Abstract
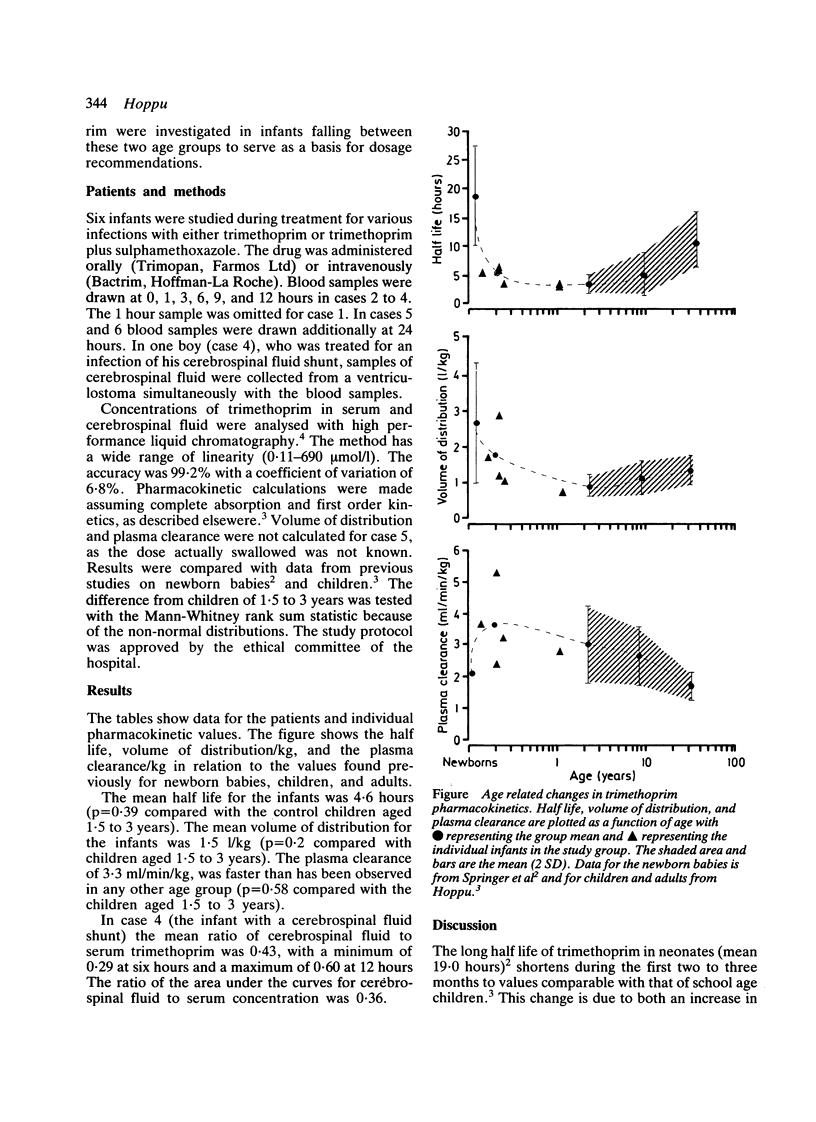
The pharmacokinetics of trimethoprim administered orally or intravenously were investigated in six infants aged 1.7 months to 1.1 years. In these infants trimethoprim had a mean half life of 4.6 hours; this was comparable with the values found in young and school age children (3.8 and 5.4 hours respectively) and about a quarter of the half life in newborns. The volume of distribution (1.5 l/kg) was smaller than in newborns but larger than in young or school age children (0.9 and 1.1 l/kg respectively). The plasma clearance in these infants (3-3 ml/min/kg) was slightly larger than in newborns or in either group of older children (2.9 and 2.4 ml/min/kg respectively). Thus the most dramatic changes in trimethoprim pharmacokinetics seem to occur during the first two months of life. A reduced daily dose of trimethoprim is necessary during the first two months only. An increased daily dose, by addition of a third dose each day, is recommended from two months.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann R. Die chronische Pankreatitis. Zur Frage der Operationsindikation und Beitrag zum Spontanverlauf der chronisch-rezidivierenden Pankreatitis. Dtsch Med Wochenschr. 1970 Jan 2;95(1):1–7. doi: 10.1055/s-0028-1108400. [DOI] [PubMed] [Google Scholar]
- Fries N., Keuth U., Braun J. S. Untersuchungen zur Liquorgängigkeit von Trimethoprim im Kindesalter. Fortschr Med. 1975 Sep 11;93(25):1178–1183. [PubMed] [Google Scholar]
- Hayton W. L., Stoeckel K. Age-associated changes in ceftriaxone pharmacokinetics. Clin Pharmacokinet. 1986 Jan-Feb;11(1):76–86. doi: 10.2165/00003088-198611010-00005. [DOI] [PubMed] [Google Scholar]
- Hoppu K. Age differences in trimethoprim pharmacokinetics: need for revised dosing in children? Clin Pharmacol Ther. 1987 Mar;41(3):336–343. doi: 10.1038/clpt.1987.36. [DOI] [PubMed] [Google Scholar]
- Hoppu K., Arjomaa P. Determination of trimethoprim in pediatric samples by high-performance liquid chromatography. Clin Chim Acta. 1987 Feb 27;163(1):81–86. doi: 10.1016/0009-8981(87)90036-2. [DOI] [PubMed] [Google Scholar]
- Sabel K. G., Brandberg A. Treatment of meningitis and septicemia in infancy with a sulphamethoxazole/trimethorpim combination. Acta Paediatr Scand. 1975 Jan;64(1):25–32. doi: 10.1111/j.1651-2227.1975.tb04376.x. [DOI] [PubMed] [Google Scholar]
- Springer C., Eyal F., Michel J. Pharmacology of trimethoprim-sulfamethoxazole in newborn infants. J Pediatr. 1982 Apr;100(4):647–650. doi: 10.1016/s0022-3476(82)80778-6. [DOI] [PubMed] [Google Scholar]
- Vree T. B., Hekster Y. A., Lippens R. J. Clinical pharmacokinetics of sulfonamides in children: relationship between maturing kidney function and renal clearance of sulfonamides. Ther Drug Monit. 1985;7(2):130–147. doi: 10.1097/00007691-198506000-00002. [DOI] [PubMed] [Google Scholar]


