Abstract
Exocrine pancreatic proteolytic activity, determined by serial measurement of faecal chymotrypsin concentration, was investigated in 21 preterm infants (23-32 weeks' gestation) during the first 28 days of life. The overall chymotrypsin concentration range was similar to that already described in term infants showing that pancreatic chymotrypsin secretion is equally well developed at birth in the preterm infant. A chymotrypsin concentration peak, seen in term infants at 4 days, did not occur in this study until day 8, suggesting a slower initiation of pancreatic exocrine function in the preterm infant. Median faecal chymotrypsin concentrations, calculated for each baby using data from stools passed between day 2 and day 12 of life, were significantly lower in infants who were small for gestational age when compared with those who were an appropriate size for gestational age. The lower chymotrypsin concentration in infants who were small for gestational age suggests a deleterious effect of intrauterine growth retardation on pancreatic exocrine function which may be a factor in limiting postnatal catch up growth.
Full text
PDF
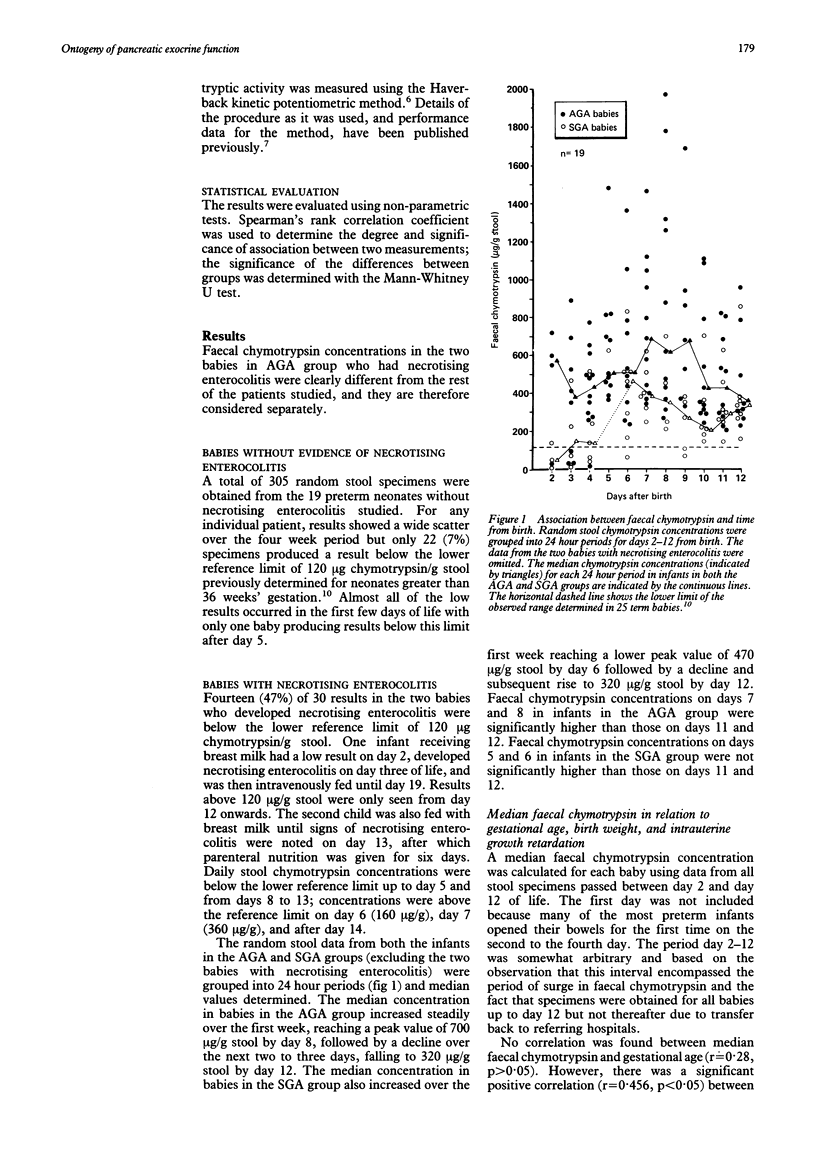
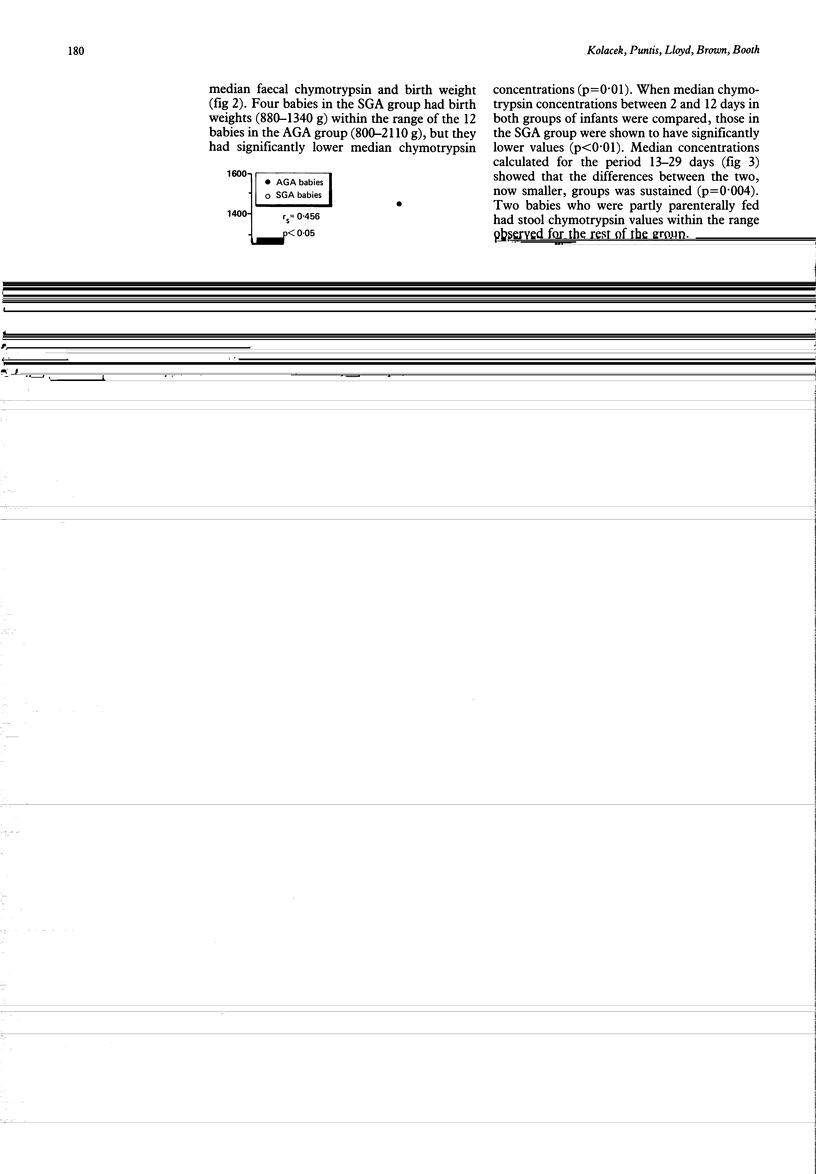

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonowicz I., Lebenthal E. Developmental pattern of small intestinal enterokinase and disaccharidase activities in the human fetus. Gastroenterology. 1977 Jun;72(6):1299–1303. [PubMed] [Google Scholar]
- Barbezat G. O., Hansen J. D. The exocrine pancreas and protein-calorie malnutrition. Pediatrics. 1968 Jul;42(1):77–92. [PubMed] [Google Scholar]
- Brown G. A., Halliday R. B., Turner P. J., Smalley C. A. Faecal chymotrypsin concentrations in neonates with cystic fibrosis and healthy controls. Arch Dis Child. 1988 Oct;63(10):1229–1233. doi: 10.1136/adc.63.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. A., Sule D., Williams J., Puntis J. W., Booth I. W., McNeish A. S. Faecal chymotrypsin: a reliable index of exocrine pancreatic function. Arch Dis Child. 1988 Jul;63(7):785–789. doi: 10.1136/adc.63.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danus O., Urbina A. M., Valenzuela I., Solimano G. The effect of refeeding on pancreatic exocrine function in marasmic infants. J Pediatr. 1970 Aug;77(2):334–337. doi: 10.1016/s0022-3476(70)80347-x. [DOI] [PubMed] [Google Scholar]
- Dubowitz L. M., Dubowitz V., Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970 Jul;77(1):1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- Ducker D. A., Hughes C. A., Warren I., McNeish A. S. Neonatal gut function, measured by the one hour blood D (+) xylose test: influence of gestational age and size. Gut. 1980 Feb;21(2):133–136. doi: 10.1136/gut.21.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. F., Lebenthal E., Krasner J., Branski D. Effect of postnatal malnutrition on pancreatic zymogen enzymes in the rat. Am J Clin Nutr. 1979 Jun;32(6):1224–1230. doi: 10.1093/ajcn/32.6.1224. [DOI] [PubMed] [Google Scholar]
- Heinonen K., Hakulinen A., Jokela V. Survival of the smallest. Time trends and determinants of mortality in a very preterm population during the 1980s. Lancet. 1988 Jul 23;2(8604):204–207. doi: 10.1016/s0140-6736(88)92300-8. [DOI] [PubMed] [Google Scholar]
- Lawrence G., Walker P. D. Pathogenesis of enteritis necroticans in Papula New Guinea. Lancet. 1976 Jan 17;1(7951):125–126. doi: 10.1016/s0140-6736(76)93160-3. [DOI] [PubMed] [Google Scholar]
- Lebenthal E., Lee P. C. Development of functional responses in human exocrine pancreas. Pediatrics. 1980 Oct;66(4):556–560. [PubMed] [Google Scholar]
- Lebenthal E., Lee P. C. The impact of intrauterine and postnatal malnutrition on the development of the exocrine pancreas and small intestine. J Pediatr Gastroenterol Nutr. 1988 Jan-Feb;7(1):1–3. [PubMed] [Google Scholar]
- Lebenthal E., Shwachman H. The pancreas--development, adaptation and malfunction in infancy and childhood. Clin Gastroenterol. 1977 May;6(2):397–413. [PubMed] [Google Scholar]
- Lucas A., Adrian T. E., Christofides N., Bloom S. R., Aynsley-Green A. Plasma motilin, gastrin, and enteroglucagon and feeding in the human newborn. Arch Dis Child. 1980 Sep;55(9):673–677. doi: 10.1136/adc.55.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas A., Bloom S. R., Aynsley-Green A. Metabolic and endocrine consequences of depriving preterm infants of enteral nutrition. Acta Paediatr Scand. 1983 Mar;72(2):245–249. doi: 10.1111/j.1651-2227.1983.tb09705.x. [DOI] [PubMed] [Google Scholar]
- Zoppi G., Andreotti G., Pajno-Ferrara F., Njai D. M., Gaburro D. Exocrine pancreas function in premature and full term neonates. Pediatr Res. 1972 Dec;6(12):880–886. doi: 10.1203/00006450-197212000-00005. [DOI] [PubMed] [Google Scholar]


