Abstract
RNaseE is the main component of the RNA degradosome of Escherichia coli, which plays an essential role in RNA processing and decay. Localization studies showed that RNaseE and the other known degradosome components (RNA helicase B, polynucleotide phosphorylase, and enolase) are organized as helical filamentous structures that coil around the length of the cell. These resemble the helical structures formed by the MreB and MinD cytoskeletal proteins. Formation of the RNaseE cytoskeletal-like structure requires an internal domain of the protein that does not include the domains required for any of its known interactions or the minimal domain required for endonuclease activity. We conclude that the constituents of the RNA degradosome are components of the E. coli cytoskeleton, either assembled as a primary cytoskeletal structure or secondarily associated with another underlying cytoskeletal element. This suggests a previously unrecognized role for the bacterial cytoskeleton, providing a mechanism to compartmentalize proteins that act on cytoplasmic components, as exemplified by the RNA processing and degradative activities of the degradosome, to regulate their access to important cellular substrates.
Keywords: RNA processing, PNPase, enolase, RNA helicase
It is well established that the eukaryotic cytoskeleton includes stable structures that are composed mainly of intermediate filament proteins and dynamic structures such as microtubules and actin filaments that can assemble and redistribute within the cell in response to specific cellular cues. It has recently been established that a variety of cytoskeletal-like structures are also present in bacterial cells (1). By analogy to the eukaryotic cytoskeleton, bacterial cytoskeletal elements are defined as filamentous structures, each based on polymers of a single class of protein, organized into long-range ordered structures within the cell. Among these elements are proteins that form membrane-associated helical structures that extend along the length of Escherichia coli cells, such as the actin bacterial homolog MreB (2, 3) and the uniquely bacterial cytoskeletal protein MinD (3).
Bacterial cytoskeletal structures play a role in a number of cell functions, including cell shape determination (4), division site selection (3), establishment of cell polarity (5, 6), and segregation of chromosome and plasmid DNA (7, 8). To accomplish these functions, the cytoskeletal structures can act as lattices for the assembly and localization of functional protein complexes. For example, the MreB helical cytoskeleton plays a role in cell shape determination by directing the helical organization of murein cell wall biosynthetic enzymes (9). Similarly, MinD helical cytoskeletal structures play a role in the proper mid-cell placement of the E. coli cell division site by serving as a scaffold for the dynamic localization of the MinC and MinE division site-selection proteins (reviewed in ref. 10).
As part of a study to identify cytoskeleton-associated elements, we used the yeast two-hybrid system to screen an E. coli genomic library for proteins that interact with the MinD protein. This identified RNaseE as a MinD-interacting protein. RNaseE is an essential endoribonuclease of 1,061 aa (11) that acts as a scaffold for the assembly of a multiprotein complex, the RNA degradosome. The degradosome includes at least three other proteins, RNA helicase B (RhlB), polynucleotide phosphorylase (PNPase), and enolase (12–15). The RNA degradosome is required for the normal maturation of transfer and ribosomal RNA and for degradation of most messenger RNAs (16–18). In degradosome-dependent mRNA decay, RhlB facilitates the degradation of structured RNA, and RNaseE provides the endoribonuclease activity that cuts the RNA into fragments that are further degraded by the 3′→5′ exoribonuclease activity of PNPase (reviewed in ref. 19). The role of enolase in this process is unclear (20). Recently, enolase was proposed to play a regulatory role in the degradation of specific RNAs such as ptsG mRNA (21).
We report here that RNaseE and the other degradosome components are all organized as helical filamentous structures that wind around the length of the cell. The structures resemble the helical structures formed by the cytoskeletal proteins MinD and MreB, but formation of the cytoskeletal-like RNaseE structures is independent of MinD or MreB. The RNaseE domain responsible for its cytoskeletal organization is separate from the RNaseE domain that contains the essential endoribonuclease activity. The present results indicate that the RNA degradosome exists as a cytoskeletal structure in E. coli, thereby compartmentalizing RNA degradative and processing activities within the cell. This type of compartmentalization could provide a general mechanism to spatially sequester proteins or protein complexes that act on cytoplasmic components and thereby regulate their access to specific substrates within the cytoplasm.
Results
Screening for MinD-Interacting Proteins.
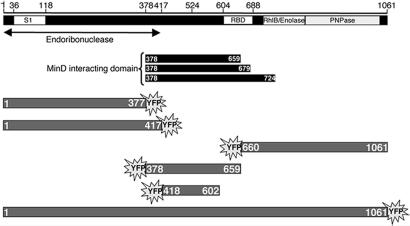
As part of a study to identify proteins that interact with bacterial cytoskeletal elements, we used the yeast two-hybrid system to screen an E. coli genomic library for genomic fragments coding for proteins that interact with MinD. Ten genomic clones that interacted with the MinD bait were identified out of a total of 12.3 × 106 yeast colonies. Six clones contained DNA coding for part of the MinC protein, and one contained MinD DNA. These are expected because MinD interacts with itself and with MinC (22, 23). The three other clones contained chromosomal inserts corresponding to the central domain of the rne gene, coding for the E. coli RNaseE protein. The three inserts started from the same position, His-378, but differed in the length of the RNaseE domains, which extended to Gln-659, Arg-679, and Gln-724, respectively (Fig. 1).
Fig. 1.
Schematic representation of RNaseE and Yfp-labeled RNaseE constructs. RNase domains are depicted as described in ref. 41. S1 domain (S1 RNA-binding domain), RBD (arginine rich RNA-binding domain), RhlB (RhlB-binding domain), enolase (enolase-binding domain), and PNPase (PNPase-binding domain) are shown. The region that includes the endoribonuclease catalytic domain is indicated (26). The black rectangles represent the RNaseE fragments that interacted with MinD in the yeast two-hybrid screen. The Yfp-labeled RNaseE constructs are shown in gray.
RNaseE Is Organized as a Cytoskeletal Structure in Vivo.
The yeast two-hybrid results suggested an interaction between RNaseE and MinD, which is organized as a helical, membrane-associated cytoskeletal structure within the cell. We therefore asked whether RNaseE showed a similar cellular organization, using RNaseE fused to yellow fluorescent protein (Yfp) to study its localization pattern in living E. coli cells. Yfp fused to either the N terminus or the C terminus of RNaseE did not interfere with the ability of the protein to correct the lethal phenotype of a Δrne mutant (data not shown).
Fluorescence microscopy revealed that RNaseE-Yfp was organized as a double-helical filamentous structure that coiled around the cell periphery and extended between the two poles (Fig. 2B). Similar results were obtained when RNaseE-Yfp was expressed under control of Plac (Fig. 2B) or when rne::yfp was substituted for the native rne gene in the chromosome under control of the normal rne promoters (data not shown).
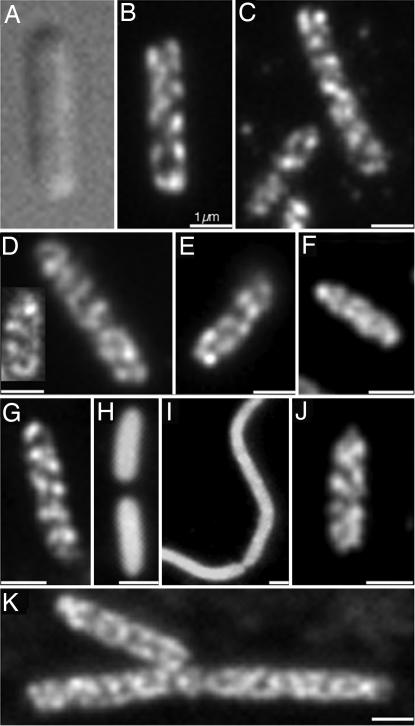
Fig. 2.
Cytoskeletal-like organization of the RNA degradosome components. (A) Differential interference contrast micrographs of strain AT1/pRNE1 [Δrne/Plac-rne::yfp]. (B) Strain AT1/pRNE1 [Δrne/Plac-rne::yfp] cell showing coiled structure of RNaseE-Yfp. (C–K) Immunofluorescence micrographs using anti-HA antibody (C and E–K) or purified anti-RhlB antibody (D). (C) Strain AT25 [rne::HA]. (D) Strain MC1000. (E) Strain AT18 [eno::HA]. (F) Strain AT19 [pnp::HA]. (G) Strain AT27 [rne1–659::HA]. (H and I) Strain AT28 [rne1–417::HA]. rne1–417 cultures contain cells of variable length, including filamentous cells (see Results). (J) A22-treated AT25 [rne::HA] (see Results for details). (K) Strain AT41 [Δmin, rne::HA]. Because of the Min− phenotype, the cells are predominantly short filaments. (Scale bars: 1 μm.)
Confirmation that the localization pattern of RNaseE-Yfp was not an artifact due to the fusion to Yfp was obtained by study of chromosomally encoded RNaseE tagged with the HA epitope (RNaseE-HA). Immunofluorescence microscopy showed that the RNaseE-HA was organized into coiled structures similar to those observed with RNaseE-Yfp (Fig. 2C). We conclude that RNaseE is organized as helical cytoskeletal-like structures in E. coli cells that resemble the previously described membrane-associated helical structures formed by cytoskeletal proteins MinD and MreB (2, 3).
Localization of the Other RNA Degradosome Components.
Within the cell, RNaseE is associated in the RNA degradosome with RhlB, PNPase, and enolase (24). Because Yfp labeling and immunofluorescence studies showed that RNaseE is organized as a cytoskeletal-like structure, we next asked whether the other RNA degradosome components are organized in a similar fashion. The other components were identified by immunofluorescence microscopy, using purified anti-RhlB antibody or antibody directed against an HA tag fused to enolase or PNPase. This showed that the three other degradosome proteins were also organized in extended coiled structures (Fig. 2 D–F). The RhlB, enolase, and PNPase structures wound around the cell and extended from one cell pole to the other, thereby resembling the structure formed by RNaseE. Thus, all of the known E. coli RNA degradosome components are organized into similar cytoskeletal-like helical structures within the cell.
RNaseE Organization Is Independent of MreB and MinD Cytoskeletal Structures.
MreB forms coiled cytoskeletal structures that can be disrupted by treatment with low concentrations of A22 [S-(3,4-dichlorobenzyl) isothiourea] without significant change in cell shape (8). To determine whether the MreB structure is required for the cytoskeletal-like organization of RNaseE, the localization pattern of RNaseE-HA was determined by immunofluorescence in cells in which the MreB helical structure was disrupted by A22 treatment. As expected from other reports (25), when exposed to 10 μg/ml A22 for 70 min, MreB coils disappeared although rod shape was retained (data not shown). Under these conditions, the RNaseE coiled structures were preserved (Fig. 2J). Thus, maintenance of the cytoskeletal-like organization of RNaseE is independent of the helical MreB cytoskeleton.
MinD also forms helical cytoskeletal structures along the length of E. coli cells (3). The fact that the yeast two-hybrid study suggested an interaction between MinD and RNaseE suggested that the RNaseE coiled structures might be secondarily associated with the MinD cytoskeleton. We therefore determined the localization pattern of RNaseE-HA in a ΔminCDE strain. As shown in Fig. 2K, the RNaseE helical distribution pattern was maintained in the absence of Min proteins. Thus, the cytoskeletal-like organization of RNaseE is independent of the MinD cytoskeleton. The biological significance of the MinD–RNaseE interaction in the yeast two-hybrid system is currently under investigation.
RNaseE Domain Required for the Cytoskeletal-Like Organization of RNaseE.
To define the RNaseE domain responsible for its cytoskeletal-like organization, different chromosomally encoded RNaseE-HA fragments and plasmid-encoded Yfp-labeled RNaseE fragments were examined (Fig. 1). (Throughout the text, numbers in parentheses refer to the RNaseE domain extending between the indicated amino acid residues.) These studies showed that the helical organization of RNaseE requires a domain located between amino acids 418 and 602 of the RNaseE protein. To avoid the possibility that localization of plasmid-encoded Yfp-labeled RNaseE fragments may be affected by endogenous chromosomally encoded RNaseE, the fragments were expressed in the absence of full-length RNaseE. Viability of Δrne cells that lack the full-length RNaseE protein requires the RNaseE(1–417) domain that contains the endoribonuclease domain of the full-length protein (26). Therefore, the constructs were expressed either in Δrne cells or, when necessary to retain viability, in strain AT8 [Prne-rne1–417], which expresses chromosomally encoded RNaseE(1–417).
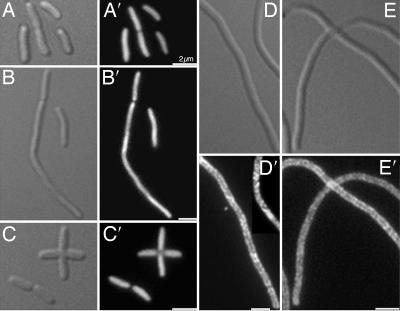
Immunofluorescence studies of RNaseE(1–659)-HA (Fig. 2G) showed the same helical localization pattern as the full-length protein (Fig. 2C) whereas RNaseE(1–417)-HA was diffusely distributed within the cytoplasm (Fig. 2 H and I). Equivalent results were obtained in fluorescence localization studies of RNaseE(1–417)-Yfp (Fig. 3B′) and RNaseE(1–659)-Yfp (data not shown). This suggested that the determinant of the helical distribution pattern of RNaseE includes sequences located between residues 417 and 659 of the protein. Evidence that RNaseE(418–602) was sufficient to form the membrane-associated coiled structure came from a study of Yfp-labeled fragments that included all or part of the RNaseE(418–659) domain. As shown in Fig. 3D′, Yfp-labeled RNaseE(378–659) was peripherally localized and formed coiled structures, although the structures were somewhat less clear than with RNaseE(1–659) (Fig. 2G). Yfp-RNaseE(418–602) showed a similar localization pattern (Fig. 3E′). In contrast, RNaseE(1–377)-Yfp, RNaseE(1–417)-Yfp, and Yfp-RNaseE(660–1061) were diffusely localized throughout the cytoplasm (Fig. 3 A′–C′). The difference in localization pattern of RNaseE domains was not due to differences in cellular concentrations of the Yfp-labeled proteins as shown by quantitation of cellular fluorescence.
Fig. 3.
Cellular localization of Yfp-labeled RNaseE domains. Differential interference contrast and YFP images are shown. (A) MC1000/Plac-rne1–377::yfp. (B) Δrne/Plac-rne1–417::yfp. (C) AT8/Plac-yfp::rne660–1061. (D) AT8/Plac-yfp::rne378–659. (E) AT8/Plac-yfp::rne418–602. (Scale bars: 2 μm.)
We conclude that the RNaseE determinant responsible for membrane association and helical cellular organization lies between amino acids 418 and 602 of the RNaseE protein. This is consistent with previous ImmunoGold electron microscopy studies showing a peripheral localization of RNaseE in wild-type cells and in a mutant strain expressing RNaseE(1–602) (24). Interestingly, the localization determinant of RNaseE overlapped the RNaseE domain that interacts with MinD in yeast two-hybrid assays [RNaseE(378–659)] (Fig. 1).
Effects of Loss of RNaseE Helical Organization.
To determine the functional significance of the cytoskeletal-like organization of RNaseE, we compared the rne mutant strains AT8 [Prne-rne1–417] and AT14 [Prne-rne1–659]. As described above, cells expressing RNaseE(1–417) are incapable of forming the coiled cellular structures (Fig. 2 H and I and 3B′) whereas cells that express RNaseE(1–659) (Fig. 2G) show a helical pattern that resembles the structure formed by full-length RNaseE.
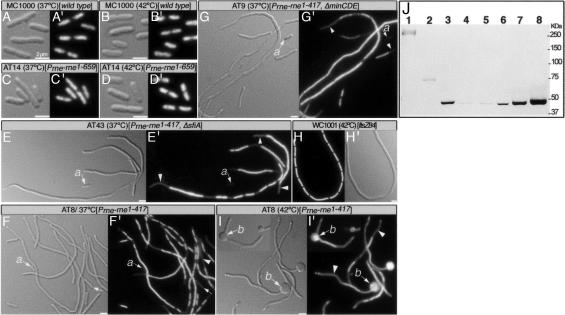
AT8 [Prne-rne1–417] cells showed the following abnormalities, which were not seen in AT14 [Prne-rne1–659] or in cells that expressed full-length RNaseE. First, AT8 cells grew slowly, with generation times in rich medium that were ≈55%, 43%, and 35% (at 30°C, 37°C, and 42°C, respectively) of the generation times of either strain AT14 or the isogenic parent strain MC1000. Second, AT8 cells showed a defect in cell division as shown by a mixed population ranging from normal-length cells to long filaments (Fig. 4F). The division block was not mediated by the endogenous division inhibitor MinC or the SOS division inhibitor SulA (27–29) as shown by the observation that filamentation was unaffected by the absence of either MinC or SulA (Fig. 4 E and G). Third, DAPI-stained AT8 cells exhibited a chromosome segregation defect, manifested by long nucleoid-free regions and extended stretches of DNA that presumably represent unseparated nucleoids (Fig. 4 E′–G′). As expected from the abnormal nucleoid distribution pattern, strain AT8 [Prne-rne1–417] produced many anucleate cells (arrows in Fig. 4 E′–G′). The chromosome segregation defect was more pronounced at elevated temperatures. At 37°C, 7.7% of AT8 cells were anucleate and 28% (of 609 cells) had unseparated nucleoids. At 42°C, 7% of cells were anucleate and 88% (of 553 cells) had unseparated nucleoids. In contrast, AT14 [Prne-rne1–659], the wild-type parent MC1000, and cells that filamented because of a defect in the unrelated FtsZ protein {WC1001 [ftsZ84ts]} showed normal chromosome segregation (Fig. 4 A′–D′ and H′). Fourth, cells of strain AT8 often contained large bulges that were frequently located at one or both cell poles and, in some cases, at the septal region (Fig. 4I). This was much more frequent at 42°C than at 37°C (34% vs. 1% of cells). The bulges usually contained chromosomal DNA, as shown by DAPI staining (Fig. 4I′). This indicates that they are formed by outward bulging of the entire cell envelope and do not result from an extrusion of the outer membrane due to a local defect in attachment of inner membrane to the murein outer-membrane layer (30, 31). We presume that a local weakening of murein, probably at division sites, leads the three cell envelope layers to bulge outward because of the turgor pressure of the cell.
Fig. 4.
Phenotypic abnormalities associated with the loss of RNaseE helical organization. Cell morphology is shown by differential interference contrast, and DNA distribution is shown by DAPI fluorescence. (A, A′, B, and B′) MC1000 grown at 37°C (A and A′) or at 42°C (B and B′). (C, C′, D, and D′) AT14 [Prne-rne1–659] grown at 37°C (C and C′) or at 42°C (D and D′). (E and E′) AT43 [ΔsfiA, Prne-rne1–417] grown at 37°C. (F, F′, I, and I′) AT8 [Prne-rne1–417] grown at 37°C (F and F′) or at 42°C (I and I′). (G and G′) AT9 [ΔminB, Prne-rne1–417] grown at 37°C. (H and H′) WC1001 [ftsZ84ts] grown for 3 h at 42°C. The arrows show positions of anucleate cells; the arrowheads mark the nucleoid-free regions; a shows anucleate cell; b shows the bulges in AT8 [Prne-rne1–417] cells grown at 42°C. (Scale bar: 2 μm.) (J) Immunoblot of total protein (54) of AT25 [Prne-rne::HA] (lane 1), AT27 [Prne-rne1–659::HA] (lane 2), AT28 [Prne-rne1–417::HA] (lane 3), and AT1/pRNE31 [Δrne/Plac-rne1–417::HA] grown in the presence of 0 μM IPTG (lane 4), 1 μM IPTG (lane 5), 10 μM IPTG (lane 6), 100 μM IPTG (lane 7), or 1 mM IPTG (lane 8). Molecular masses are indicated in kDa. Thirty micrograms (lanes 1 and 2) or 5 μg (lanes 3–8) of protein was loaded.
The defects in growth, cell division, and chromosome segregation in AT8 [Prne-rne1–417], where the cytoskeletal organization is absent, were not observed in strain AT14 [Prne-rne1–659] in which the helical RNaseE pattern was unperturbed (Fig. 4 C′ and D′). Taken together these results suggest that the cytoskeletal-like organization of RNaseE may play a significant role in its cellular function, although the possibility that the defects are related to some other effect of deleting the RNaseE(418–602) cytoskeletal localization domain has not been excluded.
It is known that the cellular concentration of RNaseE is negatively regulated by its ability to degrade its own gene transcript (32). This autoregulation seems to be lost in the rne1–417 truncation mutant, where the concentration of RNaseE(1–417) was 15- to 20-fold higher than RNaseE(1–659) or full-length RNaseE in cells in which the rne chromosomal copy was substituted by rne1–417::HA, rne1–659::HA, or rne::HA (Fig. 4J, lanes 1–3). To exclude the possibility that the increased level of RNaseE(1–417) was responsible for the abnormal phenotypes observed in AT8 [Prne-rne1–417], we varied the cellular concentration of RNaseE(1–417) by expressing rne1–417 under control of the lac promoter. This showed that the phenotypic defects were not corrected when the cellular concentration of RNaseE(1–417) was reduced to wild-type levels.
A decrease in the endoribonuclease specific activity of the RNaseE(1–417) mutant protein, suggested by studies of RNaseE(1–410) (33), could play a role in the phenotypic defects of the rne1–417 strain. If a decrease in total cellular RNaseE activity were responsible for the abnormal phenotype of AT8 [Prne-rne1–417], an increase in cellular concentration of RNaseE(1–417) would be expected to correct the defects. We therefore increased the cellular RNaseE(1–417) concentration (Fig. 4J) by expressing rne1–417 under Plac control. This failed to correct the abnormal phenotype over a 100-fold range of concentrations (data not shown), suggesting that the morphological defects are not due to a decrease in total cellular RNaseE activity. Further proof for this will require identification and study of a rne mutation that interferes with the helical cytoskeletal organization of RNaseE without affecting its enzymatic activity.
Discussion
The fact that bacteria contain several cytoskeletal elements that impart long-range order to the cell suggests that these structures play important roles in the life of the organism. In several cases, the cytoskeletal elements participate in membrane-associated functions and in cell membrane and cell wall organization. For example: MreB and MreB homologs appear to regulate the organization of murein biosynthetic enzymes and thereby regulate the shape of rod-shaped cells (34); MreB is also required for several aspects of differentiation of the cell poles (5, 6, 35); the MinD cytoskeleton and its associated proteins are responsible for placement of the bacterial division septum at mid-cell (3); the intermediate filament protein crescentin regulates cell curvature in Caulobacter crescentus (36); and the actin homolog MamK is required for positioning of the membrane-bounded magnetosome organelles of Magnetospirillum magneticum (37). Bacterial cytoskeletal elements also participate in movement of nucleoids and plasmids within the cytoplasm, as shown by the roles of MreB and ParM in chromosome and plasmid segregation (7, 8, 38). In the present work, the finding that the components of the E. coli RNA degradosome show a characteristic cytoskeletal organization indicates that cytoskeleton-associated protein complexes also participate in reactions that modify cytoplasmic molecules, such as RNA processing and degradative reactions. This represents a previously unrecognized role for the cytoskeleton in the life of the cell.
The mechanism responsible for the cytoskeletal organization of RNaseE is not known. Formation of the filamentous RNaseE structure within the cell could be an inherent property of the protein itself, based on an ability to self-assemble into filamentous structures in a manner similar to MreB and MinD (39, 40). Yeast two-hybrid studies have identified self-interacting domains in RNaseE that could participate in such a self-assembly system (41). The domains include the RNaseE(418–602) region, which we show is required for the cytoskeletal-like organization of RNaseE. However, until it is directly shown that RNaseE can actually polymerize into extended filaments, the possibility of a self-assembling RNaseE system remains conjectural. The finding that the RNaseE structures were present in cells that lacked MinD and in cells in which MreB cytoskeletal structures were disrupted indicates that maintenance of the cytoskeletal-like organization of RNaseE is independent of these cytoskeletal systems. However, RNaseE might still interact with MinD or MreB for other purposes despite the fact that the interactions are not needed for maintenance of the RNaseE helical structure. The significance of the MinD–RNaseE interaction in the yeast two-hybrid system requires further study. It is also possible that another, as yet unidentified, helical cytoskeletal system plays the role of a template or lattice for assembly of the RNaseE coiled structures.
ImmunoGold electron microscopy has shown that RNaseE is localized at the cell periphery (24). This implies that the helical structures described here are associated directly or indirectly with the cytoplasmic membrane. The ImmunoGold peripheral localization pattern required the first 602 residues of RNaseE (24). This domain includes the RNaseE(418–602) determinant, which is required for the cytoskeletal organization of the protein (Figs. 2G and 3). A direct association of RNaseE with the membrane via the RNaseE(418–602) domain could be required for its cytoskeletal-like organization within the cell. This would resemble the requirement for the MinD membrane-binding domain in formation of the helical MinD-related cytoskeletal structures (42–44). However, at present there is no evidence that RNaseE(418–602) possesses membrane-binding activity. The existence of other peripheral cytoskeletal elements without known membrane-targeting domains (reviewed in ref. 1) indicates that the membrane association of coiled cytoskeletal structures does not always require direct interaction with the membrane. Therefore, the membrane association of the RNaseE structures could be mediated by other cellular elements.
Loss of the RNaseE(418–602) cytoskeletal determinant was associated with both loss of the helical RNaseE structure and significant defects in growth rate, cell division, chromosome segregation, and cell morphology, despite the presence of the essential RNaseE endoribonuclease domain [RNaseE(1–417)]. The defects were reversed by an RNaseE fragment that included both the RNaseE endoribonucleolytic domain and the domain required for the RNaseE helical distribution pattern. These observations suggest that the cytoskeletal-like organization of RNaseE may play an important role in regulation of normal cell functions by regulating the RNA processing functions of the degradosome. Previous studies have shown an increased half-life of several mRNAs and a defect in the processing of rRNA and tRNA in cells that express only the RNaseE(1–417) fragment (17), which, as shown here, fails to assemble into the cellular cytoskeletal-like structures. It is likely that the changes in mRNA degradation and RNA processing are responsible for the various phenotypic defects of cells that express the RNaseE(1–417) domain in the absence of the domain required for RNaseE cytoskeletal organization. Further work is needed to prove that these defects all result directly from loss of the cytoskeletal organization of the protein.
These observations lead us to suggest that the cytoskeletal organization of the degradosome serves to compartmentalize essential RNA processing and degradative activities within the cell, thereby playing an important role in the regulation of their cellular activities. One function of compartmentalization may be to sequester the degradosome machinery away from sites of RNA synthesis, thereby preventing premature or uncontrolled degradation of certain essential RNA substrates. Cytoskeletal compartmentalization could also bring in close proximity other components that act cooperatively to target specific RNA substrates to the degradosome machinery. The use of the cytoskeleton to spatially compartmentalize proteins and protein complexes is illustrated by the helical MreB cytoskeleton, which interacts with MreC and with components of the cell wall biosynthetic machinery in several organisms (45, 46), and by the MinCDE cytoskeletal structure required for division site localization (3).
The present results suggest a role for the cytoskeleton in the regulation of metabolic functions that take place within the bacterial cytosol, in this case RNA processing and degradation. Because of its potential usefulness, it is likely that other cellular systems may use a similar strategy to provide the cell with additional means of regulating important cell functions.
Materials and Methods
Strains, Plasmids, Media, and Growth Condition.
E. coli strains were grown in LB medium (47) to which 100 μg/ml ampicillin, 25 μg/ml kanamycin, 30 μg/ml chloramphenicol, or 0.4% (wt/vol) glucose was added when indicated. All plasmid constructions were introduced into E. coli DH5α (47) and then transferred to another E. coli or yeast strain. Yeast strains were grown in supplemented SD medium (Clontech Yeast Two-Hybrid Manual) or in rich medium adenine-supplemented yeast extract/peptone/dextrose (48). Plasmids and strains are listed in supporting information (SI) Table 1, and the details of their construction are available upon request. Strains AT8 [Prne-rne1–417] and AT14 [Prne-rne1–659] were constructed by the λ-red recombination method (49). HA-epitope tagging was done as previously described (50), and the associated antibiotic cassettes were eliminated by use of the FLP-expressing plasmid pCP20 (51). P1-mediated transduction was used to move mutations to different strains (52). Growth rate was determined by OD at 600 nm.
Yeast Two-Hybrid E. coli Genomic Library Construction and Screening.
An E. coli genomic library for use in the yeast two-hybrid system was constructed as fusions to the yeast Gal4 activation domain (AD) of vector plasmid pAGADT7 (Clontech, Mountain View, CA). One milligram of chromosomal DNA from E. coli PB103 was partially digested at 37°C for 65 or 140 min in the presence of ATP and 0.6 or 0.3 units of CviJI (CHIMERx, Milwaukee, WI), respectively. CviJI is a blunt cutter that cleaves between GC of the RGCY recognition sequence (R, purine; Y, pyrimidine). In the presence of ATP the enzyme cuts between almost every GC pair (RGCN and YGCY) (53). Partially digested DNA was purified and fractionated on a 5–20% sucrose gradient at 25,000 rpm (SW41 Ti rotor) for 17 h at 20°C with 200 μg of DNA loaded on each gradient. Fractions containing fragments with an average size of 0.8 kb were selected from each gradient (≈0.2- to 2.5-kb size range) and were used in a 1:6 vector:insert molar ratio for ligation into 60 ng of SmaI-digested and calf intestine alkaline phosphatase (CIP)-treated pAGDAT7 vector (Clontech). Ligation products were concentrated by ethanol precipitation in the presence of 1 μg of glycogen and used to transform ELECTROMAX DH10B competent cells (Invitrogen, Carlsbad, CA). Approximately 19 × 106 colonies were collected and resuspended in 80 ml of SOC medium with ampicillin (47). One-half of the cell suspension was used to inoculate 800 ml of LB-ampicillin medium and grown for 75 min at 37°C. The cells were then used for plasmid DNA extraction using eight Qiagen-tip 500 columns (Qiagen, Valencia, CA) to prepare the genomic plasmid library. The other half was aliquoted and saved at −70°C in the presence of 20% glycerol.
Plasmid pMDB1, containing minD fused to the Gal4 binding domain, was prepared as previously described (23). To screen for DNA coding for MinD-interacting proteins, competent yeast cells [made by the lithium acetate method (48)] from a 165-ml culture of AH109/pMDB1 were transformed with 180 μg of the plasmid library. The yeast transformants were screened for interaction on supplemented SD medium without leucine, tryptophan, adenine, and histidine to select for cells that contained both the bait (BD-MinD) and the prey (AD library) plasmids (Clontech Yeast Two-Hybrid Manual). AD plasmids containing genomic fragments were separated from the binding domain (BD-MinD) plasmid by transferring the plasmid DNA isolated from each yeast clone to E. coli DH5α and selecting for the ampicillin-resistant AD library plasmid. To isolate plasmid from yeast, cells of 1.5 ml of culture grown overnight at 30°C in SD medium without leucine and tryptophan were resuspended in 60 μl of 67 mM KH2PO4 (pH 7.5) in the presence of 100 units of lyticase (Sigma, St. Louis, MO) and then incubated for 1 h at 37°C. Eleven microliters of 20% (wt/vol) SDS was added, and the lysate was vigorously vortexed for 1 min. Plasmids were extracted by using the Qiagen miniprep protocol except that only 183 μl of P1 buffer was added. The isolated AD plasmids were first screened by PCR to eliminate plasmids that contained MinD- or MinC-encoding DNA fragments because it was previously shown that MinD interacts with MinC and with itself in the yeast two-hybrid assays (22, 23).
Microscopy.
E. coli cells containing plasmids coding for Yfp-labeled proteins were grown in the presence of 10 μM isopropyl β-d-thiogalactoside. Labeled cells were examined by fluorescence microscopy as previously described (54); images were not subjected to deconvolution. For DAPI staining, cells were fixed in the presence of 0.2% glutaraldehyde and 2% formaldehyde for 20 min at room temperature, washed three times with saline, and then stained with 2 μg/ml DAPI for 10 min on ice and washed with saline solution before microscopy. Immunofluorescence methods (55) were described previously except that 100 mM phosphate buffer at pH 6.8, 6.6, 7.4, and 7.4 was used during fixation for enolase, PNPase, RhlB, and RNaseE, respectively. Fixation was done in the presence of 0.02% glutaraldehyde and 2% formaldehyde. Monoclonal mouse anti-HA tag (Sigma) was used to detect HA-tagged proteins. Rabbit antiserum directed against RhlB (kindly provided by M. Cashel, National Institutes of Health, Bethesda, MD) was purified by absorption to purified His-RhlB bound to PVDF membrane, followed by elution with 0.2 M glycine (pH 2) and renaturation with 1.5 M Tris base (pH 8.8). Alexa Fluor 488- and Alexa Fluor 594-conjugated goat anti-rabbit and Oregon green- and Alexa Fluor 488-conjugated goat anti-mouse secondary antibodies were used (Molecular Probes, Carlsbad, CA). For comparison of cellular concentrations of Yfp-labeled proteins, images were collected by using the Openlabs image acquisition program (Improvision, Lexington, MA), total fluorescence was measured for 15 individual cells to determine relative cellular concentrations by using the same software, and the mean concentration in the labeled cells was calculated for each protein sample. The concentrations of different labeled proteins were considered equivalent if they differed by <20%.
To test whether the abnormal phenotype of AT8 [Prne-rne1–417] strain can be reversed by varying the cellular concentration of RNaseE(1–417), AT1/pRNE31 [Δrne/Plac-rne1–417::HA] was grown overnight in LB medium supplemented with ampicillin and then diluted to OD600 0.05 and grown for 5 h at 37°C in LB-ampicillin medium supplemented with 0.1% glucose and 0 μM, 1 μM, 10 μM, 50 μM, 100 μM, 250 μM, 500 μM, or 1 mM IPTG. The cells were then fixed and stained with DAPI as described above.
Supplementary Material
Acknowledgments
We thank Mary Osborn and Asis Das for helpful discussions; M. Cashel, S. Cohen (Stanford University, School of Medicine, Stanford, CA), S. Uzzau (Centre National de la Recherche Scientifique, France), and M. Wachi (Tokyo Institute of Technology, Tokyo, Japan) for proving antibodies, strains, and reagents; and S. Dove for suggesting CviJI enzyme for library construction. This work was supported by National Institutes of Health Grant GM R37-06032.
Abbreviations
- PNPase
polynucleotide phosphorylase
- RhlB
RNA helicase B
- Yfp
yellow fluorescent protein
- AD
activation domain.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610491104/DC1.
References
- 1.Löwe J, van den Ent F, Amos LA. Annu Rev Biophys Biomol Struct. 2004;33:177–198. doi: 10.1146/annurev.biophys.33.110502.132647. [DOI] [PubMed] [Google Scholar]
- 2.Jones L, Carballido-Lopez R, Errington J. Cell. 2001;104:913–922. doi: 10.1016/s0092-8674(01)00287-2. [DOI] [PubMed] [Google Scholar]
- 3.Shih Y-L, Le T, Rothfield L. Proc Natl Acad Sci USA. 2003;100:7865–7870. doi: 10.1073/pnas.1232225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wachi M, Doi M, Tamaki S, Park W, Nakajima-lijima S, Matsuhashi M. J Bacteriol. 1987;169:4935–4940. doi: 10.1128/jb.169.11.4935-4940.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gitai Z, Dye N, Shapiro L. Proc Natl Acad Sci USA. 2004;101:8643–8648. doi: 10.1073/pnas.0402638101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shih YL, Kawagishi I, Rothfield L. Mol Microbiol. 2005;58:917–928. doi: 10.1111/j.1365-2958.2005.04841.x. [DOI] [PubMed] [Google Scholar]
- 7.Møller-Jensen J, Borch J, Dam M, Jensen RB, Roepstorff P, Gerdes K. Mol Cell. 2003;12:1477–1487. doi: 10.1016/s1097-2765(03)00451-9. [DOI] [PubMed] [Google Scholar]
- 8.Gitai Z, Dye NA, Reisenauer A, Wachi M, Shapiro L. Cell. 2005;120:329–341. doi: 10.1016/j.cell.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Kruse T, Bork-Jensen J, Gerdes K. Mol Microbiol. 2005;55:78–89. doi: 10.1111/j.1365-2958.2004.04367.x. [DOI] [PubMed] [Google Scholar]
- 10.Rothfield L, Taghbalout A, Shih YL. Nat Rev Microbiol. 2005;3:959–968. doi: 10.1038/nrmicro1290. [DOI] [PubMed] [Google Scholar]
- 11.Mackie GA. Nature. 1998;395:720–723. doi: 10.1038/27246. [DOI] [PubMed] [Google Scholar]
- 12.Carpousis AJ, Van Houwe G, Ehretsmann C, Krisch HM. Cell. 1994;76:889–900. doi: 10.1016/0092-8674(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 13.Py B, Causton H, Mudd EA, Higgins CF. Mol Microbiol. 1994;14:717–729. doi: 10.1111/j.1365-2958.1994.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 14.Py B, Higgins CF, Krisch HM, Carpousis AJ. Nature. 1996;381:169–172. doi: 10.1038/381169a0. [DOI] [PubMed] [Google Scholar]
- 15.Miczak A, Kaberdin VR, Wei CL, Lin-Chao S. Proc Natl Acad Sci USA. 1996;93:3865–3869. doi: 10.1073/pnas.93.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghora BK, Apirion D. Cell. 1978;15:1055–1066. doi: 10.1016/0092-8674(78)90289-1. [DOI] [PubMed] [Google Scholar]
- 17.Ow MC, Kushner SR. Genes Dev. 2002;16:1102–1115. doi: 10.1101/gad.983502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernstein JA, Lin PH, Cohen SN, Lin-Chao S. Proc Natl Acad Sci USA. 2004;101:2758–2763. doi: 10.1073/pnas.0308747101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpousis AJ. Biochem Soc Trans. 2002;30:150–155. [PubMed] [Google Scholar]
- 20.Coburn GA, Miao X, Briant DJ, Mackie GA. Genes Dev. 1999;13:2594–2603. doi: 10.1101/gad.13.19.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita T, Kawamoto H, Mizota T, Inada T, Aiba H. Mol Microbiol. 2004;54:1063–1075. doi: 10.1111/j.1365-2958.2004.04329.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu Z, Lutkenhaus J. J Bacteriol. 2000;2000:3965–3971. doi: 10.1128/jb.182.14.3965-3971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, King GF, Rothfield L. J Bacteriol. 2003;185:4948–4955. doi: 10.1128/JB.185.16.4948-4955.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liou GG, Jane WN, Cohen SN, Lin NS, Lin-Chao S. Proc Natl Acad Sci USA. 2001;98:63–68. doi: 10.1073/pnas.011535498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruse T, Blagoev B, Lobner-Olesen A, Wachi M, Sasaki K, Iwai N, Mann M, Gerdes K. Genes Dev. 2006;20:113–124. doi: 10.1101/gad.366606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callaghan AJ, Marcaida MJ, Stead JA, McDowall KJ, Scott WG, Luisi BF. Nature. 2005;437:1187–1191. doi: 10.1038/nature04084. [DOI] [PubMed] [Google Scholar]
- 27.de Boer P, Crossley RE, Rothfield LI. J Bacteriol. 1992;174:63–70. doi: 10.1128/jb.174.1.63-70.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huisman O, D'Ari R, Gottesman S. Proc Natl Acad Sci USA. 1984;81:4490–4494. doi: 10.1073/pnas.81.14.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi E, Lutkenhaus J. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fung J, MacAlister TJ, Rothfield LI. J Bacteriol. 1978;133:1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weigand RA, Vinci KD, Rothfield LI. Proc Natl Acad Sci USA. 1976;73:1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain C, Belasco JG. Genes Dev. 1995;9:84–96. doi: 10.1101/gad.9.1.84. [DOI] [PubMed] [Google Scholar]
- 33.Caruthers JM, Feng Y, McKay DB, Cohen SN. J Biol Chem. 2006;281:27046–27051. doi: 10.1074/jbc.M602467200. [DOI] [PubMed] [Google Scholar]
- 34.Daniel RA, Errington J. Cell. 2003;113:767–776. doi: 10.1016/s0092-8674(03)00421-5. [DOI] [PubMed] [Google Scholar]
- 35.Nilsen T, Yan AW, Gale G, Goldberg MB. J Bacteriol. 2005;187:6187–6196. doi: 10.1128/JB.187.17.6187-6196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ausmees N, Kuhn JR, Jacobs-Wagner C. Cell. 2003;115:705–713. doi: 10.1016/s0092-8674(03)00935-8. [DOI] [PubMed] [Google Scholar]
- 37.Komeili A, Li Z, Newman DK, Jensen GJ. Science. 2006;311:242–245. doi: 10.1126/science.1123231. [DOI] [PubMed] [Google Scholar]
- 38.Kruse T, Møller-Jensen J, Lobner-Olesen A, Gerdes K. EMBO J. 2003;22:5283–5292. doi: 10.1093/emboj/cdg504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suefuji K, Valluzzi R, RayChaudhuri D. Proc Natl Acad Sci USA. 2002;99:16776–16781. doi: 10.1073/pnas.262671699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esue O, Cordero M, Wirtz D, Tseng Y. J Biol Chem. 2005;280:2628–2635. doi: 10.1074/jbc.M410298200. [DOI] [PubMed] [Google Scholar]
- 41.Vanzo NF, Li YS, Py B, Blum E, Higgins CF, Raynal LC, Krisch HM, Carpousis AJ. Genes Dev. 1998;12:2770–2781. doi: 10.1101/gad.12.17.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taghbalout A, Ma L, Rothfield L. J Bacteriol. 2006;188:2993–3001. doi: 10.1128/JB.188.8.2993-3001.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z, Lutkenhaus J. Mol Microbiol. 2003;47:345–355. doi: 10.1046/j.1365-2958.2003.03321.x. [DOI] [PubMed] [Google Scholar]
- 44.Szeto T, Rowland S, Rothfield L, King GF. Proc Natl Acad Sci USA. 2002;99:15693–15698. doi: 10.1073/pnas.232590599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Divakaruni AV, Loo RR, Xie Y, Loo JA, Gober JW. Proc Natl Acad Sci USA. 2005;102:18602–18607. doi: 10.1073/pnas.0507937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dye NA, Pincus Z, Theriot JA, Shapiro L, Gitai Z. Proc Natl Acad Sci USA. 2005;102:18608–18613. doi: 10.1073/pnas.0507708102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1982. [Google Scholar]
- 48.Gietz R, Woods R. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 49.Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. Proc Natl Acad Sci USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uzzau S, Figueroa-Bossi N, Rubino S, Bossi L. Proc Natl Acad Sci USA. 2001;98:15264–15269. doi: 10.1073/pnas.261348198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Datsenko KA, Wanner BL. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller JH. A Short Course on Bacterial Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1992. [Google Scholar]
- 53.Swaminathan N, George D, McMaster K, Szablewski J, Van Etten JL, Mead DA. Nucleic Acids Res. 1994;22:1470–1475. doi: 10.1093/nar/22.8.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shih Y-L, Fu X, King GF, Le T, Rothfield LI. EMBO J. 2002;21:3347–3357. doi: 10.1093/emboj/cdf323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Justice SS, Garcia-Lara J, Rothfield L. Mol Microbiol. 2000;37:410–423. doi: 10.1046/j.1365-2958.2000.02007.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.