Summary
The sticky spiral of araneoid spider orb webs consists of silk fibers coated with adhesive droplets. The droplets contain a variety of low-molecular-mass compounds (LMM). Within a species, a fairly consistent ratio of LMM is often observed, but substantial variability can exist. To gain insight into factors influencing LMM composition, spiders of three araneid species were starved and LMM from their webs were analyzed for changes in composition. To determine if these changes were consistent with the spider’s ability to synthesize the different organic LMM, synthetic capacities were estimated following the feeding of radiolabeled metabolites. Some changes in droplet composition were broadly consistent with differing synthetic capacities: molar percentages of less readily synthesized compounds (e.g., choline, isethionate, n-acetyltaurine) typically declined with starvation, at least during a portion of the imposed fast, while more readily synthesized compounds (e.g., GABamide, glycine) tended to increase. Most striking was the apparent partial substitution of n-acetylputrescine by the more readily synthesized GABamide in fasting Argiope trifasciata. However, departures from expected compositional shifts demonstrated that synthetic capacity alone does not adequately predict sticky droplet compositional shifts with starvation. Moreover, feeding controls exhibited some changes in composition similar to starving spiders. As the webs of both feeding and starving spiders were removed for chemical analysis and could not be recycled, the loss of LMM contained in these webs likely contributed to similarities between treatments. In addition, feeding spiders molted, oviposited, and/or built heavier webs. The added metabolic demands of these activities may have contributed to changes in composition similar to those resulting from starvation.
Keywords: 4-aminobutyramide, Araneus cavaticus, choline, glycine, glycine betaine, isethionic acid, orb web, putrescine, resource allocation, spider web recycling, sticky spiral, taurine
List of Abbreviations: Ala alanine, Bet glycine betaine, Cho choline, 2D-TLC two-dimensional thin layer chromatography, GAB 4-aminobutyramide, Gly glycine, 1H-NMR proton nuclear magnetic resonance spectroscopy, HVE high-voltage paper electrophoresis, Ise isethionic acid (2-hydroxyethane sulfonic acid), LMM sticky droplet low-molecular-mass compounds, NAP n-monoacetylputrescine, NAT n-acetyltaurine, PES post-egg-sac, Pro proline, Put putrescine, Tau taurine
Introduction
In the construction of its orb web an araneoid spider first builds a non-sticky scaffolding using ampullate silk gland fibers and piriform silk gland junctional cements/attachment disks. One part of the scaffolding, the radii, are arranged like the spokes of a wheel. Subsequently, the sticky spiral (= adhesive or viscid spiral, among other names; Zschokke, 1999) is attached to the radii. The sticky spiral consists of a pair of flagelliform silk gland fibers coated with an adhesive, aqueous secretion from the aggregate glands (Sekiguchi, 1952; Peters, 1955). This aggregate gland secretion contains organic and inorganic low-molecular-mass compounds (LMM; <200 Da), at least one high-molecular-mass phosphorylated glycoprotein, and, likely, lipids (see references in Higgins et al., 2001:82; Schulz, 2001). Organic LMM include 11 of the 12 compounds monitored in this study; GABamide (= 4-aminobutyramide) (GAB), n-monoacetylputrescine (NAP), isethionic acid (= 2-hydroxyethane sulfonic acid) (Ise), n-acetyltaurine (NAT), glycine (Gly), choline (Cho), glycine betaine (Bet), alanine (Ala), proline (Pro), putrescine (Put), taurine (Tau) (Fischer and Brander, 1960; Tillinghast and Christenson, 1984; Vollrath et al., 1990; Townley et al., 1991; Higgins et al., 2001).
The aggregate gland secretion, initially deposited as an unstable liquid cylinder, spontaneously re-distributes into a pattern of linked droplets (Boys, 1889; Warburton, 1890; Edmonds and Vollrath, 1992; Ball, 1999:18–19). Concurrent with droplet formation is the formation of discrete nodules at the center of the droplets that contain the aforementioned glycoprotein (Vollrath and Tillinghast, 1991). While it is believed the nodules are the principal adhesive agents in the droplets (Richter, 1956; Vollrath and Tillinghast, 1991), the functions of the LMM have not been established, though one role, adsorbing atmospheric moisture, is indicated by the hygroscopic properties of certain LMM (Fischer and Brander, 1960; Schildknecht et al., 1972; Vollrath et al., 1990; Townley et al., 1991).
The LMM account for about 40–70% of the desiccated weight of orb webs, indicating they are important to web function (Fischer and Brander, 1960; Anderson and Tillinghast, 1980; Tillinghast, 1984; Tillinghast and Christenson, 1984; Townley et al., 1991). They may contribute to the elastomeric mechanical properties of the sticky spiral; directly, by inhibiting crystallization within the flagelliform fibers (Gosline et al., 1995), or indirectly, by adsorbing moisture, thereby keeping the fibers elastomeric through hydration (Vollrath and Edmonds, 1989; Bonthrone et al., 1992; Hayashi and Lewis, 1998). Direct and indirect effects of the LMM may also be important to the functioning of the adhesive glycoprotein within the nodules. The conformation adopted by the glycoprotein and the formation of nodules may depend on direct interactions between the glycoprotein and LMM, and the ability of the glycoprotein to spread out on contact with an insect (Richter, 1956; Vollrath and Tillinghast, 1991) presumably relies on the presence of water. Water adsorbed by LMM also promotes droplet formation, which increases adhesiveness (Edmonds and Vollrath, 1992), and contributes to aerodynamic drag following the impact of an insect, thus helping to dissipate the prey’s kinetic energy (Lin et al., 1995).
The LMM composition of sticky droplets often differs quantitatively and qualitatively among araneoid species (Vollrath et al., 1990; Townley et al., 1991; Higgins et al., 2001; M. A. Townley and E. K. Tillinghast, unpublished). The significance of these differences is unknown. One attempt to determine if differences in composition translate into differences in web hygroscopicity among three araneid species did not demonstrate such a relationship (Townley et al., 1991). Nor do we know the extent to which the composition of the droplets is tailored to the physical environment in which a spider forages, the prey captured, or the metabolic needs of the spider. And while LMM composition within a species is consistent enough that analyses of pooled web collections from groups of individuals generally yield similar results (Vollrath et al., 1990; Townley et al., 1991), substantial intraspecific differences have also been observed within and among populations, between the sexes, and following a change in environment/diet (Vollrath et al., 1990; Townley et al., 1991; Higgins et al., 2001).
The goal of the present study was to examine the influence of starvation on the LMM composition of the sticky droplets and to determine if observed changes reflect differences in the spider’s capacity to synthesize the various organic LMM. Specifically, we anticipated that the total mass of LMM would decrease in webs of starving spiders, but that there would be greater relative declines in those organic LMM the spider is less able to synthesize. Therefore, in addition to analyzing series of webs built by starving and feeding spiders of three araneid species, we fed radiolabeled compounds to two of these species to determine to what extent the spiders can synthesize the different organic LMM (Kasting and McGinnis, 1966). Given the results of these synthetic capacity measurements, some of the changes in composition observed with starvation conformed to our expectations, but others did not. Unexpected results also came from the control feeding spiders, which also exhibited changes in droplet composition, in some respects similar to trends seen in webs of starving spiders. Possible explanations for these results are discussed. In addition, the construction of egg sacs by some of the study spiders allowed us to make a preliminary examination of the influence of the egg laying cycle on droplet composition.
Materials and methods
Synthesis of organic LMM by Argiope
Spider collection, maintenance, and radioisotope feeding
Adult female Argiope aurantia Lucas, 1833 and Argiope trifasciata (Forskål, 1775) were collected in southern New Hampshire, USA from late July to late August and in the latter half of September, respectively. Spiders were housed individually in wood or aluminum frames (51 x 51 x 9 cm) with glass plates on the front and back. They were exposed to the prevailing natural light:dark cycle in a room facing east at temperatures and relative humidities that approximated outside conditions.
Four groups of A. aurantia (5–7 spiders/group) and three groups of A. trifasciata (6–7 spiders/group) were formed. For each A. aurantia, 3 μCi d-[U-14C]glucose (ICN Biomedicals, Irvine, CA, USA) in 3 μl water was placed on the mouthparts using a 10 μl Hamilton syringe (Reno, NV, USA) and the spider was observed until the droplet was completely imbibed. Argiope trifasciata were likewise fed d-[U-14C]glucose, but in quantities of 1–3 μCi/spider.
The synthesis of the three sulfonic acids in the webs’ sticky droplets (Ise, NAT, Tau) was also investigated by feeding adult female A. aurantia (4 groups; 4–6 spiders/group; 1–4 μCi/spider) a solution containing L-[35S]methionine and L-[35S]cysteine (TRAN35S-Label, ICN).
Spiders were either fed radioisotope within 3 days of being captured or, if held longer prior to isotope feeding, were fed crickets or grasshoppers. They were not fed after isotope feeding, but orbs built on the day of isotope feeding were partially collapsed and the spiders were allowed to recycle them. Water was given daily, except for the day before and the day of isotope feeding to encourage drinking of the labeled solution.
Handling of radiolabeled orb webs
The first 5 webs built by each spider after ingesting radioisotope were collapsed, wound onto one end of a glass micropipet, and stored at −20°C. All webs built by members of the same group were pooled on a single micropipet (19–30 webs/group). Not all spiders built 5 webs. While still on their micropipets, each group’s web collection was extracted twice in 4 ml distilled water for 1.5 hr with occasional gentle vortexing. The two extracts were pooled, dried, and analyzed by proton nuclear magnetic resonance spectroscopy (1H-NMR) as described below using the Argiope acquisition parameters (see ‘1H-NMR analysis’). From these analyses molar percentages of the 11 organic LMM (listed in Introduction) in each of the 11 extracts (seven 14C-labeled, four 35S-labeled) were calculated. Extracts were then fractionated individually by high-voltage paper electrophoresis (HVE).
HVE and chromatography of radiolabeled water-soluble web fractions
Each water-soluble extract was fractionated by HVE at 3000 V on 23 x 57 cm sheets of Whatman 3MM chromatography paper (Brentford, England), with the extract applied in 75 μl of electrolyte solution over a 13 cm long origin. For the 14C-labeled extracts, the origin was 28 cm from the positive pole and electrophoresis was carried out for 35 min. For the 35S-labeled extracts, the origin was 41 cm from the positive pole and the run lasted 55 min. The electrolyte solution used was pyridine:glacial acetic acid:water (133:4.6:1862.4, v/v), pH 6.4. Coolant at 2°C was passed through the unit’s lower plate.
After electrophoresis, the paper was air dried and autoradiograms were generated using Kodak BioMax MR film (Rochester, NY, USA). Using the autoradiograms as a guide, the electrophoretograms were cut into radioactive and nonradioactive bands. All bands were eluted overnight with distilled water containing 0.001% sodium azide in a chamber saturated with water vapor. Eluates were dried, resuspended in 1 ml distilled water, and 50 μl removed for scintillation counting. Selected eluates were examined by 1H-NMR to establish locations of organic LMM.
To determine what percentage of the radioisotope in a HVE eluate was incorporated into an organic LMM of interest, portions of radioactive eluates were further fractionated by two-dimensional thin layer chromatography (2D-TLC) on 20 x 20 cm cellulose plates (0.1 mm thickness, Merck KGaA, Darmstadt, Germany) using pyridine:acetone:ammonium hydroxide:water (45:30:5:20, v/v) in the first dimension and 2-propanol:formic acid:water (75:12.5:12.5, v/v) in the second dimension (Schmidt, 1974). This was necessary because HVE did not resolve all of the organic LMM measured in this study and because these compounds, though they constitute the bulk of the organic LMM, could not be assumed to be the only organic or sulfur-containing compounds extracted by water from orb webs (and, indeed, they are not). Autoradiograms were prepared from the 2D-TLC plates as above. If an autoradiogram revealed two or more radioactive compounds, these were individually scraped off the plate and their radioactivities were measured by scintillation counting. Identifications of the organic LMM on the plates were based on NMR of the HVE eluates and on experience in this system with the migration characteristics of most of the organic LMM of interest. Where any doubt remained, radioactive compounds were scraped off plates and examined by NMR to confirm identity.
Using pre- and post-fractionation NMR data and the radioactivity data obtained following HVE and 2D-TLC, relative specific radioactivities for the organic LMM were estimated (initially in counts per minute/mole %, then, for 14C-labeled LMM, converted to cpm/molar quantity of carbon).
For the two n-acetylated LMM, NAP and NAT, estimates were also made of the relative specific activities of their acetyl groups versus their Put or Tau moieties. Because NAP was not detected in webs of A. aurantia, only the three A. trifasciata water-soluble extracts were used to make this determination for this compound. After identifying by NMR the HVE eluates containing NAP and NAT, 70% of each was hydrolyzed under vacuum (50 mtorr) in 6 N HCl at 115°C for 20 hr. At the same time, commercial samples of Put and Tau (two each) were treated likewise. Based on the % recovery of these standards (Put, 83.8%; Tau, 84.3%), a correction for losses occurring during hydrolysis was made. Hydrolyzed and unhydrolyzed portions of eluates were fractionated by HVE and 2D-TLC and autoradiograms were generated. Of the total 14C present in these eluates, the percentage incorporated into the Put/Tau moieties versus the intact compounds was determined by scintillation counting of compounds scraped off the 2D-TLC plates. The difference between these was taken to be the percentage of the 14C incorporated into the acetyl groups.
Effects of starvation and web removal on orb web weights and LMM composition in Araneus and Argiope
Collection and maintenance of spiders
Juvenile Araneus cavaticus (Keyserling, 1882) were collected from barns in southern New Hampshire between early April and mid-July. Juvenile female Argiope aurantia and Argiope trifasciata were also collected locally from mid-July to early August and throughout August, respectively. Spiders were housed as described above.
Before spiders molted and were placed in an experimental group, they were fed 1–3 flies on days they built webs, were allowed to recycle (i.e., consume) their webs freely, and were given water daily. Both before and after assigning spiders to groups, A. cavaticus and A. aurantia were fed house flies (Musca domestica Linnaeus, 1758) and other dipterans (primarily Phaenicia sericata (Meigen, 1826) and Phormia regina (Meigen, 1826)), while A. trifasciata received house flies exclusively.
Formation of study groups and collection of orb webs
During this study, 21 groups were formed (Supplemental Fig. 1), with the spiders composing a group receiving the same treatment (fed or starved) and being of the same species, sex, and stage (juvenile or adult), and, for adult females, having made the same number of egg sacs (0–3). All spiders were added to a group at the same point in the molt/intermolt cycle (beginning of intermolt). With A. cavaticus, webs of male and female juveniles (all penultimate instars) and adult females were collected. With the two Argiope species only webs built by adult females were collected. An individual spider could belong to only one group at a time, but to two or more groups over the course of the study, as explained below.
Orb webs were collected from a spider only once it had reached the desired stage following its most recent ecdysis. From 1–3 molts were required in the laboratory before the desired stage was reached. Spiders were initially divided into a feeding group and a starving group, with the first individual to reach the desired stage randomly assigned to one of these groups. The next individual that molted to the desired stage was then assigned to the other group and this alternation continued as other spiders subsequently molted to the desired stage.
Following ecdysis, with the building of the first orb web, the spider, whether assigned to a feeding or starving group, was fed 2 or more flies (see above) totaling about 50 mg wet weight. The web was then partially collapsed and the spider was allowed to recycle it. Thus, no compositional or weight data were obtained from the first web. All subsequent webs, however, were collected on micropipets and stored at −20°C. All second webs built by members of the same group were collected on the same micropipet and are referred to as the web 2 collection of the group. Likewise, all third webs built by the spiders within a group were pooled to yield the web 3 collection and so on. The complete set of web collections from a group is referred to as a ‘series’.
Spiders in starving groups were treated differently from spiders in feeding groups after the construction of the second orb web. In both groups, the second webs were collected as described above, but then only feeding group spiders were handed one or more flies totaling about 25–35 mg wet weight. Feeding group spiders were fed only on days they built a web. All spiders were given water daily. With starving groups, spiders were stressed until resultant changes in behavior/physiology were readily apparent (e.g. sluggishness, construction of incomplete webs), then feeding was resumed, at which point the spiders became members of a ‘resumed feeding’ group (Supplemental Fig. 1).
All constructed orb webs, unless they contained no sticky spiral whatsoever, were collected from each member of a group and added to that group’s series of web collections until one of four events occurred (Supplemental Fig. 1): 1) the spider was transferred to another group, 2) the spider died, 3) the spider escaped, or 4) the date arrived, 30 September, on which other obligations forced us to end web collecting for the year. This lattermost event was not too detrimental as many of the spiders involved, all adult A. cavaticus or A. aurantia, had already become relatively inactive by this time and would have built few additional webs. However, the series from the A. aurantia PES3 group (see next paragraph) was ended prematurely. Argiope trifasciata webs were collected during a different year when there was no need to terminate the study on a particular date.
There were three reasons a spider was transferred to another group. Transfer of starving group spiders to a resumed feeding group has already been described. The other two reasons were egg sac construction and molting. Egg sac construction resulted in a spider being transferred to a post-egg-sac (PES) group. Because some individuals of both A. aurantia and A. trifasciata produced three egg sacs (containing nonviable eggs), the construction of each of which was followed by the spinning of additional orb webs, there are three successive PES groups for both species (PES1, 2, and 3) (Supplemental Fig. 1). Molting pertains to the penultimate instar A. cavaticus only since adults do not molt. On reaching adulthood males lack the ability to build orb webs (Sekiguchi, 1955) so the final molt marked the end of their period of service. Female A. cavaticus, on the other hand, by the procedure described above, were assigned to either a feeding adult or a starving adult group after their final molt (Supplemental Fig. 1).
Following the transfer of a spider to either a resumed feeding or PES group we did not allow it to recycle its first web, as spiders in starving or feeding groups were allowed, but collected this web and fed the spider one or more flies totaling about 25–35 mg wet weight. In all other ways web collection and feeding were carried out as for members of feeding groups.
Gravimetric measurements of orb webs
Each web collection was scraped off its micropipet with a razor blade, desiccated in vacuo over phosphorus pentoxide for at least 2 days, weighed to the nearest 0.01 mg, then extracted three times in 2 ml distilled water for 1 hr with occasional gentle vortexing. The three extracts were pooled and dried on a Savant Speed Vac Concentrator (Hicksville, NY, USA), then transferred with two aliquots of distilled water totaling 300 μl to a pre-weighed cup fashioned from the cap of a microcentrifuge tube. After desiccating as above for at least 3 days, this water-soluble fraction of the web, containing the LMM, was weighed and then transferred back to its sample tube with three aliquots of distilled water totaling 1 ml. After drying, the sample was analyzed by 1H-NMR. The desiccated water-insoluble fraction was also weighed.
Within a group, not all individuals built the same number of webs (Supplemental Table 1). Consequently, later web collections in a series contained fewer webs than earlier web collections. Initially, therefore, anticipating difficulty with 1H-NMR analysis on the smaller samples (due to unacceptably large numbers of scans being required to achieve a good signal-to-noise ratio), we typically pooled two or more end-of-series web collections to generate water-soluble extracts that would be large enough to allow for analysis in a reasonable amount of time. Note that this more infrequent ‘horizontal pooling’ of, e.g., web collections 16–19 is in addition to the invariable practice of ‘vertical pooling’ of, e.g., all second webs built by the members of a group. We found, however, that our first horizontal poolings were too generous and that smaller samples (0.3–1 mg) could be analyzed within an acceptable amount of time (3 h to overnight). Thus, we did less horizontal pooling as the study progressed. As the first webs analyzed were those built by Araneus cavaticus, more horizontal pooling was carried out on webs of this species.
Gravimetric measurements of spiders
Fresh and dry weight data on the three study species were obtained to gain some measure of the percentage of a spider’s dry weight that it typically invests in its orb web, thus providing an indication of the loss incurred when, as in this study, a spider is denied the opportunity to recycle its web. Freshly captured local penultimate instar and adult female Araneus cavaticus, and adult female Argiope aurantia and Argiope trifasciata were weighed immediately on return to the laboratory. Some of these individuals were anesthetized with CO2, immersed in 80% ethanol for 1 h, and dried to constant weight in a 43°C oven. Only adult females that were not conspicuously gravid were collected for these measurements.
Composition of orb web water-soluble fractions
1H-NMR analysis
The water-soluble fraction of each web collection was dissolved in 0.5–1.0 ml 99.96% D2O (Cambridge Isotope Laboratories, Andover, MA, USA) and analyzed on a Bruker AM-360 spectrometer (Billerica, MA, USA) with a 5 mm proton selective probe. 360-MHz 1H spectra were obtained at a temperature of 300K with 2-methyl-2-propanol added as an internal standard (δ= 1.2200 ppm). Following Fourier transformation, peak areas were integrated and used to calculate the molar percentages of the 11 organic LMM dealt with in this study. 1H-NMR chemical shifts and coupling constants for each of these LMM in D2O have been reported previously (Townley et al., 1991; Higgins et al., 2001).
In all NMR analyses a spectral width of 5000 Hz was examined and a pulse width of 4.3 μsec, yielding about a 53° flip angle, was used. Various numbers of scans (128 to 5064) were accumulated depending on sample size. Several other acquisition parameters differed between A. cavaticus and Argiope samples. With the A. cavaticus extracts, analyzed first, the pulse repetition time was the same as the acquisition time, 3.28 sec, during which 32K data points were acquired and later zero-filled to 64K prior to Fourier transformation. However, analyses of a standard solution containing the 11 organic LMM indicated that a longer time between pulses would yield more accurate molar percentages (Supplemental Table 2), particularly for Pro. Therefore, with the Argiope extracts, a longer pulse repetition time was adopted (8.28 sec) that still allowed samples to be analyzed within a reasonable amount of instrument time, with 64K data points acquired over 6.55 sec, later zero-filled to 128K. We did not attempt to apply corrections to the A. cavaticus data. Thus, more than anything else, Pro is likely somewhat underrepresented in the A. cavaticus webs, though consistently so.
In addition to measuring relative quantities of the organic LMM in molar %, we wanted to estimate absolute quantities of the organic LMM. With the A. cavaticus web collections, this was achieved by a standard addition method whereby each sample was analyzed by NMR twice, before and after the addition of synthetic GAB (Kleemann et al., 1980) (1 μmol/mg water-soluble fraction). Integrations from the two analyses allowed us to calculate average μg/web for each organic LMM in each web collection. We also calculated the percentage of the water-soluble fraction’s weight that could be accounted for by the eleven organic LMM. For the two Argiope species, we obtained approximate μg/web of the organic LMM by assuming the percentage of the water-soluble fraction’s weight that could be accounted for by the eleven organic LMM was the same as the mean in A. cavaticus.
Phosphorus analysis
After NMR analysis, each water-soluble web fraction was assayed for inorganic phosphorus (Chen et al., 1956) using KH2PO4 as a standard.
Statistical Analyses
Pearson correlation coefficients were calculated from molar % data: 1) for all pair-wise combinations of individual LMM, with data from all non-radioactive web collections from each species pooled for the analyses, 2) between H2PO4− and total positively-charged LMM, total negatively-charged organic LMM, and ‘excess’ positive charge (see ‘H2PO4− and charge balance’ in Results), and 3) for all pair-wise combinations of web collection number and total readily synthesized, moderately synthesized, and poorly synthesized LMM (see ‘Synthesis of organic LMM by Argiope’ in Results) in web collections from the starving and feeding groups. As in an earlier study (Higgins et al., 2001), all molar percentages were arcsine transformed prior to analysis and Bonferroni-corrected P values are reported.
Linear regression analysis was used to evaluate changes in three quantities over the series of web collections for a group: 1) the % of web weight that was solubilized by water, 2) the % of the water-soluble fraction’s weight that was accounted for by the 11 organic LMM in A. cavaticus (see ‘1H-NMR analysis’ above), and 3) the ‘excess’ positive charge. A t-test was used to determine if slopes of regressions differed significantly from zero. Slopes of ‘excess’ positive charge regressions were compared using a Tukey’s multiple comparison test (Zar, 1999:372).
Specific radioactivities of LMM from radiolabeled webs were normalized as described in Table 1, log10-transformed, and compared using ANOVA and Tukey’s HSD multiple comparisons test. The specific radioactivities reported in Table 1 are back-transformed means and 95% confidence intervals.
Table 1.
Molar percentages and relative specific radioactivities of organic LMM in water extracts of radiolabeled orb webs from Argiope fed [U-14C]glucose or a solution containing l-[35S]methionine and l-[35S]cysteine
|
A. aurantia |
A. trifasciata |
|||||
|---|---|---|---|---|---|---|
|
35S-Labeled Webs
(N = 4 web collections) |
14C-Labeled Webs
(N = 4 web collections) |
14C-Labeled Webs
(N = 3 web collections) |
||||
| Mole % (X ± SEM) | Relative Specific Radioactivity (Normalized CPM/mole sulfur) X (95% CI) | Mole % (X ± SEM) | Relative Specific Radioactivity (Normalized CPM/mole carbon) X (95% CI) | Mole % (X ± SEM) | Relative Specific Radioactivity (Normalized CPM/mole carbon) X (95% CI) | |
| Readily synthesized LMM | ||||||
| Alanine | 0.01 ± 0.010 | NA | 0.05 ± 0.027 | — | 0.57 ± 0.122 | 88.2 (51.4 – 151)i |
| Glycine | 0.53 ± 0.306 | NA | 2.3 ± 0.49 | 37.8 (27.1 – 52.9)d | 2.7 ± 0.97 | 40.2 (23.4 – 69.0)h,i |
| GABamide | 57.6 ± 2.51 | NA | 53.9 ± 1.89 | 19.6 (14.0 – 27.5)c,d | 11.0 ± 2.58 | 10.2 (5.97 – 17.6)e,f,g |
| Proline | ND | NA | ND | — | ND | — |
| Moderately synthesized LMM | ||||||
| N-Acetylputrescine | ND | NA | ND | — | 24.9 ± 3.17 | 3.53 (2.06 – 6.05)c,d,e |
| Putrescine moiety | 2.70 (1.57 – 4.63)c,d | |||||
| Acetyl moiety | 5.05 (2.94 – 8.67)d,e,f | |||||
| Putrescine | ND | NA | ND | — | ND | — |
| Taurine | 1.5 ± 0.45 | 66.91 (58.0 – 77.2)b | 0.95 ± 0.207 | — | 1.7 ± 0.21 | — |
| N-Acetyltaurine | 12.6 ± 0.66 | 52.9 (47.8 – 58.5)a | 15.2 ± 1.22 | 12.9 (9.19 – 18.0)c | 5.6 ± 0.54 | 14.1 (8.24 – 24.2)f,g,h |
| Taurine moiety | 2.24 (1.60 – 3.14)b | 1.15 (0.67 – 1.97)b,c | ||||
| Acetyl moiety | 23.1 (16.5 – 32.3)c,d | 27.0 (15.7 – 46.3)g,h,i | ||||
| Isethionic acid | 8.3 ± 1.07 | 47.1 (42.5 – 52.1)a | 7.6 ± 1.21 | 2.08 (1.49 – 2.91)b | 26.7 ± 1.49 | 0.69 (0.40 – 1.18)b |
| Poorly synthesized LMM | ||||||
| Choline | 15.7 ± 2.00 | NA | 17.2 ± 0.96 | 0.19 (0.14 – 0.27)a | 23.3 ± 0.62 | 0.16 (0.09 – 0.27)a |
| Glycine betaine | 3.8 ± 0.35 | NA | 2.8 ± 0.23 | — | 3.5 ± 0.77 | — |
See text for explanation of different categories of LMM (Readily, Moderately, and Poorly synthesized).
In calculating molar %, inorganic phosphate was not included as a constituent, unlike Table 2.
For 35S-labeled web extracts, specific radioactivities were normalized within each web collection by expressing the specific activity of each LMM as a percentage of the sum of the specific activities of NAT + Ise. For 14C-labeled web extracts, normalized specific activities were similarly expressed as a percentage of the sum of the specific activities of Cho + GAB + Gly + NAT + Tau moiety of NAT + acetyl moiety of NAT + Ise (i.e., the constituents for which we have specific activity data in both Argiope species). Thus, the mean specific activities reported from the 14C-labeled webs are directly comparable between the two species.
Within the webs of a single species labeled with a single radioisotope, specific radioactivities differed significantly (P < 0.05) from each other if they show no superscript letters in common. See ‘Statistical Analyses’ in Materials and methods for more details.
95% CI, 95% confidence interval; NA, not applicable; ND, none detected by 1H-NMR.
N = 2 (taurine was not adequately resolved in the other two web collections).
Transformations and slope comparisons were done in Microsoft Excel 2002 (Redmond, WA, USA); correlations, regressions, ANOVA’s and multiple comparisons of means were carried out in Systat 10 (Point Richmond, CA, USA).
Results
Synthesis of organic LMM by Argiope
Relative specific radioactivities of the organic LMM from the labeled web collections are presented in Table 1, along with the molar percentages of the organic LMM in these webs. Put and free Pro were not detected by NMR in any of the radiolabeled web collections. Thus, specific activity data were not obtained for these compounds, though the specific activity of the Put component of NAP was measured. The specific activity of free Ala was determined only from the A. trifasciata web extracts because of the very small amounts of Ala in the A. aurantia extracts. Specific activities for Bet and 14C-labeled Tau were not obtained because we were not convinced these compounds had been adequately resolved. However, relative specific activities were determined for 35S-labeled Tau and for the Tau moiety of 14C-labeled NAT.
In 14C-labeled webs, the specific activity of NAT was significantly higher than that of Ise (Table 1). Hydrolysis of NAT, however, revealed that the bulk of the 14C incorporated into this compound was restricted to its acetyl group and that the specific activities of Ise and the Tau moiety of NAT were not significantly different. Neither were the specific activities of NAT and Ise significantly different in 35S-labeled webs. In contrast to NAT, the label in NAP was more evenly divided between the acetyl and Put moieties, though on a per mole carbon basis, the acetyl group’s specific activity was again higher than that of Put, though not significantly so. This difference between the two N-acetylated compounds was in greater measure due to the significantly higher specific activity of the acetyl group in NAT as compared with the acetyl group in NAP, and to a lesser extent due to the higher (though not significantly so) specific activity of the Put moiety as compared with the Tau moiety. Recall that comparisons between these two LMM could be made only in A. trifasciata since NAP was not detected in A. aurantia.
Based on the specific radioactivities obtained, we divided the LMM in Table 1 into three categories: readily synthesized LMM (Ala, Gly, GAB), moderately synthesized LMM (NAP, Put, NAT, Tau, Ise), and poorly synthesized LMM (Cho, Bet). Assuming the acetyl moieties of NAP and NAT can be readily synthesized, LMM with mean specific activities greater than that of the acetyl group of NAP were taken to be readily synthesized. As we did not obtain specific activities for Pro, its placement is uncertain, but it is likely at least a moderately synthesized compound. Free Put and Tau were assumed to have specific activities comparable to those of the Put and Tau moieties of NAP and NAT, respectively, and it was the data for these moieties that dictated the inclusion of NAP and NAT in the moderately synthesized category. Though we also did not obtain data for Bet, we tentatively consider it a poorly synthesized LMM given the observations of Higgins and Rankin (1999) and the likelihood of its synthesis from Cho (see Discussion).
We predicted that, with starvation, molar percentages of readily synthesized LMM would tend to increase while those of poorly synthesized LMM would decrease and these trends would be reversed when feeding was resumed. The fate of moderately synthesized LMM was more uncertain, but our suspicion was that their molar percentages would either decrease or just be maintained with starvation.
Molar percentages of organic LMM were measured in the radiolabeled web collections simply to allow us to express radioisotope incorporation in terms of relative specific radioactivities. We assumed LMM compositions in these webs would be similar to those in some of the non-radioactive web collections. In some respects they were, but there were also differences. The molar percentages of Cho and Bet are especially noteworthy in this regard. The lowest molar % of Cho in the 8 radiolabeled A. aurantia web extracts (10.9 mole %) was higher than the highest molar % of Cho in the 102 unlabeled A. aurantia web extracts (8.9 mole %). (Note that these two molar percentages were calculated based on the 11 organic LMM constituting 100% of the LMM. The highest molar % for Cho in A. aurantia given in Table 3 is 7.4 mole % because the percentages in that table were calculated based on the 11 organic LMM plus H2PO4− constituting 100% of the LMM.) Likewise, the lowest molar % of Cho in the 3 labeled A. trifasciata web extracts (22.6 mole %) was higher than the highest molar % of Cho in the 123 unlabeled A. trifasciata web extracts (13.9 mole %). The same sort of discrepancy, albeit not as extreme, was also observed in A. aurantia with regard to molar % of Bet. Unlabeled webs were built by adult female Argiope maintained on a diet of flies. They were collected as juveniles and raised in the laboratory through 1–2 molts and were unmated. Labeled webs were built by adult female Argiope that had recently been feeding in the field or, if fed in captivity, were given crickets or grasshoppers. They were collected as adults and were likely mated.
Table 3.
Relative quantities (in mole %) of the organic LMM and inorganic phosphate in orb webs of the three species studied, as measured in the non-radioactive web collections
|
Mean ± SEM1 (Range)2 |
|||
|---|---|---|---|
| Araneus cavaticus | Argiope aurantia | Argiope trifasciata | |
| (means: N = 21) | (means: N = 7) | (means: N = 7) | |
| (ranges: N = 86) | (ranges: N = 102) | (ranges: N = 123) | |
| putrescine | 0.58 ± 0.067 (ND - 1.24) | ND (ND) | 1.11 ± 0.157 (ND - 1.85) |
| choline | 7.5 ± 0.94 (0.3 – 15.9) | 4.2 ± 0.90 (0.2 – 7.4) | 6.7 ± 1.28 (ND - 12.2) |
| GABamide | 42.1 ± 1.05 (31.6 – 58.3) | 52.6 ± 1.73 (47.6 – 70.8) | 3.9 ± 0.93 (0.6 – 31.8) |
| N-acetylputrescine | 3.13 ± 0.186 (ND - 6.60) | ND (ND) | 32.8 ± 0.97 (14.7 – 43.0) |
| glycine betaine | 0.37 ± 0.083 (ND - 2.42) | 0.65 ± 0.227 (ND - 2.00) | 2.4 ± 0.61 (ND - 5.0) |
| glycine | 1.33 ± 0.157 (0.25 – 15.5) | 2.5 ± 0.55 (0.7 – 19.9) | 8.9 ± 0.55 (2.6 – 31.6) |
| alanine | 0.45 ± 0.043 (ND - 2.76) | 0.16 ± 0.030 (ND - 2.85) | 2.82 ± 0.207 (0.18 – 6.06) |
| proline | 0.76 ± 0.159 (ND - 8.83) | ND (ND - 1.65) | ND (ND) |
| taurine | ND (ND) | 0.36 ± 0.048 (ND - 0.58) | 0.32 ± 0.101 (ND - 0.80) |
| N-acetyltaurine | 6.0 ± 0.50 (2.0 – 14.4) | 12.7 ± 0.64 (2.5 – 17.2) | 10.3 ± 1.25 (0.2 – 13.2) |
| isethionate | 24.5 ± 0.75 (6.0 – 33.1) | 8.8 ± 0.77 (1.5 – 11.6) | 19.1 ± 1.71 (9.2 – 39.8) |
| H2PO4− | 13.2 ± 0.37 (10.0 – 22.7) | 18.1 ± 0.46 (8.7 – 22.8) | 11.6 ± 0.28 (4.8 – 15.8) |
All inorganic phosphorus was assumed to be present as H2PO4−.
ND, none detected by 1H-NMR.
Means were calculated using only data from web collections 2–6 from Feeding groups and web collections 2–3 from Starving groups. This selection is somewhat arbitrary, but averages calculated from these data are probably closer to typical molar percentages in field-built webs than are means calculated using data from all web collections. Even so, some of the means presented (e.g. Cho) may still deviate substantially from average field-built webs.
Ranges consider data from all non-radioactive web collections.
Effects of starvation and web removal on orb web weight and LMM composition
The number of webs composing each web collection is given in Supplemental Table 1.
Gravimetric data
Weight per orb web
Withholding prey and removing webs resulted in an immediate drop in mean orb web weight in four of the five starving groups and the downward trend continued until feeding was resumed (Fig. 4, Supplemental Figs. 3–5). Only in the Argiope aurantia starving adult group was there a slight delay before web weight likewise declined (Supplemental Fig. 6). When feeding was resumed, web weights quickly increased, though, among adults, Argiope trifasciata were slower to recover web weight than A. aurantia or Araneus cavaticus. Among A. cavaticus, juvenile males and females were slower to recover web weight than adult females. Web weights among resumed feeding adults, but not juveniles, ultimately returned to or exceeded weights at the start of the stadium.
Fig. 4.
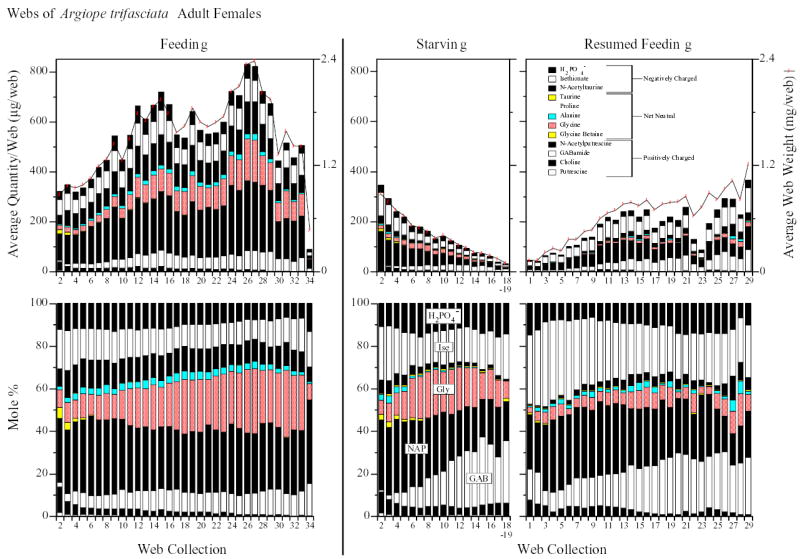
Absolute (upper panel) and relative (lower panel) quantities of LMM in water extracts of webs built by Argiope trifasciata adult females in feeding and starving/resumed feeding groups. Average desiccated web weights within web collections are indicated by line graphs in the upper panel (right y-axis). The legend (within the upper ‘resumed feeding’ panel) identifies the LMM, arranged in the same order, represented in all six bar charts. Several major LMM are also directly labeled on the lower ‘starving’ bar chart to further aid orientation. LMM with a net charge are represented by solid black or white while net neutral LMM are shown in color. Proline was not detected in any A. trifasciata web collections. GAB, GABamide; Gly, glycine; Ise, isethionate; NAP, n-acetylputrescine.
Among feeding group juvenile A. cavaticus, web weights were maintained or slightly increased in the earlier part of the stadium for both females and males. As they approached their final molt, web weights for females remained high (Supplemental Fig. 4), while those for males declined (Supplemental Fig. 3). Among feeding adult and A. aurantia PES groups, an upward trend followed by a decline in the days leading up to egg sac construction was typical (Fig. 4, Supplemental Figs. 5–7). In the A. trifasciata PES groups, an initial upward trend was less in evidence, but decreases in web weight prior to construction of the next egg sac were observed (Supplemental Fig. 7). Web weight in the three resumed feeding adult groups, however, did not tend to drop near the end of the series (Fig. 4, Supplemental Figs. 5, 6).
Web weight as a percentage of spider weight
Table 2 presents data on fresh weights of the three species of spiders and their dry weight fractions. It also presents desiccated weights of webs built by feeding group spiders and the total weights of the 12 measured LMM in these webs. From these data we estimate that these spiders typically invested about 1–3% of their dry weight into the materials composing an orb web and about 0.5–1% of their dry weight into the 12 measured LMM per web.
Table 2.
Spider and f eeding group or b web weight data.
| Spider Fresh | Spider Dry Weight | Feeding Group | Feeding Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Instar | Sex | Weight (mg) (X ± SEM) | N | Spider Fresh Weight (X ± SEM) | N | Web Weight (mg) (X ± SEM) | N | Weight of LMM (mg) (X ± SEM) | N |
| Acav | Pen | M | 1.05 ± 0.028 | 5 | 0.52 ± 0.025 | 5 | ||||
| web collections 7–10 excluded | ||||||||||
| Acav | Pen | F | 327 ± 18.5 | 22 | 0.270 ± 0.009 | 7 | 1.18 ± 0.037 | 10 | 0.56 ± 0.023 | 10 |
| Acav | Adult | F | 518 ± 62.6 | 11 | 0.268 ± 0.008 | 4 | 1.59 ± 0.041 | 15 | 0.74 ± 0.020 | 15 |
| web collections 17–19 excluded | ||||||||||
| Aau r | Adult | F | 315 ± 41.1 | 7 | 0.313 ± 0.026 | 7 | 1.89 ± 0.073 | 25 | 0.74 ± 0.030 | 25 |
| web collections 27–29 excluded | ||||||||||
| Atri | Adult | F | 265 ± 16.7 | 12 | 0.265 ± 0.011 | 6 | 1.59 ± 0.070 | 32 | 0.56 ± 0.025 | 32 |
| web collection 34 excluded | ||||||||||
Aaur = Argiope aurantia, Acav = Araneus cavaticus, Atri = Argiope trifasciata, F = female, M = male, Pen = penultimate instar, LMM = low- molecular-mass compound s (include s 11 org ani c co mpounds listed in Introduc tion p lus H2PO4−), N = number of observa tions, SEM = standa rd error of the mean.
Spider weights for penu ltimate instar male A. cavaticus were not obtained but are probably comparable to those of penu ltimate instar females. No te that some of the variation in spider weight in all three species may be attributable to variation in the number of stadia spiders pass through before arriving at the penultimate instar and, consequently, variation also in the number of stadia preceding adulthood.
The feeding group desiccated web weights and total weights for the 12 measured LMM are presented here to provide an indication of weights that are typical for these spiders, at least in the laboratory, when they are capturing prey (but not allowed to recycle their webs beyond the first post-ecdysial web). Webs with especially low weights at the end of a series have been excluded from the presented means since they are viewed as being more a typical, compromised webs resulting from intern al preparations for molting or egg laying.
Water-soluble percentages
For A. cavaticus, the mean percentage of the desiccated web weight extracted by water ± SD was 71.0% ± 4.24%, N = 86 web collections (juveniles 72.4% ± 4.06%, N = 51; adults 68.9% ± 3.57%, N = 35). For A. aurantia and A. trifasciata, as observed previously (Townley et al., 1991), water-soluble percentages were usually lower, the means ± SD being 54.6% ± 5.70% (N = 105) and 52.8% ± 7.45% (N = 123), respectively. Water-soluble fractions undoubtedly include some non-LMM components, such as nodular glycoprotein (see Introduction). Very low water-soluble percentages, far below the averages, were seen in some web collections built at or near the end of the series in the A. trifasciata feeding, resumed feeding, PES2, and PES3 groups, and in the A. aurantia PES1 group. It is likely that additional examples of very low water-soluble percentages were masked as a result of end-of-series ‘horizontal’ pooling (see “Gravimetric measurements of orb webs” in Materials and Methods and Supplemental Table 1).
Water-soluble percentages tended to decrease over the series. Slopes of linear regressions differed significantly from zero in 14 of the 21 groups, and these 14 slopes were all negative (range: −0.22 to −1.65) and spread across all species and treatments (Supplemental Table 3).
The percentage of the water-soluble weight accounted for by the 11 organic LMM in A. cavaticus was 57.8% ± 5.4% (X ± SD, N = 86). There was a tendency for this percentage to decrease over the series irrespective of treatment, but linear regression slopes departed significantly from zero in only 3 of the 9 groups of A. cavaticus (feeding juvenile females, P = 0.005; starving adult females, P = 0.030; resumed feeding adult females, P = 0.030). When the data from all 9 groups were pooled, the slope of the regression was significantly different from zero (P = 0.039), with an average decrease from one web collection to the next of 0.27%.
LMM compositional data
Molar % data (averages and ranges) for the 11 organic LMM and inorganic phosphate in the non-radioactive webs of each species are summarized in Table 3. These data were also analyzed for correlations between LMM (Table 4). Some significant correlations showed agreement among our three species and/or with Nephila clavipes (Linnaeus, 1767) (Higgins et al., 2001) and these are indicated in Table 4.
Table 4.
Pearson correlation matrices for arcsine-transformed molar percentages of the 12 LMM in all non-radioactive web collections from each species (A. cavaticus N = 86; A. aurantia N = 102; A. trifasciata N = 123)
| Put | Cho | GAB | NAP | Bet | Gly | Ala | Pro | Tau | NAT | Ise | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. cav. | 0.454 *** | |||||||||||
| Cho | A. aur. | – | ||||||||||
| A. tri. | 0.366 ** | |||||||||||
| A. cav. | −0.604*** | −0.745*** | ||||||||||
| GAB | A. aur. | – | −0.272 | |||||||||
| A. tri. | −0.470*** | −0.149 | ||||||||||
| A. cav. | 0.662*** | 0.080 | −0.497*** | |||||||||
| NAP | A. aur. | – | – | – | ||||||||
| A. tri. | 0.020 | −0.099 | −0.519*** | |||||||||
| A. cav. | 0.346 | 0.558 *** | −0.574*** | 0.298 | ||||||||
| Bet | A. aur. | – | 0.828 *** | −0.397** | – | |||||||
| A. tri. | 0.364** | 0.513 *** | −0.356** | 0.046 | ||||||||
| A. cav. | −0.615*** | −0.386* | 0.372 * | −0.677*** | −0.172 | |||||||
| Gly | A. aur. | – | −0.283 | −0.304 | – | −0.197 | ||||||
| A. tri. | 0.359** | −0.437*** | −0.303* | −0.198 | −0.247 | |||||||
| A. cav. | −0.462*** | −0.647*** | 0.426** | −0.147 | −0.408** | 0.457 *** | ||||||
| Ala | A. aur. | – | −0.480*** | −0.021 | – | −0.335* | 0.598 *** | |||||
| A. tri. | 0.096 | −0.293 | −0.544*** | 0.077 | −0.139 | 0.459 *** | ||||||
| 72 | ||||||||||||
| A. cav. | −0.489*** | −0.494*** | 0.256 | −0.421** | −0.195 | 0.834 *** | 0.546 *** | |||||
| Pro | A. aur. | – | −0.377** | −0.143 | – | −0.275 | 0.456 *** | 0.650 *** | ||||
| A. tri. | – | – | – | – | – | – | – | |||||
| A. cav. | – | – | – | – | – | – | – | – | ||||
| Tau | A. aur. | – | 0.504 *** | −0.223 | – | 0.325 * | −0.112 | −0.356* | −0.182 | |||
| A. tri. | −0.027 | 0.640 *** | 0.257 | −0.160 | 0.307 * | −0.598*** | −0.512*** | – | ||||
| A. cav. | 0.038 | −0.413** | −0.061 | 0.552*** | −0.006 | −0.068 | 0.498*** | 0.209 | – | |||
| NAT | A. aur. | – | −0.339* | −0.412*** | – | −0.150 | 0.006 | 0.280 | 0.143 | −0.129 | ||
| A. tri. | 0.312* | −0.459*** | −0.508*** | 0.113 | −0.094 | 0.615*** | 0.754*** | – | −0.722*** | |||
| A. cav. | 0.695*** | 0.534*** | −0.637*** | 0.715*** | 0.395** | −0.871*** | −0.588*** | −0.735*** | – | 0.025 | ||
| Ise | A. aur. | – | 0.258 | −0.329* | – | 0.246 | −0.554*** | −0.535*** | −0.395** | 0.277 | 0.253 | |
| A. tri. | −0.454*** | 0.396*** | 0.203 | 0.130 | 0.143 | −0.838*** | −0.369** | – | 0.664*** | −0.730*** | ||
| A. cav. | −0.376* | −0.025 | 0.247 | −0.539*** | −0.099 | 0.360* | −0.030 | 0.171 | – | −0.521*** | −0.469*** | |
| PO4− | A. aur. | – | 0.342* | −0.082 | – | 0.244 | −0.466*** | −0.600*** | −0.323* | 0.172 | −0.451*** | 0.198 |
| A. tri. | 0.179 | 0.424*** | 0.259 | −0.326* | 0.404*** | −0.296* | −0.418*** | – | 0.335** | −0.190 | −0.065 |
Missing correlation coefficients indicate LMM not detected in webs of that species. Underlined coefficients indicate significant correlations that agree among the three species (i.e., all three negative or all three positive), or between two species if either of the LMM being compared went undetected in the third species. Coefficients in bold indicate significant correlations that agree with significant correlations seen in webs of N. clavipes (Higgins et al., 2001). In that study, NAP and Ise were not detected in web washes, H2PO4− was not assayed, and Pro and Tau, while detected in some web washes, were not included in statistical analyses.
A. cav. = Araneus cavaticus; A. aur. = Argiope aurantia; A. tri. = Argiope trifasciata.
Bonferroni probabilities: P • 0.05;
P < 0.01;
P • 0.001.
Changes in molar percentages of 8 of the organic LMM with starvation and subsequent resumed feeding are shown in Figs. 1–3. Fig. 1 contains the readily synthesized LMM, Ala, Gly, and GAB, Fig. 2 the three most abundant moderately synthesized LMM, NAP, NAT, and Ise, and Fig. 3 the poorly synthesized LMM, Cho and Bet (see ‘Synthesis of organic LMM by Argiope’ above). These figures also present the corresponding data from the feeding groups.
Fig. 1.
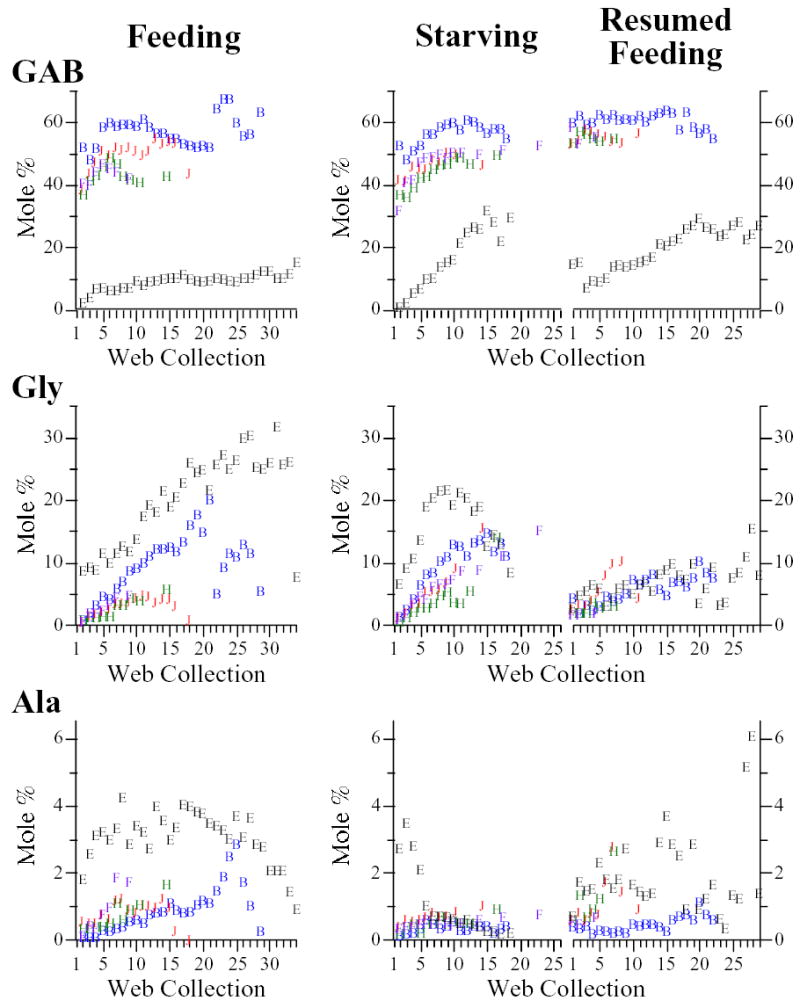
Molar percentages of GABamide (GAB), free glycine (Gly), and free alanine (Ala) in water extracts of webs built by feeding and starving/resumed feeding groups. In this and the next two figures, a data point representing horizontally pooled web collections, e.g., web collections 14–19, was assigned an x-coordinate midway in this range, i.e., web collection 16.5 (see Materials and methods for explanation of ‘horizontal pooling’ and Supplemental Table 1). Note the very different molar % ranges for different compounds. Purple diamonds: juvenile male Araneus cavaticus; green triangles: juvenile female A. cavaticus; red circles: adult female A. cavaticus; blue squares: adult female Argiope aurantia; open circles: adult female Argiope trifasciata.
Fig. 3.
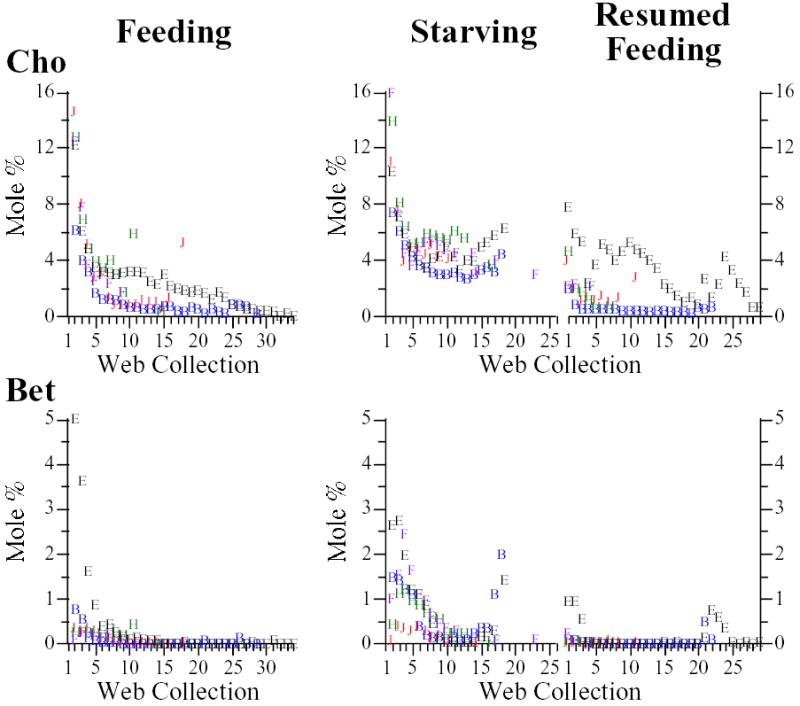
Molar percentages of choline (Cho) and glycine betaine (Bet) in water extracts of webs built by feeding and starving/resumed feeding groups. Purple diamonds: juvenile male Araneus cavaticus; green triangles: juvenile female A. cavaticus; red circles: adult female A. cavaticus; blue squares: adult female Argiope aurantia; open circles: adult female Argiope trifasciata.
Fig. 2.
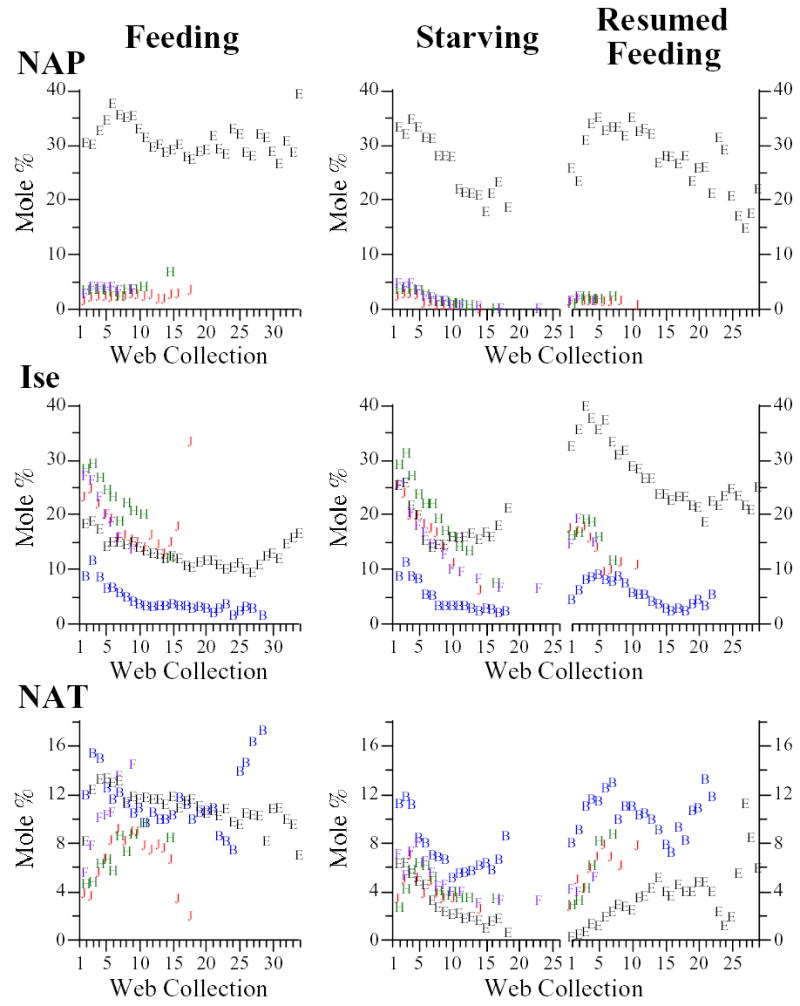
Molar percentages of n-acetylputrescine (NAP), isethionate (Ise), and n-acetyltaurine (NAT) in water extracts of webs built by feeding and starving/resumed feeding groups. Purple diamonds: juvenile male Araneus cavaticus; green triangles: juvenile female A. cavaticus; red circles: adult female A. cavaticus; blue squares: adult female Argiope aurantia; open circles: adult female Argiope trifasciata.
Bar charts showing both the absolute and relative quantities of all 12 measured LMM in web collections from the A. trifasciata starving, resumed feeding, and feeding groups are presented in Fig. 4. Supplemental Figs. 3–6 show the corresponding bar charts for A. cavaticus and A. aurantia (legend in Supplemental Fig. 2). Absolute and relative quantity data for the 12 LMM in each web collection from all groups are also available in tabular form in Supplemental Tables 4–6.
In all tables and figures except Table 1, molar percentages were calculated based on the 12 LMM composing 100% of the LMM. (In Table 1, molar % was calculated based on the 11 organic LMM composing 100% of the LMM). In all three species, however, several as yet unidentified and generally minor organic LMM were detected in many of the spectra. There are undoubtedly other inorganic LMM components as well, such as NO3−, K+, Na+, and Ca2+ reported from webs of other araneoid species (Schildknecht et al., 1972; Work, 1981; Tillinghast and Christenson, 1984; Patel and Nigam, 1996).
Organic LMM
We predicted that molar percentages of Ala, Gly, and GAB would increase with starvation and then drop when feeding was resumed. Conforming to expectations, percentages of Gly and GAB increased with starvation in all three species, at least in the earlier part of the series (Fig. 1). It is especially noteworthy that not only molar percentages, but also absolute quantities of Gly and GAB increased in the webs of starving spiders; Gly in all three species and GAB in Argiope trifasciata, again, at least in the first portion of the series (Fig. 4, Supplemental Figs. 3–6, Supplemental Tables 4–6). With resumed feeding, molar percentages of Gly immediately dropped in all three species, as did GAB in A. trifasciata, though, unexpectedly, they again tended to increase as the resumed feeding series progressed. Other trends were also not expected: free Ala remained a minor component in starving Araneus cavaticus and Argiope aurantia, increasing only slightly (indeed, less than in feeding groups), and in A. trifasciata Ala clearly declined with starvation (Fig. 1). Also, GAB did not decrease with resumed feeding in A. cavaticus and A. aurantia. Even more unanticipated, increases in Gly and GAB were also seen in the feeding groups, where more stable compositions had been expected. These increases are reflected in positive correlations in feeding groups between web collection number and the summed molar percentages of the three readily synthesized LMM, very similar to correlations seen in the starving groups (Table 5).
Table 5.
Pearson correlation matrices describing relationships among web collection number and arcsine-transformed summed molar percentages of readily synthesized (RS: GABamide, glycine, alanine), moderately synthesized (MS: n-acetylputrescine, n-acetyltaurine, isethionate, putrescine, taurine), and poorly synthesized (PS: choline, glycine betaine) LMM in the starving and feeding groups of each species
| Web number | RS | MS | ||
|---|---|---|---|---|
| A. cavaticus starving | 0.883*** | |||
| A. aurantia starving | 0.773** | |||
| RS | A. trifasciata starving | 0.815*** | ||
| A. cavaticus feeding | 0.606** | |||
| A. aurantia feeding | 0.727*** | |||
| A. trifasciata feeding | 0.808*** | |||
| A. cavaticus starving | −0.926*** | −0.947*** | ||
| A. aurantia starving | −0.821*** | −0.987*** | ||
| MS | A. trifasciata starving | −0.888*** | −0.982*** | |
| A. cavaticus feeding | −0.533* | −0.841*** | ||
| A. aurantia feeding | −0.498 | −0.880*** | ||
| A. trifasciata feeding | −0.609** | −0.898*** | ||
| A. cavaticus starving | −0.702*** | −0.873*** | 0.748*** | |
| A. aurantia starving | −0.476 | −0.888*** | 0.848*** | |
| PS | A. trifasciata starving | −0.444 | −0.846*** | 0.752** |
| A. cavaticus feeding | −0.688*** | −0.874*** | 0.645*** | |
| A. aurantia feeding | −0.700*** | −0.876*** | 0.789*** | |
| A. trifasciata feeding | −0.906*** | −0.781*** | 0.466 |
Molar % data from adult female, juvenile female, and juvenile male starving and feeding groups used to calculate A. cavaticus correlation coefficients. Coefficients for both Argiope species calculated using molar % data from adult female starving and feeding groups.
Bonferroni probabilities: P < 0.05;
P < 0.01;
P < 0.001.
We suspected that the moderately synthesized LMM would decrease or at most be maintained in starving spiders. The low specific activities of Ise and NAT (Table 1) made it especially likely that they would decline. With starvation we observed trends toward decreased molar percentages of not only Ise and NAT, but NAP as well (Fig. 2). In starving A. cavaticus females, however, decreases in NAT were preceded by increases such that percentages of NAT at the end of the series were similar to those at the beginning. Starving spiders responded to resumed feeding with increased molar percentages of NAP, NAT, and Ise, though subsequent trends varied as the resumed feeding series progressed and include examples of declines in molar percentages after the initial rise (e.g., Ise in all 3 species) as well as more prolonged upward trends (e.g., NAT in A. trifasciata and A. cavaticus). Interestingly, in contrast to the rapid and substantial recovery of Ise in resumed feeding A. trifasciata, resulting in high molar percentages of Ise relative to feeding group A. trifasciata, NAT’s recovery was slow and modest, resulting in low percentages of NAT relative to the feeding group (Figs. 2, 4).
In agreement with expectations, molar percentages of NAP were essentially maintained by feeding spiders and, while not necessarily maintained at a constant level, percentages of NAT in webs of feeding spiders were generally higher than in the corresponding webs of starving spiders. Ise, however, exhibited molar % declines in webs of feeding spiders that were sometimes very similar to those of starving spiders. These contributed to negative correlations between web collection number and summed molar percentages of the moderately synthesized LMM in feeding groups, though these correlations were less significant than those of the starving groups (Table 5). Negative correlations between total readily synthesized and moderately synthesized LMM, and positive correlations between moderately synthesized and poorly synthesized LMM, in both starving and feeding groups, indicate that the moderately synthesized LMM generally exhibit shifts in relative abundance more like poorly synthesized, rather than readily synthesized, LMM (Table 5). Several correlations between NAT and other LMM (Table 4), however, demonstrate that this is not invariably the case (though Table 4, unlike Table 5, considers data from resumed feeding and PES groups as well as from starving and feeding groups).
Cho and Bet were expected to decline with starvation and, indeed, declines were observed in all three species (Fig. 3). But as with GAB, Gly, and Ise, similar trends were also unexpectedly exhibited by the feeding groups (Table 5). Moreover, in the starving groups, after a steep initial decline, Cho tended to level off (or partially rebound) at molar percentages that exceeded the percentages of Cho in the corresponding feeding group webs. In some instances, even μg Cho/web was greater in starving groups than in the corresponding feeding group webs (Supplemental Figs. 3–6, Supplemental Tables 4, 5). This contrasted with what we generally observed: greater absolute quantities of LMM per web (often considerably so) in feeding groups than in starving groups due to the usually greater total web weights (Fig. 4, Supplemental Figs. 3–6) and weights of water-soluble fractions in feeding groups. In both Argiope species, negative correlations between web collection number and Cho + Bet were not significant in starving groups (Table 5) primarily due to unexpected end-of-series increases in Cho and Bet. In starving A. cavaticus groups (juveniles especially), Bet molar % decreases occurred only after initial unanticipated increases (Fig. 3). Comparable increases were not seen in feeding A. cavaticus. Also contrary to expectations, resumed feeding of starving spiders yielded little or no resurgence in Cho and Bet.
Of the remaining 3 organic LMM measured in this study, Put and Tau were invariably minor components (< 2 mole %) and free Pro’s contribution exceeded 2 mole % only in some A. cavaticus webs (Table 3). Each went undetected in all webs from one of the 3 study species, though it may be that small quantities were not detected because of overlapping peaks from other compounds, including minor unidentified compounds. It now appears that the compound identified as Tau in earlier analyses of A. cavaticus webs (Townley et al., 1991) was not Tau but a minor as yet unidentified compound. Shifts in Pro were reminiscent of Gly with upward trends in both starving and feeding A. cavaticus, and a drop when feeding was resumed by starving spiders (Supplemental Figs. 3–5). Absolute quantities of Pro per web were generally maintained or increased in starving A. cavaticus (Supplemental Table 4).
Post-egg-sac (PES) webs
The construction of up to 3 egg sacs by some Argiope aurantia and Argiope trifasciata and the building of orb webs after each of these gave us the opportunity to examine changes in LMM composition in webs built between egg laying episodes by feeding spiders (though, as throughout this study, determining LMM composition meant that spiders were not allowed to recycle webs). Cyclical changes in LMM molar percentages were evident when the data from successive PES groups were examined (Fig. 5; absolute quantities of LMM shown in Supplemental Fig. 7). To mention just two examples, in both species Gly was relatively low following egg sac construction, but increased subsequently and then declined with the approach of the next oviposition. Cho, on the other hand, was highest in the first webs built after an egg sac was made and tended to decline as the series progressed.
Fig. 5.
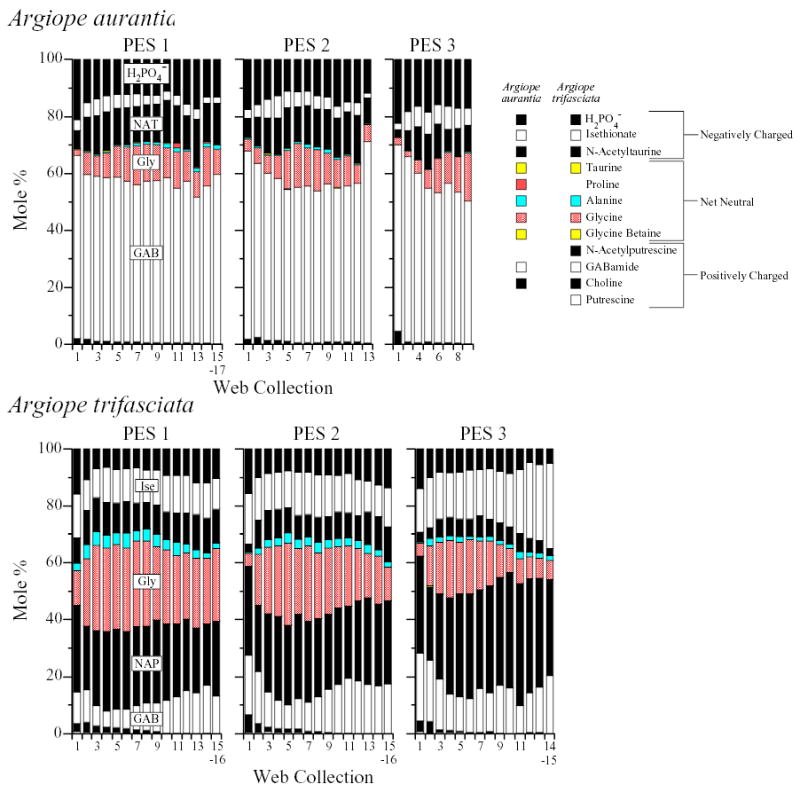
Changes in LMM composition in webs built after construction of first (PES 1), second (PES 2), and third (PES 3) egg sacs by Argiope aurantia (upper panel) and Argiope trifasciata (lower panel). The PES 3 series from A. aurantia had to be ended prematurely (see Materials and methods). The legend at top right identifies the LMM, arranged in the same order, represented in the bar charts. Note that some LMM were either not detected in individual web collections or too minor to be visible in these plots. Glycine betaine and taurine, though not clearly visible in the bar charts, have been retained in the legend as a reminder that these LMM were detected in some of these web collections and for consistency with Fig. 4 and Supplemental Figs. 2–7. GAB, GABamide; Gly, glycine; Ise, isethionate; NAP, n-acetylputrescine; NAT, N-acetyltaurine; PES, post-egg-sac group.
H2PO4− and charge balance
In the bar charts in Figs. 4, 5 and Supplementary Figs. 3–7, LMM carrying a net positive charge at the pH of the sticky droplets [about 4 in Araneus diadematus Clerck 1757 (Schildknecht et al., 1972); 7.9 in Argiope anasuja Thorell 1887 (Patel and Nigam, 1996)] are grouped together, as are net negative and net neutral LMM.
When the molar percentages of the positively-charged organic LMM — Put, Cho, GAB, and NAP — were added (doubling Put because of its +2 charge) and compared with the sums of the negative organic LMM — NAT and Ise — there was an ‘excess’ of positive charge in all 311 web collections analyzed during this study. This excess was greatest in Argiope aurantia (43.6 mole % ± 0.70 mole %, X ± SEM; N = 102) and least in Argiope trifasciata (21.5 mole % ± 0.65 mole %; N = 123), with Araneus cavaticus intermediate (31.0 mole % ± 0.86 mole %; N = 86). Analyzing the data from each group separately revealed a highly significant (P • 0.001) increase over the series in the excess positive charge in all 5 starving groups and these increases were greater than and significantly different (P • 0.05) from increases seen in any other groups. In A. cavaticus, there was no significant difference between the starving adult group and either of the starving juvenile groups. Much of the increase in excess positive charge in the starving groups was due to a drop in negatively-charged organic LMM (NAT + Ise) over the series rather than to an increase in positively-charged organic LMM. In the 3 starving A. cavaticus groups, > 91% of the increased excess positive charge was due to a decrease in NAT + Ise. For the A. aurantia and A. trifasciata starving groups, this percentage was 74% and 48%, respectively.
All web extracts were assayed for inorganic phosphorus to estimate inorganic phosphate’s contribution as a counter-ion to the excess positive charge. In only 9 of the 311 web collections did the molar % of H2PO4− essentially balance or even slightly exceed the molar % of excess positive charge (6 of the 9 were in the A. trifasciata resumed feeding series and were due primarily to the relatively rapid recovery of Ise near the start of the series decreasing the excess positive charge). In the remaining 302 web collections the molar % of H2PO4− was insufficient to balance the molar % of excess positive charge. On average, the percentage of the excess positive charge balanced by inorganic phosphate was least in the species with the greatest excess positive charge, A. aurantia (36.3%), and about equal in the other two species (A. trifasciata 47.7%, A. cavaticus 48.8%). As noted above, the 12 LMM quantitated in this study do not provide a complete inventory of the LMM, especially of inorganic ions. And a more complete accounting of charges in this system should also consider components other than LMM, such as the nodular glycoprotein (see Introduction).
There was a positive correlation between the molar percentages of H2PO4− and excess positive charge when the data from all three species were pooled (r = 0.651, P < 0.001, N = 311), but only A. cavaticus (r = 0.546, P < 0.001, N = 86) and A. trifasciata (r = 0.386, P < 0.001, N = 123) showed significant correlations when each species was analyzed separately. In A. cavaticus, this correlation was attributable to a negative correlation between H2PO4− and negatively-charged organic LMM (NAT + Ise) (r = −0.643, P < 0.001), as opposed to the situation in A. trifasciata in which there were significant correlations between H2PO4− and both negatively-charged (r = −0.235, P = 0.009) and positively-charged (r = 0.274, P = 0.002) organic LMM. The inverse relationship between H2PO4− and the two sulfonic acids was mostly clearly exhibited in juvenile A. cavaticus (males and females) and contributed to significant inverse correlations between H2PO4− and both NAT and Ise in A. cavaticus (Table 4).
Discussion
Synthesis of sticky droplet organic LMM
Radiolabeled glucose and methionine/cysteine were fed to spiders to assess their ability to synthesize sticky droplet LMM. Label from [14C]glucose appeared in high specific radioactivity in web droplet Ala, Gly, and GAB, while only a meager amount was associated with Cho (Table 1), indicating that Cho is nutritionally essential. Ise, Tau (free and as a component of NAT) and the Put moiety of NAP occupied positions between these extremes, with the relatively low specific activities of the two sulfonic acids in particular raising the possibility that, without adequate dietary intake of these LMM (or more immediate precursors), the spider’s synthetic rate may not always be sufficient to meet the requirements for optimal web construction. These results are essentially in agreement with the literature, as detailed in the following sections.
GABamide, glycine, and alanine (Readily synthesized LMM)
Relatively high specific activities for Ala, Gly, and GAB are in keeping with previous studies on Araneus cavaticus fed [14C]glucose (Townley and Tillinghast, 1988) and Nephila clavipes fed [14C]glucose or [14C]acetate (Higgins and Rankin, 1999). That Gly and Ala were readily synthesized is not surprising as these amino acids are generally dispensable in arthropods (Dall and Moriarty, 1983; Dadd, 1985; Guillaume, 1997). Nor was it surprising that in each of the three 14C-labeled Argiope trifasciata web collections the specific activity of Ala was higher than that of Gly. Such a disparity in animals administered [U-14C]glucose has often been observed, including in other arachnids (Rodriguez and Hampton, 1966; Rodriguez and Lasheen, 1971), insects (see references in Kasting and McGinnis, 1966:100; also Rock and Hodgson, 1971; Widmer, 1973), and crustaceans (e.g., Shewbart et al., 1972; van Marrewijk and Zandee, 1975; Lasser and Allen, 1976; Miyajima et al., 1976).
In an earlier study with N. clavipes webs (Higgins et al., 2001), molar percentages of free Gly and Ala tended to be positively correlated. Likewise, in this study, Gly and Ala were positively correlated in the three species and, in A. cavaticus and Argiope aurantia, Pro was positively correlated with both Gly and Ala (Table 4) (Pro was not detected in webs of A. trifasciata).
n-Acetyltaurine, isethionate, and n-acetylputrescine (Moderately synthesized LMM)
Earlier studies examined the incorporation of radioisotope into LMM from webs built by Argiope fed [14C]Tau or [35S]cysteine (Anderson and Tillinghast, 1980; Tillinghast, 1984). However, as those studies preceded the identifications of NAT and Ise (Vollrath et al., 1990), only tentative conclusions may be drawn from them. Nevertheless, it appeared that, after feeding either radioactive precursor, labeled NAT (‘compound I’ of Anderson and Tillinghast (1980)), Ise (consistent with ‘compound II’), and Tau were present in webs built by A. trifasciata, while in A. aurantia only NAT was unambiguously labeled. This difference may have been due to different relative quantities of sulfonates on the webs: A. aurantia webs typically contain lower molar percentages of Ise and higher NAT than A. trifasciata webs (Vollrath et al., 1990; Townley et al., 1991) (Table 3). In webs of N. clavipes, Ise has not been detected by NMR (Higgins et al., 2001), but radiolabeling of NAT by spiders fed [14C]glucose or [14C]acetate has been observed, albeit inconsistently (Higgins and Rankin, 1999). Results obtained to date from Argiope fed [35S]cysteine (Anderson and Tillinghast, 1980) (Table 1) indicate that these spiders can, like many other animals (Jacobsen and Smith, 1968; Allen and Garrett, 1971; Chen, 1985:195–196; Huxtable, 1986), synthesize Tau from cysteine, though we have no information on which pathway(s ) is(are) used in this conversion.
The incorporation of radioisotope into Ise by Argiope fed [14C]Tau, as indicated in Anderson and Tillinghast (1980), needs verification, but if true would suggest that Ise is formed from Tau. Though this conversion apparently occurs in some organisms (Braun and Fromageot, 1962), it has not been established that Ise synthesis from Tau occurs in animal tissues (Huxtable, 1986:167–170; Fellman, 1987). More recent studies with human neutrophils have again raised the possibility of a Tau to Ise conversion in animals (Cunningham et al., 1998; Cunningham and Tipton, 2000), but the evidence is inconclusive.
Given the comparable specific activities obtained for Ise and the Tau moiety of NAT in the present study (Table 1), one possible alternative pathway to Ise (Cavallini et al., 1978; Scandurra et al., 1978) warrants mention. In this pathway, cystamine would give rise to equal amounts of cysteamine and 2-mercaptoethanol, from which the former can be converted to Tau (Cavallini et al., 1976; Huxtable and Bressler, 1976; Read et al., 1976) and the latter to Ise (Federici et al., 1976; Dupré et al., 1978). Here, Tau is not a precursor, but a co-product of the pathway to Ise.
NMR analyses have not revealed NAP in webs of N. clavipes, but Put is present in quantity (Higgins et al., 2001), as it is in some other araneoids (e.g., Metepeira incrassata F. O. P.-Cambridge, 1903 (Higgins et al., 2001), Micrathena gracilis (Walckenaer, 1805) (M. A. Townley and E. K. Tillinghast, unpublished)). Put was radiolabeled in some webs built by N. clavipes fed [14C]glucose or [14C]acetate, but not as consistently as GAB, Gly, and Ala (Higgins and Rankin, 1999). Likewise, in this study the specific activity of the Put component of NAP was significantly lower than the specific activities of GAB, Gly, and Ala (Table 1).
Choline and glycine betaine (Poorly synthesized LMM)
In earlier studies, radioisotope was not observed in Cho from webs built by A. aurantia fed [14C]glucose (Tillinghast and Townley, 1994) or N. clavipes fed [14C]glucose or [14C]acetate (Higgins and Rankin, 1999). Insects, likewise, are either unable to synthesize Cho or synthesize insufficient Cho (via methylation of phosphatidylethanolamine), making it a nutritional essential (Dadd, 1985). Limited synthesis of Cho from phosphatidylethanolamine also occurs in at least some crustaceans (Bilinski, 1962; Shieh, 1969; Brichon et al., 1980; D’Abramo and Baum, 1981), but again most evidence suggests that crustaceans have a dietary requirement for Cho (D’Abramo and Baum 1981; Catacutan and de la Cruz, 1989; Kanazawa et al., unpublished, cited in Boonyaratpalin, 1996:21; Shiau 1998; Shiau and Lo, 2001). Indeed, Cho’s essential status may apply to arthropods generally (Morris, 1991).
In the present study, the specific radioactivity of Bet was not determined because of suspected contamination, but our observations indicate that labeling of this compound was slight at most. Higgins and Rankin (1999) reported that Bet from webs of N. clavipes fed [14C]glucose or [14C]acetate was not radiolabeled and that this compound is likely essential. A significant positive correlation between molar percentages of Bet and Cho was reported in one population of N. clavipes (Higgins et al., 2001) and was likewise observed in all three species used in this study (Table 4). Such observations are consistent with Bet synthesis from Cho via betaine aldehyde, to our knowledge the only established pathway to Bet in both chelicerate (Dragolovich and Pierce, 1994) and mandibulate (Bilinski, 1960; Weiher and Komnick, 1997) arthropods.
Is there agreement between synthetic capacity and LMM compositional changes with starving?
Assuming an organic LMM’s specific radioactivity is a reliable indicator of the spider’s ability to synthesize that LMM, we anticipated that, with fasting, there would be decreased molar percentages of those LMM showing lower incorporation of radioisotope. Was this expectation met? In some respects, yes. Downward trends in starving spiders were most evident among ‘poorly synthesized’ (Cho, Bet) and ‘moderately synthesized’ (Ise, NAT, NAP, Put) LMM, with the exception of the decline in Ala in A. trifasciata. Extended upward trends were seen only among ‘readily synthesized’ LMM (GAB, Gly, and, to a far lesser extent, Ala in A. cavaticus) and Pro (presumed at least a moderately synthesized LMM). Even more than increases in molar %, increases in μg/web of Gly, GAB, and Pro, as seen over at least part of a starving series in one or more species, suggest that starving spiders use these LMM to compensate to some extent for decreases in less readily synthesized and less available LMM.
Argiope trifasciata provided a particularly striking example of a compositional shift accompanying starvation that may have resulted from differences in synthetic capacity; specifically, the decline in molar % of NAP and coincident increase in GAB. In webs of this species, the mean specific radioactivity of GAB was almost four times higher than that of the Put moiety of NAP (Table 1). Unlike webs of A. cavaticus and A. aurantia, webs of A. trifasciata contain NAP as a major constituent whereas GAB is generally less abundant (Townley et al., 1991) and sometimes even a minor component, as at the start of the feeding and starving series (Figs. 1, 4). This suggests that these two similar compounds may fulfill the same, as yet unknown, function in the sticky droplets and that when A. trifasciata are starved and their webs removed the more readily synthesized GAB is increasingly recruited to stand in for NAP. An inverse correlation between molar percentages of NAP and GAB in A. trifasciata (and A. cavaticus) is consistent with this interpretation (Table 4).
There were, on the other hand, a number of trends in the webs of starving spiders that did not conform to expectations based on specific radioactivities. Ala’s unexpected decline in starving A. trifasciata has already been mentioned, but even the slight molar % increases in Ala in A. cavaticus and A. aurantia were scarcely commensurate with its specific radioactivity. Perhaps Ala is more in demand in starving spiders than Gly (e.g., as a substrate for gluconeogenesis (Felig, 1973)) and, thus, is less available for use in the web. We also did not anticipate the initial rise in Bet or NAT at the start of the series in starving A. cavaticus, when weight of the water-soluble fraction was already on the decline, nor the resurgence in Bet at the end of the series in both Argiope species. Also unexpected, Ise partially rebounded at the end of the starving A. trifasciata series, but the other major sulfonate, NAT, did not. Conversely, NAT partially rebounded at the end of the starving A. aurantia series, but Ise did not (Fig. 2). Neither rebounded in A. cavaticus. Probably not coincidentally, Ise is generally more abundant than NAT in webs of A. trifasciata while the opposite is true of A. aurantia webs (Vollrath et al., 1990; Townley et al., 1991). Given Cho’s very low specific radioactivity, we were especially surprised that, after an initial decline, its molar % tended to level out higher than in the corresponding webs of the feeding groups. If the spiders’ ability to synthesize Cho is so poor, where was it coming from in the starving spiders? One possibility is the store of Cho residing in membrane phospholipids, mobilized as tissue reserves were broken down to meet the energy and material needs of vital tissues.
The most unexpected results, however, came from webs of the feeding controls. LMM composition was not as stable in these webs as we had anticipated. As detailed above, some compositional shifts in feeding group webs, especially of Gly, GAB, Pro, Cho, Bet, Ise, and Put, were similar to trends observed in starving groups. Thus, some changes in composition with starvation are likely attributable, at least in part, to factors other than starvation shared by feeding group spiders. One such factor that almost certainly contributed to similar trends was the absence of web recycling, discussed in the following section. Another possibility, however, is that starving and feeding spiders were both responding to limited resources, but arising for different reasons; an absence of prey in starving groups versus allocation of resources to other activities, or the allocation of more resources to web building, in feeding groups. Examples of other activities include molting in juveniles and egg laying in adult females. These different possibilities are not mutually exclusive and the relative importance of each may differ among the different LMM.
For the present we are proceeding from the assumption that shifts in LMM composition seen in starving groups were shifts away from what would generally be a more effective composition for securing prey. This assumption may not be correct. At present we know almost nothing about how LMM compositional differences affect sticky spiral functioning.
Possible effects of web recycling on LMM composition
Spiders in the field often have the opportunity to recycle at least a portion of their old orb web by ingestion before they construct a new one (Hingston, 1922; Peakall, 1971; Carico, 1986 and references therein; Craig, 1989). In this study, however, spiders were only allowed to recycle their first post-ecdysial web; all other webs were collected for analysis. Web recycling or its lack clearly influences some web parameters (Breed et al., 1964) and this influence appears to extend to LMM composition. It seems likely that similar trends seen between feeding and starving groups were at least partly the result of these spiders being deprived of web material, particularly LMM, that they normally would have been able to recoup. It is especially likely that some differences seen between earlier and later web collections within a series were related to web recycling having occurred just before the start of the series, but not subsequently.
For example, we have evidence that the relatively steep decline in Cho early in the series in feeding and starving groups was the result of webs being recycled before, but not after, the start of the series. This evidence comes from an experiment in which we fed 4 male and 4 female penultimate instar A. cavaticus a solution containing 6.54 x 106 cpm [1,2-14C]Cho chloride (NEN, Boston, MA, USA) and 43.9 μg Cho. These spiders had all built 2 post-ecdysial webs, both of which we had removed, and had not been fed since prior to ecdysis. After radioisotope feeding, spiders received 1 fly (P. sericata or P. regina) after each of the first 4 webs built. We found that 76.6% ± 2.42% (X ± SEM, N = 8) of the 14C ingested was present in the water-soluble fraction of the first 2 webs built; 81.5% ± 2.59% was present in the first 5 webs built. No significant difference was found between males and females comparing data from the first 2 webs (P = 0.646) or webs 3–5 (P = 0.265; unpaired t-test). Using the same protocol, we also attempted to feed 4 males and 1 female the same amount of [14C]Cho to which an additional 224 μg unlabeled Cho was added, but only 1 male drank the solution quickly and without incident. The other spiders did eventually drink comparable, but imprecisely known, volumes. Nevertheless, with the 1 cooperative male, 75.0% of the ingested 14C was present in the first 2 webs built; 78.3% was in the first 6 webs built. The results from the other 4 spiders, while only approximate, indicate that this result is representative.
Thus, it appears that a large percentage of ingested free Cho, such as the spider receives when it recycles an old web, is incorporated into future webs, with the bulk of this going into the first web. This high percentage of incorporation can occur even when relatively large quantities of free Cho (268 μg) are consumed, resulting in webs with unusually high molar percentages of Cho. For example, we analyzed the first labeled web built by the 1 female fed [14C]Cho spiked with unlabeled Cho. Based on the 11 organic LMM constituting 100% of the LMM, Cho accounted for 60.6 mole % of the LMM in this web (!), much higher than we have ever seen in webs built by spiders fed only insects. By the second web, which contained only 13.8% as much 14C as the first web, Cho had dropped to a more typical 11.8 mole %.
Considering the large contribution made by LMM to total web weight (see ‘Gravimetric data’ in Results) and the spider’s limited capacity for synthesizing some of the LMM, the principal selective advantage in web recycling behavior may come from retrieval of LMM rather than silk protein residues (T. A. Blackledge, personal communication). As further suggested to us by Blackledge, this possibility is supported by observations indicating that those orb weavers that build webs lacking sticky droplets tend not to recycle their webs (e.g., the araneids Cyrtophora and Mecynogea (Lubin, 1986; Carico, 1986), and some, though apparently not all, uloborids (Eberhard 1971:341; Opell, 1982; Lubin, 1986; Watanabe, 2001:586)). However, the benefits from consuming water, very small insects (Nentwig, 1985:592), or pollen (Smith and Mommsen, 1984) when recycling webs cannot be discounted.
Possible effects of the molt/intermolt and egg laying cycles on LMM composition and web weight
We anticipated that the feeding group spiders might be less than ideal controls since they would be more apt to molt and lay eggs than starving group spiders. Indeed, most starving group juveniles did not molt and no starving group adults oviposited until feeding was resumed whereas all feeding group juveniles molted and some feeding group adults, though unmated, oviposited (Supplemental Fig. 1). If these factors affect the allocation of LMM to the web, then differences seen between feeding and starving groups may not be attributable solely to changes resulting from starvation. For example, as noted earlier, the molar % of Cho tended to be higher in starving groups than in feeding groups as series progressed and in some of these later web collections even μg Cho/web was higher in the starving groups. Was this difference due entirely to starvation, with free Cho liberated from membrane phospholipids as starving spiders tapped tissue reserves, or was it to some extent due to Cho in feeding spiders being diverted into reproduction or growth/molting?
Support for the second of these possibilities was provided by webs of PES group Argiope and juvenile Araneus cavaticus. In both Argiope species, Cho levels were highest in the first or second webs built after an egg sac was constructed. They dropped again with the approach of the next egg sac’s construction (Fig. 5), suggesting that available Cho was being diverted away from foraging and into reproduction. In juvenile male and female A. cavaticus, μg Cho/web were lower in the last web collections from feeding spiders than in the corresponding webs from starving spiders (Supplemental Table 4), suggesting that Cho was being diverted or held in reserve due to the impending molt.
The influence of reproduction and molting on LMM composition apparently extends beyond the above example with Cho. Cyclical changes in quantities of other LMM, synchronized to the egg laying cycle, are also evident in the data from the PES groups (Fig. 5). Certain end-of-series departures from earlier trends seen in feeding adults, but not feeding juveniles, such as declines in Gly, Ala, and Pro (Fig. 1), may also reflect the influence of egg laying. And in the latter half of the series from the A. cavaticus feeding juvenile male group (Supplemental Fig. 3), the drop in μg/web of several LMM indicates that the re-allocation of resources away from foraging applies to LMM in addition to Cho.
Growth/molting and reproduction may have contributed not only to differences between the webs of feeding and starving groups, but also to similarities. The reallocation of LMM due to growth/molting or reproduction in feeding groups may have produced shortages for web construction that in some ways resembled the effects of starvation. Perhaps some of the same changes in LMM composition made necessary by starvation are also favored in some circumstances by feeding spiders endeavoring to lay eggs or ecdyse. Re-allocation of resources may be most evident in spiders subsisting on a diet that is quantitatively or qualitatively sub-optimal, a topic we consider below.
The molt/intermolt and egg laying cycles also bring about changes in other web parameters, including, as seen in previous studies, web size, and as seen in this study, web weight. In a study with juvenile Nephila clavipes (where webs were not removed), orb web size typically increased in the earlier part of an intermolt and then decreased with the approach of the next ecdysis (Higgins, 1990). Reed et al. (1969) have likewise noted that A. aurantia build smaller webs around the time of molting. In our feeding juvenile A. cavaticus (where webs were removed), web weights did not change substantially in the earlier part of the intermolt, but a clear decline in web weight with the approach of ecdysis was seen in males, though not females (Supplemental Figs. 3, 4). Presumably, this difference between the sexes reflects a major difference following the final molt; males are unable to build orb webs (Sekiguchi 1955) and thus much less likely to feed as adults than females. An initial increase in web weight in feeding adults, especially Argiope (Fig. 4, Supplemental Fig. 6), following the final molt may also reflect the influence of the molt/intermolt cycle. In N. clavipes (Higgins, 1990) and Larinioides cornutus (Clerck, 1757) (Sherman, 1994), web size declined with the approach of egg laying. In this study, measurements of web weight indicated the same trend, seen most convincingly in the two Argiope species (the two species that produced egg sacs in the laboratory). Following oviposition, web size has been observed to increase in L. cornutus (Sherman, 1994) and we likewise observed web weights rebound in the two Argiope species after an egg sac was made (Supplemental Fig. 7).
The changes in web parameters apparently associated with the molt/intermolt and egg laying cycles may be considered in terms of resource allocation, with relative investments in foraging (the web primarily), growth, and reproduction changing over time. But with molting and ovipositing, the influence of more tangible, anatomical changes should be considered as well. Several types of silk glands are remodeled during a molt, including those that produce the sticky spiral, the aggregate and flagelliform glands. At the height of remodeling these silk glands are nonfunctional (Townley et al., 1993:37) and orb web construction ceases for up to several days before ecdysis (e.g., Witt, 1971; Higgins, 1990). But beyond this clear-cut effect on web building, it is possible that the last webs built before ecdysis and/or the first webs built after are the products of silk glands that are in the earliest or final phase of remodeling, respectively. If so, changes taking place in these silk glands might put constraints on certain web parameters such that some structural or compositional options, available at other times, are not available close to ecdysis.
There are also anatomical changes in the silk glands associated with egg development. It is not unusual for a large part of the abdomen to become increasingly crowded by eggs and cylindrical silk glands (major sources of egg sac silk) during this time. As a result, other tissues become compressed, including the aggregate glands, source of the web’s sticky droplets. This compression may render these and other silk glands temporarily nonfunctional, forcing a suspension of web building until oviposition (Kovoor, 1972, 1977). Thus, comparable to silk gland remodeling during molting, the structure and composition of last webs built before oviposition may reflect silk glands experiencing reduced functionality.
Note that both molting and egg laying are often accompanied by a hiatus in web building extending before and/or after the day on which ecdysis or egg sac construction occur. This effect on the time between bouts of web building may itself influence LMM composition in the first post-ecdysial or post-ovipositional webs by affecting quantities of LMM that can be synthesized or otherwise amassed for use in these webs.
Possible effects of feeding regimen on LMM composition among feeding group spiders
When setting the feeding regimen for the feeding groups it was our intention that spiders not gain weight so fast that juveniles molted or adults oviposited after building only a few webs. There was a tendency for web weight to increase as the series progressed in feeding group spiders, particularly in the two Argiope species. How did the feeding regimen affect decisions regarding foraging investment? Did maintained or increased web weights resulting from such decisions contribute to compositional shifts similar in some respects to those seen in starving groups? We consider and provide some background to these questions in the following paragraphs.
Models presented by Sherman (1994) and Higgins (1995) predict that foraging investment, represented principally by the web, increases as foraging success decreases, so long as the increased investment does not result in weight loss by the spider. At that point, further decreases in foraging success are accompanied by decreased foraging investment. Consequently, assuming average mesh size does not change, the largest webs are built by spiders consuming moderate amounts of food. Spiders consuming either very little or large amounts of food build smaller webs. Data from some studies on araneoid orb-weavers essentially agree with this model (Higgins and Buskirk, 1992; Sherman, 1994; Higgins, 1995; Herberstein et al., 1998, 2000; Tso, 1999; de Crespigny et al., 2001), as do studies on a uloborid orb-weaver (Watanabe, 2001). Other data are more ambiguous or in conflict with the model (Witt, 1963; Benforado and Kistler, 1973; Vollrath and Samu, 1997; Blackledge, 1998; Herberstein and Heiling, 1999:1245), perhaps in part (1) because of constraints imposed on study animals (e.g., Blackledge, 1998:26), (2) because it is not well established what rates of consumption constitute low, moderate, and high food diets, or (3) because a change in web size is not necessarily the only way a spider may manifest a change in foraging effort. An increase in foraging effort with decreased prey capture took the form of increased web size and web building frequency in a laboratory study with A. trifasciata (Tso, 1999), but only an increase in web building frequency in a laboratory study with A. diadematus (Vollrath and Samu, 1997).
Thus, was the tendency for web weight to increase in feeding group spiders, especially Argiope, an indication that the feeding rate was only moderate, a condition exacerbated by our removal of webs, and consequently foraging investment was increased? Or was the feeding rate more than moderate, resulting in a response (increased web weight) that would allow the spiders to better exploit the relatively abundant prey (Benforado and Kistler, 1973:98; Vollrath and Samu, 1997:296)? Or were increased web weights a response to a consistent, as opposed to a sporadic, return on the foraging investment (Herberstein et al., 2000)? There is also the possible influence of the molt/intermolt cycle on web weight, mentioned earlier. Whichever, if any, of these four explanations apply, such increases in web weight may have spread certain resources too thin, such that compositional compromises were made by feeding group spiders. If resources were not only lost in the webs we collected, but were increasingly diverted into reproduction and/or growth by feeding group spiders, it would presumably have been even more difficult to maintain an optimal LMM composition while maintaining or increasing web weight. Thus, certain shifts in composition in feeding groups (e.g., the growing molar % of Gly) that were mirrored in starving groups may have reflected a compromise made by feeding spiders that enabled them to build heavier webs. Whether because of dietary deficiencies, synthetic capacity limitations, or diversion of resources into reproduction or growth, it may not always be possible to allocate the ‘ideal’ quantities of LMM to webs containing more aggregate gland secretion. Perhaps it is sometimes more advantageous to build heavier webs with a less-than-ideal LMM composition than to build lighter webs with the ‘ideal’ LMM composition.
Indeed, compositional compromises made under certain circumstances have previously been indicated in non-sticky web components. Madsen et al. (1999) found that starvation may result in decreased extensibility of major ampullate silk. They suggested that decreased availability of amino acids with starvation may result in compromised silks with compositions and mechanical properties different from those of well-nourished spiders. A related suggestion was made by Craig et al. (2000) based on data indicating that qualitative differences in diet can influence the amino acid composition of major ampullate silk. The observation of considerable intraspecific and intraindividual variability in major ampullate silk amino acid composition lends support to these possibilities (Work and Young, 1987 and references therein; Craig et al., 2000), while the observation of a uniform intraspecific composition does not (Lombardi and Kaplan, 1990).
In comparing our study to earlier studies we should bear in mind differences in web parameters measured. In this study, web weights were measured but dimensional parameters such as web size and mesh size were not, while in most earlier studies the converse is true. Ideally, the monitoring of foraging investment would include measures of both to help prevent or resolve seeming contradictions that can arise between studies. For example, in this study decreases in web weight were observed soon after starvation and web removal began, whereas Witt (1963) observed no significant decrease in orb web size after six days of starvation and web removal. And with resumed feeding of starved spiders there was no significant increase in web size even after 10 days of feeding (Witt, 1963), again in contrast to our weight results. However, Witt also obtained a measure of web weight in the form of total web nitrogen content which, divided by total thread length, was used to calculate “thread thickness”. Witt notes that, despite web size remaining large after six days of starvation, there was a decrease in thread thickness in these webs, consistent with our weight data. Parenthetically, given the large contribution the sticky spiral’s aggregate gland cover makes to orb web weight and the recognition that many of the organic LMM contain nitrogen, we suggest that changes reported in “thread thickness” with different feeding and/or web removal protocols (Witt, 1963; Breed et al., 1964) may be partly or largely attributable to changes in the quantity of aggregate gland secretion applied per length of sticky spiral. In our study, the tendency for the water-soluble % of the web to decrease over the series in many groups (Supplemental Table 3) indicates a decrease in this quantity. More direct observations have confirmed that this quantity varies, even within a single web (Eberhard, 1988:300; Vollrath and Edmonds, 1989; Edmonds and Vollrath, 1992).
In discussing the influence of the feeding regimen on compositional changes we should consider not only feeding rate but also quality of the diet. Of the three species used in this study, A. cavaticus is probably the most reliant on dipterans as a natural source of food (e.g., Olive, 1980; Riechert and Cady, 1983; Horton and Wise, 1983; Howell and Ellender, 1984). But even in this species a diet consisting of just one or a few species of brachyceran flies, as given to all feeding group spiders, is not typical of spiders in the field. Perhaps the same quantity of food, but from more varied or typical prey items would have resulted in more stable LMM compositions in feeding group spiders. Qualitative aspects of the diet may also have contributed to differences in composition seen between radiolabeled and non-radiolabeled webs. As noted earlier, the molar % of Cho in the webs of Argiope given radioisotope was invariably higher than in the non-radioactive webs of conspecifics. The former had either recently been feeding in the field or were fed orthopterans whereas the latter were fed only muscid and calliphorid flies. We note that Craig et al. (2000) found a significant difference in the amino acid composition of A. keyserlingi dragline silk depending on whether the spiders had been feeding on blowflies or crickets.
Consistency amidst variation
While recognizing the significant intraspecific variation that clearly exists in LMM composition, and the validity of the statement made earlier (Higgins et al., 2001:82), that “the composition of the organic low-molecular-weight solution is not fixed”, we should also recognize that there are features to a species’ LMM composition that are at least typically maintained. The inventory of principal LMM is generally consistent within a species, at least within one sex, and some quantitative relationships among the LMM are often observed. In this study, GAB was invariably the most abundant LMM in webs of A. aurantia and A. cavaticus on a molar % basis (Supplemental Fig. 8). In A. cavaticus webs the molar % of NAT was almost invariably lower than that of Ise, often by a considerable margin (Supplemental Figs. 3–5), while the opposite was observed in A. aurantia (Fig. 5, Supplemental Fig. 6). Some quantitative relationships were observed in all three species. For example, Cho was usually (A. trifasciata) or always (A. aurantia, A. cavaticus) more abundant than Bet, and Gly was usually (A. cavaticus) or always (A. trifasciata, A. aurantia) more abundant than Ala or Pro. Many other such relationships were demonstrated by ranking molar % data for each species (Supplemental Fig. 8).
When LMM carrying the same charge were grouped together, a consistency at a higher level was also evident within a species. Thus, the total molar % of positively-charged organic LMM (GAB + NAP + Cho + Put) was generally maintained within a fairly narrow range even with starvation (X ± SD for: A. cavaticus, 54.4 mole % ± 3.08 mole %, N = 86; A. aurantia, 59.0 mole % ± 4.07 mole %, N = 102; A. trifasciata, 46.0 mole % ± 5.92 mole %, N = 123) (Figs. 4, 5, Supplemental Figs. 2–6). Relative to the means, standard deviations were larger for the total molar % of negatively-charged organic LMM (Ise + NAT) (X ± SD for: A. cavaticus, 23.7 mole % ± 6.33 mole %; A. aurantia, 15.4 mole % ± 4.05 mole %; A. trifasciata, 24.8 mole % ± 4.94 mole %), apparently because of the low synthetic rates of Ise and the Tau portion of NAT. However, this greater variability was partially offset by compensatory changes in inorganic phosphate (X ± SD of Ise + NAT + H2PO4− for: A. cavaticus, 38.8 mole % ± 5.05 mole %; A. aurantia, 31.2 mole % ± 4.41 mole %; A. trifasciata, 35.0 mole % ± 5.02 mole %).
Conclusions
We investigated how starvation affects LMM composition in orb web sticky droplets, anticipating that with fasting there would be decreases in the molar percentages of those organic LMM the spider is least able to synthesize and increases in those that are more readily synthesized. Many shifts in composition were basically consistent with differing synthetic capacities. Thus, declines in the molar percentages of LMM with lower rates of synthesis (Cho, Ise, NAT, and, presumably, Bet) were observed at least over part of a web series in starving group spiders while molar percentages of some LMM with higher rates of synthesis increased (Gly, GAB, and, in Araneus cavaticus, Pro). The most convincing indications that certain more readily synthesized LMM were increasingly relied upon by starving spiders were increases observed in μg/web of Gly, GAB (in Argiope trifasciata), and Pro (in A. cavaticus) in webs of starving group spiders. These increases in absolute quantities per web contrasted sharply with the decreases seen in most LMM in starving spiders and give the impression that these LMM were used to fill in for other, perhaps more desirable, but unavailable or costly LMM. In A. trifasciata, it appeared that GAB was increasingly used to substitute for the less readily synthesized NAP.
However, synthetic capacity was not an entirely reliable predictor of compositional changes with starvation. Some shifts in LMM molar percentages in starving group spiders were not predicted based on specific radioactivity measurements (e.g., the decrease in Ala in A. trifasciata). We also found a number of similarities between starving and feeding group spiders with respect to changes in LMM molar percentages, making interpretation of results uncertain. Possible explanations for these parallel changes include a factor common to both the feeding and starving groups. It is likely that one such factor was web recycling. By not allowing spiders to recycle their webs, a form of nutritional stress was imposed on feeding as well as starving group spiders and may have influenced LMM composition in a similar manner in both. It may also have contributed to decreases observed both in the water-soluble % of the web and the % of water-soluble weight that could be accounted for by the organic LMM. Paradoxically, it is also possible that the difference imposed on feeding and starving group spiders, availability of insect prey or lack thereof, contributed to similar molar % shifts. Feeding group spiders allocated resources to molting, egg laying, and increased foraging (increased web weight) and these expenditures may have resulted in shortages of some LMM and consequent shifts in LMM composition reminiscent of shifts due to fasting in starving group spiders. Analyses of webs of juveniles and adults indicated that both the molt/intermolt and egg laying cycles, respectively, influenced LMM composition.
Additional studies are needed that focus on individual factors that probably influence LMM composition, including those touched on in this report (web recycling, the molt/intermolt and egg laying cycles, qualitative and quantitative aspects of diet, time between web building episodes), as well as studies that focus on the synthesis, allocation, and transport of individual LMM.
Supplementary Material
Acknowledgments
This study would not have taken place without the aid of Kathy Gallagher (UNH Instrumentation Center), who went well above the call of duty in placing her expertise with NMR at our disposal, providing training and making large blocks of instrument time available to us. Nor would this study have been possible without partial financial support from the National Institutes of Health, grant R15DK/OD51291, to EKT. We are also very grateful to Dr. Stacia Sower (UNH) for the use of laboratory equipment and to Dr. Todd Blackledge (University of Akron) and an anonymous reviewer for their many valuable suggestions for improving the manuscript.
References
- Allen JA, Garrett MR. Taurine in marine invertebrates. Adv Mar Biol. 1971;9:205–253. [Google Scholar]
- Anderson CM, Tillinghast EK. GABA and taurine derivatives on the adhesive spiral of the orb web of Argiope spiders, and their possible behavioural significance. Physiol Entomol. 1980;5:101–106. [Google Scholar]
- Ball, P. (1999). The Self-Made Tapestry: Pattern Formation in Nature. Oxford: Oxford University Press.
- Benforado J, Kistler KH. Growth of the orb weaver, Araneus diadematus, and correlation with web measurements. Psyche. 1973;80:90–100. [Google Scholar]
- Bilinski E. Biosynthesis of trimethylammonium compounds in aquatic animals. I Formation of trimethylamine oxide and betaine from C14-labelled compounds by lobster (Homarus americanus) J Fish Res Board Canada. 1960;17:895–902. [Google Scholar]
- Bilinski E. Biosynthesis of trimethylammonium compounds in aquatic animals. III Choline metabolism in marine Crustacea. J Fish Res Board Canada. 1962;19:505–510. [Google Scholar]
- Blackledge TA. Stabilimentum variation and foraging success in Argiope aurantia and Argiope trifasciata (Araneae: Araneidae) J Zool, Lond. 1998;246:21–27. [Google Scholar]
- Bonthrone KM, Vollrath F, Hunter BK, Sanders JKM. The elasticity of spiders’ webs is due to water-induced mobility at a molecular level. Proc R Soc Lond B. 1992;248:141–144. [Google Scholar]
- Boonyaratpalin, M. (1996). Nutritional requirements of commercially important shrimps in the tropics. In Feeds for Small-Scale Aquaculture: Proceedings of the National Seminar-Workshop on Fish Nutrition and Feeds, Tigbauan, Iloilo, Philippines, 1–2 June 1994 (ed. C. B. Santiago, R. M. Coloso, O. M. Millamena and I. G. Borlongan), pp. 10–28. Iloilo: SEAFDEC Aquaculture Department.
- Boys, C. V. (1889). Quartz fibres. Nature 247–251.
- Braun R, Fromageot P. Désamination de la taurine par Aspergillus niger. Biochim Biophys Acta. 1962;62:548–555. doi: 10.1016/0006-3002(62)90237-8. [DOI] [PubMed] [Google Scholar]
- Breed AL, Levine VD, Peakall DB, Witt PN. The fate of the intact orb web of the spider Araneus diadematus Cl. Behaviour. 1964;23:43–60. [Google Scholar]
- Brichon G, Chapelle S, Zwingelstein G. Phospholipids composition and metabolism in the hemolymph of Carcinus maenas (Crustacea, Decapoda)—Effect of temperature. Comp Biochem Physiol. 1980;67B:647–652. [Google Scholar]
- Carico, J. E. (1986). Web removal patterns in orb-weaving spiders. In Spiders: Webs, Behavior, and Evolution (ed. W. A. Shear), pp. 306–318. Stanford: Stanford University Press.
- Catacutan MR, de la Cruz M. Growth and mid-gut cells profile of Penaeus monodon juveniles fed water-soluble-vitamin deficient diets. Aquaculture. 1989;81:137–144. [Google Scholar]
- Cavallini, D., Scandurra, R., Duprè, S., Federici, G., Santoro, L., Ricci, G. and Barra, D. (1976). Alternative pathways of taurine biosynthesis. In Taurine (ed. R. Huxtable and A. Barbeau), pp. 59–66. New York: Raven Press.
- Cavallini, D., Duprè, S., Federici, G., Solinas, S., Ricci, G., Antonucci, A., Spoto, G. and Matarese, M. (1978). Isethionic acid as a taurine co-metabolite. In Taurine and Neurological Disorders (ed. A. Barbeau and R. J. Huxtable), pp. 29–34. New York: Raven Press.
- Chen, P. S. (1985). Amino acid and protein metabolism. In Comprehensive Insect Physiology, Biochemistry and Pharmacology, Vol. 10: Biochemistry (ed. G. A. Kerkut and L. I. Gilbert), pp. 177–217. Oxford: Pergamon Press Ltd.
- Chen PS, Jr, Toribara TY, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- Craig CL. Alternative foraging modes of orb web weaving spiders. Biotropica. 1989;21:257–264. [Google Scholar]
- Craig CL, Riekel C, Herberstein ME, Weber RS, Kaplan D, Pierce NE. Evidence for diet effects on the composition of silk proteins produced by spiders. Mol Biol Evol. 2000;17:1904–1913. doi: 10.1093/oxfordjournals.molbev.a026292. [DOI] [PubMed] [Google Scholar]
- Cunningham, C. M. and Tipton, K. F. (2000). Metabolism of taurine to sulphoacetaldehyde during oxidative stress. In Taurine 4: Taurine and Excitable Tissues (Advances in Experimental Medicine and Biology, Vol. 483) (ed. L. Della Corte, R. J. Huxtable, G. Sgaragli and K. F. Tipton), pp. 383–388. New York: Kluwer Academic/Plenum Publishers. [DOI] [PubMed]
- Cunningham C, Tipton KF, Dixon HBF. Conversion of taurine into N-chlorotaurine (taurine chloramine) and sulphoacetaldehyde in response to oxidative stress. Biochem J. 1998;330:939–945. doi: 10.1042/bj3300939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Abramo LR, Baum NA. Choline requirement of the microcrustacean Moina macrocopa: A purified diet for continuous culture. Biol Bull. 1981;161:357–365. [Google Scholar]
- Dadd, R. H. (1985). Nutrition: Organisms. In Comprehensive Insect Physiology, Biochemistry and Pharmacology, Vol. 4: Regulation: Digestion, Nutrition, Excretion (ed. G. A. Kerkut and L. I. Gilbert), pp. 313–390. Oxford: Pergamon Press Ltd.
- Dall, W. and Moriarty, D. J. W. (1983). Functional aspects of nutrition and digestion. In The Biology of Crustacea, Vol. 5: Internal Anatomy and Physiological Regulation (ed. L. H. Mantel), pp. 215–261. New York: Academic Press.
- de Crespigny FEC, Herberstein ME, Elgar MA. The effect of predator-prey distance and prey profitability on the attack behaviour of the orb-web spider Argiope keyserlingi (Araneidae) Aust J Zool. 2001;49:213–221. [Google Scholar]
- Dragolovich J, Pierce SK. Characterization of partially purified betaine aldehyde dehydrogenase from horseshoe crab (Limulus polyphemus) cardiac mitochondria. J Exp Zool. 1994;270:417–425. [Google Scholar]
- Dupré S, Federici G, Ricci G, Spoto G, Antonucci A, Cavallini D. Enzymatic oxidation of mercaptoethanol to isethinic acid and isethionic acid. Enzyme. 1978;23:307–313. doi: 10.1159/000458594. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. The ecology of the web of Uloborus diversus (Araneae: Uloboridae) Oecologia. 1971;6:328–342. doi: 10.1007/BF00389107. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. Behavioral flexibility in orb web construction: Effects of supplies in different silk glands and spider size and weight. J Arachnol. 1988;16:295–302. [Google Scholar]
- Edmonds DT, Vollrath F. The contribution of atmospheric water vapour to the formation and efficiency of a spider’s capture web. Proc R Soc Lond B. 1992;248:145–148. [Google Scholar]
- Federici G, Ricci G, Dupré S, Antonucci A, Cavallini D. The metabolism of mercaptoethanol by the living rat. Biochem Exp Biol. 1976;12:341–345. [Google Scholar]
- Felig P. The glucose-alanine cycle. Metab Clin Exp. 1973;22:179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Fellman JH. Isethionic acid. Meth Enzymol. 1987;143:172–177. doi: 10.1016/0076-6879(87)43032-2. [DOI] [PubMed] [Google Scholar]
- Fischer FG, Brander J. Eine Analyse der Gespinste der Kreuzspinne. Hoppe-Seyler’s Z Physiol Chem. 1960;320:92–102. doi: 10.1515/bchm2.1960.320.1.92. [DOI] [PubMed] [Google Scholar]
- Gosline, J., Nichols, C., Guerette, P., Cheng, A. and Katz, S. (1995). The macromolecular design of spiders’ silks. In Biomimetics: Design and Processing of Materials (ed. M. Sarikaya and I. A. Aksay), pp. 237–261. Woodbury, New York: AIP Press.
- Guillaume, J. (1997). Protein and amino acids. In Crustacean Nutrition (Advances in World Aquaculture, Vol. 6) (ed. L. R. D’Abramo, D. E. Conklin and D. M. Akiyama), pp. 26–50. Baton Rouge: World Aquaculture Society.
- Hayashi CY, Lewis RV. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. J Mol Biol. 1998;275:773–784. doi: 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- Herberstein ME, Heiling AM. Asymmetry in spider orb webs: a result of physical constraints? Anim Behav. 1999;58:1241–1246. doi: 10.1006/anbe.1999.1255. [DOI] [PubMed] [Google Scholar]
- Herberstein ME, Abernethy KE, Backhouse K, Bradford H, de Crespigny FE, Luckock PR, Elgar MA. The effect of feeding history on prey capture behaviour in the orb-web spider Argiope keyserlingi Karsch (Araneae: Araneidae) Ethology. 1998;104:565–571. [Google Scholar]
- Herberstein ME, Craig CL, Elgar MA. Foraging strategies and feeding regimes: Web and decoration investment in Argiope keyserlingi Karsch (Araneae: Araneidae) Evol Ecol Res. 2000;2:69–80. [Google Scholar]
- Higgins LE. Variation in foraging investment during the intermolt interval and before egg-laying in the spider Nephila clavipes (Araneae: Araneidae) J Insect Behav. 1990;3:773–783. [Google Scholar]
- Higgins LE. Direct evidence for trade-offs between foraging and growth in a juvenile spider. J Arachnol. 1995;23:37–43. [Google Scholar]
- Higgins LE, Buskirk RE. A trap-building predator exhibits different tactics for different aspects of foraging behaviour. Anim Behav. 1992;44:485–499. [Google Scholar]
- Higgins L, Rankin MA. Nutritional requirements for web synthesis in the tetragnathid spider Nephila clavipes. Physiol Entomol. 1999;24:263–270. [Google Scholar]
- Higgins LE, Townley MA, Tillinghast EK, Rankin MA. Variation in the chemical composition of orb webs built by the spider Nephila clavipes (Araneae, Tetragnathidae) J Arachnol. 2001;29:82–94. [Google Scholar]
- Hingston RWG. The snare of the giant wood spider (Nephila maculata). Parts II, III. J Bombay Nat Hist Soc. 1922;28:911–923. [Google Scholar]
- Horton CC, Wise DH. The experimental analysis of competition between two syntopic species of orb-web spiders (Araneae: Araneidae) Ecology. 1983;64:929–944. [Google Scholar]
- Howell FG, Ellender RD. Observations on growth and diet of Argiope aurantia Lucas (Araneidae) in a successional habitat. J Arachnol. 1984;12:29–36. [Google Scholar]
- Huxtable, R. J. (1986). Biochemistry of Sulfur New York: Plenum Press.
- Huxtable, R. and Bressler, R. (1976). The metabolism of cysteamine to taurine. In Taurine (ed. R. Huxtable and A. Barbeau), pp. 45–57. New York: Raven Press.
- Jacobsen JG, Smith LH., Jr Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968;48:424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Kasting R, McGinnis AJ. Radioisotopes and the determination of nutrient requirements. Ann N Y Acad Sci. 1966;139:98–107. doi: 10.1111/j.1749-6632.1966.tb41188.x. [DOI] [PubMed] [Google Scholar]
- Kleemann A, Leuchtenberger W, Martens J, Weigel H. A new preparation of 4-aminobutyramide. Angew Chem Int Ed Engl. 1980;19:627. [Google Scholar]
- Kovoor J. Étude histochimique et cytologique des glandes séricigènes de quelques Argiopidae. Ann Sci Nat, Zool Biol Anim, Sér 12. 1972;14:1–40. [Google Scholar]
- Kovoor J. La soie et les glandes séricigènes des arachnides. Ann Biol. 1977;16:97–171. [Google Scholar]
- Lasser GW, Allen WV. The essential amino acid requirements of the Dungeness crab, Cancer magister. Aquaculture. 1976;7:235–244. [Google Scholar]
- Lin LH, Edmonds DT, Vollrath F. Structural engineering of an orb-spider’s web. Nature. 1995;373:146–148. [Google Scholar]
- Lombardi SJ, Kaplan DL. The amino acid composition of major ampullate gland silk (dragline) of Nephila clavipes (Araneae, Tetragnathidae) J Arachnol. 1990;18:297–306. [Google Scholar]
- Lubin, Y. D. (1986). Web building and prey capture in the Uloboridae. In Spiders: Webs, Behavior, and Evolution (ed. W. A. Shear), pp. 132–171. Stanford: Stanford University Press.
- Madsen B, Shao ZZ, Vollrath F. Variability in the mechanical properties of spider silks on three levels: interspecific, intraspecific and intraindividual. Int J Biol Macromol. 1999;24:301–306. doi: 10.1016/s0141-8130(98)00094-4. [DOI] [PubMed] [Google Scholar]
- Miyajima, L. S., Broderick, G. A. and Reimer, R. D. (1976). Identification of the essential amino acids of the freshwater shrimp, Macrobrachium ohione In Proceedings of the Seventh Annual Meeting, World Mariculture Society (ed. J. W. Avault, Jr.), pp. 699–704. Baton Rouge: Louisiana State University Division of Continuing Education.
- Morris, J. G. (1991). Nutrition. In Comparative Animal Physiology, 4thed.: Environmental and Metabolic Animal Physiology (ed. C. L. Prosser), pp. 231–276. New York: Wiley-Liss Inc.
- Nentwig W. Prey analysis of four species of tropical orb-weaving spiders (Araneae: Araneidae) and a comparison with araneids of the temperate zone. Oecologia. 1985;66:580–594. doi: 10.1007/BF00379353. [DOI] [PubMed] [Google Scholar]
- Olive CW. Foraging specializations in orb-weaving spiders. Ecology. 1980;61:1133–1144. [Google Scholar]
- Opell BD. Post-hatching development and web production of Hyptiotes cavatus (Hentz) (Araneae, Uloboridae) J Arachnol. 1982;10:185–191. [Google Scholar]
- Patel SK, Nigam S. Biochemical studies on certain spider’s silk. J Anim Morph Physiol. 1996;43:51–53. [Google Scholar]
- Peakall DB. Conservation of web proteins in the spider, Araneus diadematus. J Exp Zool. 1971;176:257–264. doi: 10.1002/jez.1401760302. [DOI] [PubMed] [Google Scholar]
- Peters HM. Über den Spinnapparat von Nephila madagascariensis (Radnetzspinnen, Fam. Argiopidae) Z Naturforsch. 1955;10b:395–404. [Google Scholar]
- Read, W. O., Schramm, M. A. and Welty, J. D. (1976). Metabolic pathway of taurine synthesis in heart. In Taurine (ed. R. Huxtable and A. Barbeau), pp. 165–168. New York: Raven Press.
- Reed CF, Witt PN, Scarboro MB. The orb web during the life of Argiope aurantia (Lucas) Dev Psychobiol. 1969;2:120–129. doi: 10.1002/dev.420020212. [DOI] [PubMed] [Google Scholar]
- Richter G. Untersuchungen über Struktur und Funktion der Klebefäden in den Fanggeweben ecribellater Radnetzspinnen. Naturwissenschaften. 1956;43:23. [Google Scholar]
- Riechert SE, Cady AB. Patterns of resource use and tests for competitive release in a spider community. Ecology. 1983;64:899–913. [Google Scholar]
- Rock GC, Hodgson E. Dietary amino requirements for Heliothis zea determined by dietary deletion and radiometric techniques. J Insect Physiol. 1971;17:1087–1097. [Google Scholar]
- Rodriguez JG, Hampton RE. Essential amino acids determined in the two-spotted spider mite, Tetranychus urticae Koch (Acarina, Tetranychidae) with glucose-U-C14. J Insect Physiol. 1966;12:1209–1216. [Google Scholar]
- Rodriguez JG, Lasheen AM. Axenic culture of Tyrophagus putrescentiae in a chemically defined diet and determination of essential amino acids. J Insect Physiol. 1971;17:979–985. [Google Scholar]
- Scandurra R, Federici G, Dupré S, Cavallini D. Taurine and isethionic acid production in mammals. Bull Mol Biol Med. 1978;3:141–147. [Google Scholar]
- Schildknecht H, Kunzelmann P, Krauß D, Kuhn C. Über die Chemie der Spinnwebe, I. Arthropodenabwehrstoffe, LVII. Naturwissenschaften. 1972;59:98–99. [Google Scholar]
- Schmidt, L. (1974). The biochemical detection of metabolic disease: Screening tests and a systematic approach to screening. In Heritable Disorders of Amino Acid Metabolism: Patterns of Clinical Expression and Genetic Variation (ed. W. L. Nyhan), pp. 675–697. New York: John Wiley & Sons Inc.
- Schulz S. Composition of the silk lipids of the spider Nephila clavipes. Lipids. 2001;36:637–647. doi: 10.1007/s11745-001-0768-7. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K. On a new spinning gland found in geometric spiders and its functions. Annot Zool Japon. 1952;25:394–399. [Google Scholar]
- Sekiguchi K. The spinning organs in sub-adult geometric spiders and their changes accompanying the last moulting. Sci Rep Tokyo Kyoiku Daigaku, Sect B. 1955;8:33–40. [Google Scholar]
- Sherman PM. The orb-web: an energetic and behavioural estimator of a spider’s dynamic foraging and reproductive strategies. Anim Behav. 1994;48:19–34. [Google Scholar]
- Shewbart KL, Mies WL, Ludwig PD. Identification and quantitative analysis of the amino acids present in protein of the brown shrimp Penaeus aztecus. Mar Biol. 1972;16:64–67. [Google Scholar]
- Shiau SY. Nutrient requirements of penaeid shrimps. Aquaculture. 1998;164:77–93. [Google Scholar]
- Shiau SY, Lo PS. Dietary choline requirement of juvenile grass shrimp (Penaeus monodon) Anim Sci. 2001;72:477–482. [Google Scholar]
- Shieh HS. The biosynthesis of phospholipids in the lobster, Homarus americanus. Comp Biochem Physiol. 1969;30:679–684. doi: 10.1016/0010-406x(69)92146-x. [DOI] [PubMed] [Google Scholar]
- Smith RB, Mommsen TP. Pollen feeding in an orb-weaving spider. Science. 1984;226:1330–1332. doi: 10.1126/science.226.4680.1330. [DOI] [PubMed] [Google Scholar]
- Tillinghast EK. The chemical fractionation of the orb web of Argiope spiders. Insect Biochem. 1984;14:115–120. [Google Scholar]
- Tillinghast EK, Christenson T. Observations on the chemical composition of the web of Nephila clavipes (Araneae, Araneidae) J Arachnol. 1984;12:69–74. [Google Scholar]
- Townley MA, Tillinghast EK. Orb web recycling in Araneus cavaticus (Araneae, Araneidae) with an emphasis on the adhesive spiral component, GABamide. J Arachnol. 1988;16:303–319. [Google Scholar]
- Townley MA, Bernstein DT, Gallagher KS, Tillinghast EK. Comparative study of orb web hygroscopicity and adhesive spiral composition in three araneid spiders. J Exp Zool. 1991;259:154–165. [Google Scholar]
- Townley MA, Tillinghast EK, Cherim NA. Moult-related changes in ampullate silk gland morphology and usage in the araneid spider Araneus cavaticus. Phil Trans R Soc Lond B. 1993;340:25–38. doi: 10.1098/rstb.1993.0046. [DOI] [PubMed] [Google Scholar]
- Tso IM. Behavioral response of Argiope trifasciata to recent foraging gain: A manipulative study. Amer Midl Nat. 1999;141:238–246. [Google Scholar]
- van Marrewijk WJA, Zandee DI. Amino acid metabolism of Astacus leptodactylus (Esch. )—II Biosynthesis of the non-essential amino acids. Comp Biochem Physiol. 1975;50B:449–455. doi: 10.1016/0305-0491(75)90257-6. [DOI] [PubMed] [Google Scholar]
- Vollrath F, Edmonds DT. Modulation of the mechanical properties of spider silk by coating with water. Nature. 1989;340:305–307. [Google Scholar]
- Vollrath F, Samu F. The effect of starvation on web geometry in an orb-weaving spider. Bull Brit Arachnol Soc. 1997;10:295–298. [Google Scholar]
- Vollrath F, Tillinghast EK. Glycoprotein glue beneath a spider web’s aqueous coat. Naturwissenschaften. 1991;78:557–559. [Google Scholar]
- Vollrath F, Fairbrother WJ, Williams RJP, Tillinghast EK, Bernstein DT, Gallagher KS, Townley MA. Compounds in the droplets of the orb spider’s viscid spiral. Nature. 1990;345:526–528. [Google Scholar]
- Warburton C. The spinning apparatus of geometric spiders. Quart J Microsc Sci, New Ser. 1890;31:29–39. [Google Scholar]
- Watanabe T. Effects of web design on the prey capture efficiency of the uloborid spider Octonoba sybotides under abundant and limited prey conditions. Zool Sci. 2001;18:585–590. [Google Scholar]
- Weiher B, Komnick H. Digestion of phosphatidylcholines, absorption, and esterification of lipolytic products by Aeshna cyanea larvae as studied in vivo and in vitro. Arch Insect Biochem Physiol. 1997;36:273–293. doi: 10.1002/(SICI)1520-6327(1997)36:4<273::AID-ARCH3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Widmer B. Untersuchungen zur Synthese und zum Metabolismus von Aminosäuren in Larven des Wildtyps und der Letalmutanten l(3)tr und l(2)me von Drosophila melanogaster. Insect Biochem. 1973;3:181–203. [Google Scholar]
- Witt PN. Environment in relation to behavior of spiders. Arch Environ Health. 1963;7:4–12. doi: 10.1080/00039896.1963.10663490. [DOI] [PubMed] [Google Scholar]
- Witt PN. Instructions for working with web-building spiders in the laboratory. BioScience. 1971;21:23–25. [Google Scholar]
- Work RW. Web components associated with the major ampullate silk fibers of orb-web-building spiders. Trans Amer Microsc Soc. 1981;100:1–20. [Google Scholar]
- Work RW, Young CT. The amino acid compositions of major and minor ampullate silks of certain orb-web-building spiders (Araneae, Araneidae) J Arachnol. 1987;15:65–80. [Google Scholar]
- Zar, J. H. (1999). Biostatistical Analysis, Fourth Edition Upper Saddle River, NJ: Prentice Hall.
- Zschokke S. Nomenclature of the orb-web. J Arachnol. 1999;27:542–546. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


