenium red. The sensitivities to specific stimuli and physiological functions of these neurons are likely influenced by the relative degree of expression of these TRPV channels in each individual neuron.
ACKNOWLEDGEMENTS
The authors thank Robert F. Morton and Wen-Bin Yang for their technical assistance.
Footnotes
GRANTS
This study was supported by National Institutes of Health Grants HL58686 (to L.-Y. L.), HL67379 (to L.-Y. L.) and NS42183 (to M. X. Z.).
REFERENCES
- 1.Adriaensen D, Timmermans JP, Brouns I, Berthoud HR, Neuhuber WL, Scheuermann DW. Pulmonary intraepithelial vagal nodose afferent nerve terminals are confined to neuroepithelial bodies: an anterograde tracing and confocal microscopy study in adult rats. Cell Tissue Res. 1998;293:395–405. doi: 10.1007/s004410051131. [DOI] [PubMed] [Google Scholar]
- 2.Agostoni E, Chinnock JE, Daly M, De B, Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lung and abdominal viscera in the cat. J Physiol. 1957;135:182–205. doi: 10.1113/jphysiol.1957.sp005703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol. 1992;319:586–598. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- 4.Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42:873–888. doi: 10.1016/s0028-3908(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 5.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 6.Braun FJ, Aziz O, Putney JW. 2-aminoethoxydiphenyl borane activates a novel calcium-permeable cation channel. Mol Pharmacol. 2003;63:1304–1311. doi: 10.1124/mol.63.6.1304. [DOI] [PubMed] [Google Scholar]
- 7.Carr MJ, Undem BJ. Ion channels in airway afferent neurons. Respir Physiol. 2001;125:83–97. doi: 10.1016/s0034-5687(00)00206-1. [DOI] [PubMed] [Google Scholar]
- 8.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Ann Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. 2-aminoethoxydiphenyl borane activates and sensitizes the heat-gated ion channel TRPV3. J Neurosci. 2004;24:5177–5182. doi: 10.1523/JNEUROSCI.0934-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapham DE, Montell C, Schultz G, Julius D. International Union of Pharmacology. International Union of Pharmacology. XLIII. Compendium of voltage-gated ion channels: transient receptor potential channels. Pharmacol Rev. 2003;55:591–596. doi: 10.1124/pr.55.4.6. [DOI] [PubMed] [Google Scholar]
- 13.Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 14.Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- 15.Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethoxydiphenyl borane is a noval inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosthate receptors. Biochem J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 17.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. 1998;250:177–180. doi: 10.1016/s0304-3940(98)00475-3. [DOI] [PubMed] [Google Scholar]
- 18.Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- 19.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacological Reviews. 1991;43:143–201. [PubMed] [Google Scholar]
- 20.Hu HZ, Gu Q, Wang C, Colton CK, Tang J, Kinoshita-Kawada M, Lee LY, Wood JD, Zhu MX. 2-aminoethoxydiphenyl borane is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 21.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 23.Kanzaki M, Zhang YQ, Mashima H, Li L, Shibata H, Kojima I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu T, Yamamoto M, Shimokata K, Nagura H. Distribution of substance P-immunoreactive and calcitonin gene-related peptide-immunoreactive nerves in normal human lungs. Int Arch Allergy Appl Immunol. 1991;95:23–28. doi: 10.1159/000235449. [DOI] [PubMed] [Google Scholar]
- 25.Lee LY, Kwong K, Lin YS, Gu Q. Hypersensitivity of bronchopulmonary C-fibers induced by airway mucosal inflammation: cellular mechanisms. Pulm Pharmacol Ther. 2002;15:199–204. doi: 10.1006/pupt.2002.0338. [DOI] [PubMed] [Google Scholar]
- 26.Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- 27.Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J Physiol. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lundberg JM, Saria A. Polypeptide-containing neurons in airway smooth muscle. Annu Rev Physiol. 1987;49:557–572. doi: 10.1146/annurev.ph.49.030187.003013. [DOI] [PubMed] [Google Scholar]
- 29.Ma HT, Venkatachalam K, Parys JB, Gill DL. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J Biol Chem. 2002;277:6915–6922. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borane, a membrane-permeable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 31.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 32.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–598. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 33.Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, Clapham DE, Harteneck C, Heller S, Julius D, Kojima I, Mori Y, Penner R, Prawitt D, Scharenberg AM, Schultz G, Shimizu N, Zhu MX. A unified nomenclature for the superfamily of TRP cation channels. Molecular Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- 34.Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- 35.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Phsyiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 36.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002;296:2046–2049. doi: 10.1126/science.1073140. [DOI] [PubMed] [Google Scholar]
- 37.Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs in dependently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- 39.Solway J, Leff AR. Sensory neuropeptides and airway function. J Appl Physiol. 1991;71:2077–2087. doi: 10.1152/jappl.1991.71.6.2077. [DOI] [PubMed] [Google Scholar]
- 40.Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004;61:3–12. doi: 10.1002/neu.20079. [DOI] [PubMed] [Google Scholar]
- 41.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 42.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Voets T, Nilius B. TRPs make sense. J Membr Biol. 2003;192:1–8. doi: 10.1007/s00232-002-1059-8. [DOI] [PubMed] [Google Scholar]
- 44.Wananable H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 45.Wananable H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Kamimura N, Takeo T, Suga S, Wakui M, Maruyama T, Mikoshiba K. 2-aminoethoxydiphenyl borane modulates kinetics of intracellular Ca2+ signals mediated by inositol 1,4,5-trisphospaphate-sensitive Ca2+ stores in single pancreatic acinar cells of mouse. Mol Pharmacol. 2000;58:1368–1374. doi: 10.1124/mol.58.6.1368. [DOI] [PubMed] [Google Scholar]
- 47.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol. 2004;286:G983–G991. doi: 10.1152/ajpgi.00441.2003. [DOI] [PubMed] [Google Scholar]