Abstract
Seed development in flowering plants is initiated by the fusion of two male gametes with two female gametes—the egg cell and the central cell—which leads to the formation of an embryo and an endosperm, respectively. Fertilization-independent seed formation is actively repressed by the FERTILIZATION-INDEPENDENT SEED (FIS) Polycomb group (PcG) proteins, an evolutionarily conserved class of proteins that ensures the stable transmission of developmental decisions. The FIS proteins act together in a complex and modify their target genes by applying repressive methylation on histone H3 lysine 27. In addition to its function before fertilization, the FIS complex restricts endosperm proliferation. This function is likely to be achieved by imprinting the maternal alleles of FIS target genes. However, imprinting in the endosperm is controlled not only by the FIS complex but also by DNA methylation, and the interconnections between these two processes are now being investigated.
Keywords: imprinting, seed development, Polycomb group proteins, MEDEA, PHERES1
Introduction
The fusion of two male gametes with two female gametes—the haploid egg cell and the diploid central cell—marks the start of seed development in flowering plants, and results in the formation of the diploid embryo and the triploid endosperm, respectively. The embryo and the endosperm are surrounded by the maternally derived seed coat, which provides a protective shield for the developing seed. In most plant species, fertilization is required for seed development, and premature divisions of the egg cell and the central cell are actively suppressed. Insight into this suppressive mechanism has been provided by the identification of the fertilization-independent seed (fis) mutants that have the ability to form seed-like structures without fertilization (Ohad et al, 1996; Chaudhury et al, 1997). These fertilization-independent seed-like structures are characterized by ovule integuments that develop into a seed coat, a central cell that starts nuclear divisions to form a diploid multi-nuclear endosperm, and an egg cell that occasionally starts to divide to form an embryo-like structure (Chaudhury et al, 1997; Guitton & Berger, 2005a). The FIS genes that have been identified in plants are MEDEA (MEA; Grossniklaus et al, 1998; Kiyosue et al, 1999), FERTILIZATION-INDEPENDENT ENDOSPERM (FIE; Ohad et al, 1999), FIS2 (Luo et al, 1999) and MULTI-COPY SUPPRESSOR OF IRA1 (MSI1; Köhler et al, 2003a; Guitton et al, 2004). The FIS genes encode Polycomb group (PcG) proteins that have close homologues in animals, such as insects and mammals, but not yeast. The corresponding homologues in Drosophila and mammals are E(z) (Ezh1, Ezh2), Esc (Eed), Su(z)12 and Nurf55 (RbAp46/48), respectively (reviewed by Cao & Zhang, 2004; Guitton & Berger, 2005b). In animals, PcG proteins ensure the stable propagation of established developmental decisions through mitotic cell cycles.
The FIS proteins MEA, FIE and MSI1 interact and form a protein complex of about 650 kDa (Köhler et al, 2003a), which probably contains FIS2 as well (Chanvivattana et al, 2004). Similarly, the PcG proteins in animals interact and are localized in large protein complexes that change their composition during development (reviewed by Lund & Lohuizen, 2004; Bantignies & Cavalli, 2006). One of the best-characterized PcG complexes in animals is the Polycomb repressive complex 2 (PRC2), which is similar in size and composition to the plant FIS complex (reviewed by Lund & Lohuizen, 2004). Insect and mammalian PRC2 complexes have intrinsic histone methyltransferase activity and target lysine (Lys) 27 of histone H3 (H3Lys27), suggesting that the maintenance of cellular memory involves the methylation of histones (reviewed by Cao & Zhang, 2004).
fis mutants are maternal gametophytic mutants
In addition to their ability to form seeds independently of fertilization, fis mutants have a striking maternally determined phenotype after fertilization. Every seed that inherits a fis mutant allele from the mother fails to complete development and eventually aborts, containing an embryo that has arrested at the late heart stage—5–6 days after fertilization—and an uncellularized endosperm that forms more nuclei than wild-type endosperm (Ohad et al, 1996; Chaudhury et al, 1997; Grossniklaus et al, 1998; Kiyosue et al, 1999; Ingouff et al, 2005). This phenotype can be interpreted in two ways: either the paternal alleles of FIS genes are not expressed and therefore cannot complement the mutant fis phenotype, or FIS genes act before fertilization in the female gametophyte and the phenotype observed after fertilization is a consequence of the missing expression in the female gametophyte. The first hypothesis has been tested and it has been found that only the maternal alleles of MEA and FIS2 are expressed in the endosperm, whereas the paternal alleles are silenced throughout seed development; therefore, MEA and FIS2 are regulated by genomic imprinting (Vielle-Calzada et al, 1999; Luo et al, 2000; Kinoshita et al, 1999; Jullien et al, 2006a).
Imprinting of MEA is established by two, probably independent, mechanisms. The paternal MEA allele is decorated with repressive histone methylation on H3Lys27 (Gehring et al, 2006) and becomes reactivated in the endosperm of fis mutants (Baroux et al, 2006; Gehring et al, 2006; Jullien et al, 2006b). The mechanism that prevents recruitment of the FIS complex to the maternal MEA allele is unknown. One additional distinctive feature of MEA endosperm alleles is DNA hypermethylation of the paternal MEA allele, mediated by DNA METHYLTRANSFERASE 1 (MET1; Gehring et al, 2006). MET1 targets methylation at CG residues in the MEA promoter as well as in the 3′ untranslated region. The DNA glycosidase DEMETER (DME) is specifically expressed within the central cell and excises 5-methylcytosine from the maternal MEA alleles, establishing a parent-of-origin specific methylation pattern (Choi et al, 2002; Gehring et al, 2006). DME activity in the central cell is necessary for MEA activation, which provides a link between DNA methylation and MEA imprinting (Choi et al, 2002). It is possible that the presence of DNA methylation at the paternal MEA allele is the distinctive mark that specifically recruits the FIS PcG complex to the paternal MEA endosperm allele. However, even the paternal MEA allele contributed by a met1 mutant is not reactivated in the endosperm, although in this background DNA methylation is mostly lacking (Gehring et al, 2006). Thus, it is unlikely that DNA methylation of the paternal MEA allele provides the distinguishing marks for recruiting the FIS complex. Nonetheless, the importance of DNA methylation for the regulation of imprinted genes is supported by the observation that repression of the paternal alleles of FWA and FIS2 is mediated by DNA methylation. In met1 mutants the paternal alleles of FWA and FIS2 are expressed, and expression of the maternal FWA and FIS2 alleles depends on DME activity in the female gametophyte (Kinoshita et al, 2004; Jullien et al, 2006a). Thus, paternal imprinting of MEA, FWA and FIS2 requires DME-mediated DNA demethylation to activate maternal alleles, whereas silencing of paternal alleles requires either DNA methylation (FWA and FIS2) or histone methylation on H3Lys27 (MEA; Fig 1).
Figure 1.
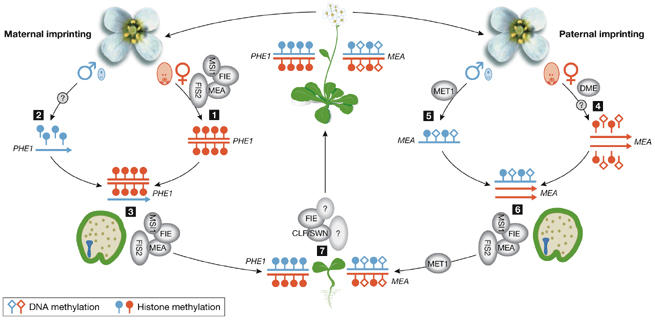
Model of molecular mechanisms controlling maternal and paternal imprinting in Arabidopsis. The maternally imprinted gene PHERES 1 (PHE1; red) is repressed in the female gametophyte by the FERTILIZATION INDEPENDENT SEED (FIS) Polycomb group (PcG) complex, which contains MEDEA (MEA), FERTILIZATION INDEPENDENT ENDOSPERM (FIE), FIS2 and MULTI-COPY SUPPRESSOR OF IRA1 (MSI1) and applies methylation on histone H3 lysine 27 (H3Lys27; 1). From the active paternal PHE1 allele (blue) H3Lys27 methylation needs to be removed, but it is not known whether this is an active or passive process (2). Repression of the maternal PHE1 allele in the endosperm is maintained by the FIS complex (3). In the central cell of the female gametophyte, DEMETER (DME) actively erases DNA methylation from the maternal allele (red) of the paternally imprinted gene MEA (4). It is not known whether repressive H3Lys27 methylation is actively erased from the maternal MEA allele as well. The paternal MEA allele (blue) is methylated by DNA METHYLTRANSFERASE 1 (MET1). Whether repressive histone methylation is maintained on the paternal allele is unknown (5). Repression of the paternal MEA allele in the endosperm is maintained by the FIS complex (6). In vegetative tissues, the silent state of both genes, PHE1 and MEA is maintained by H3Lys27 methylation (7), which depends on PcG complexes containing CURLY LEAF (CLF), SWINGER (SWN) and FIE.
PHERES 1 is regulated by parental imprinting
Microarray expression profiling of fis mutants and chromatin immunoprecipitation (ChIP) experiments identified the type I MADS-box gene PHERES 1 (PHE1) as a direct target gene of the FIS PcG complex (Köhler et al, 2003b). The paternal PHE1 allele is readily expressed in the developing embryo and endosperm, whereas the maternal PHE1 allele is silent or only weakly expressed. Mutations in mea cause increased expression of the maternal PHE1 allele, but have little effect on the activity of the paternal PHE1 allele. This suggests that the FIS complex specifically represses the maternal PHE1 allele (Köhler et al, 2005). The PHE1 locus is decorated with repressive H3Lys27 tri-methylation marks, which accumulate most strongly before fertilization (Makarevich et al, 2006). Therefore, it is likely that the FIS complex establishes PHE1 imprinting by the methylation of histones at the maternal PHE1 allele in the female gametophyte. Notably, the FIS genes continue to be expressed during early seed development (Luo et al, 2000), indicating that a functional FIS complex is also present after fertilization. It is not yet known which mechanism prevents the FIS complex from silencing the paternal PHE1 allele after fertilization.
Imprinting has evolved independently in mammals and flowering plants (reviewed by Haig & Westoby, 1989). It has been hypothesized that one of the main forces for the evolution of imprinted genes is the conflict over resource allocation from the mother to the many developing offspring. In both mammals and flowering plants, the mother provides all the nutrients to the offspring without substantial paternal contribution. Therefore, evolution would favour the expression of growth-promoting genes when paternally contributed and silencing of these genes when maternally contributed (reviewed by Haig & Westoby, 1989; Tilghman, 1999). PHE1 is an imprinted gene, but whether the PHE1 transcription factor has a function in resource allocation from the mother to the developing seed is unknown. Similarly, the imprinted genes MEA and FIS2 have not been shown to regulate maternal resource allocation; however, the endosperm overproliferation in mea and fis2 mutants would be consistent with this role. Interestingly, some autosomal imprinted genes and X-chromosome inactivation in mammals are also regulated by PcG proteins (Wang et al, 2001; Mager et al, 2003; Silva et al, 2003). Therefore, although imprinting has evolved independently in mammals and flowering plants, evolutionarily conserved PcG complexes have been recruited for the regulation of imprinted genes in both taxa.
Impact of imprinted genes on seed development
In mammals, embryos containing only maternally or only paternally derived genomes do not complete development, suggesting that functions specific to each of the maternal and paternal genomes are required (reviewed by Haig & Westoby, 1989). By contrast, several angiosperm species can reproduce asexually and form viable maternal embryos without paternal contribution (reviewed by Köhler & Grossniklaus, 2005). However, many asexually derived embryos require a sexual endosperm to complete development (Koltunow, 1993), indicating that imprinted genes are acting mainly in the endosperm. Support for this hypothesis comes from studies in maize, in which gene expression dependent on the parent-of-origin has been shown to occur in the endosperm (reviewed by Köhler & Grossniklaus, 2005). Furthermore, interploidy crosses in Arabidopsis affect endosperm development more than embryo development (Scott et al, 1998). Seeds from crosses of a diploid mother plant with a tetraploid father contain an overproliferated endosperm with an increased number of nuclei. By contrast, seeds from the reciprocal cross contain endosperm with fewer nuclei (Scott et al, 1998). Thus, an overdose of paternally derived genes (paternal excess) enhances growth, whereas an overdose of maternally derived genes (maternal excess) restricts growth, supporting the predictions proposed by Haig & Westoby (1989). Furthermore, imprinting of MEA occurs specifically in the endosperm and MEA is biparentally expressed in the embryo, at least from the torpedo stage onwards (Kinoshita et al, 1999). The specific function of MEA and other FIS genes in the endosperm is evident from the phenotype of fis mutants, which develop highly overproliferated chalazal endosperm domains (Kiyosue et al, 1999; Ingouff et al, 2005), suggesting that the FIS complex represses the maternal allele of paternally expressed genes acting in the endosperm. This hypothesis is fully supported by the observed regulation of PHE1, which is a paternally expressed, maternally repressed FIS target gene. However, it is not yet known whether PHE1 imprinting occurs specifically in the endosperm.
DNA methylation regulates imprinted genes in plants
As discussed above, it is likely that one of the main functions of the FIS PcG complex is the imprinting of paternally expressed genes in the endosperm. In fis mutants, loss of imprinting and increased expression of these genes confers the endosperm overgrowth phenotype. Similarly, at least one cluster of imprinted genes in the mammalian placenta is regulated by PRC2-mediated histone methylation, and it has been proposed that regulation of imprinting by PRC2 is an ancient mechanism acting in this organ (Lewis et al, 2004; Umlauf et al, 2004). Imprinting by PRC2 and histone methylation is not particularly stable, but as the placenta is short-lived, stable silencing might not be necessary. By contrast, stable imprinting of genes in the embryo might have been achieved by the additional recruitment of DNA methyltransferases (Lewis et al, 2004).
Similar to the placenta, the endosperm in plants is short-lived. Therefore, imprinting in the endosperm does not need to be particularly stable. This is indeed the case for PHE1, for which the ‘silent' maternal allele is weakly transcribed (Köhler et al, 2005). However, there is evidence that DNA methylation has a substantial role in the regulation of imprinted genes in the endosperm. Seeds derived from a hypomethylated pollen donor have an underproliferated endosperm and resemble seeds with a maternal excess derived from a 4n × 2n interploidy cross. The opposite phenotype is obtained when a hypomethylated mother plant is pollinated with wild-type pollen. Here, the endosperm overproliferates and seeds phenocopy paternal excess phenotypes obtained after 2n × 4n crosses (Adams et al, 2000). These results suggest that hypomethylation activates the expression of imprinted genes. In hypomethylated pollen, paternally silenced genes would be reactivated causing a maternal excess phenotype, whereas in hypomethylated female gametes maternally silenced genes would be reactivated causing a paternal excess phenotype (Adams et al, 2000; Fig 2). Further support for this hypothesis comes from experiments showing that hypomethylated pollen can rescue heterozygous fie mutant seeds (Vinkenoog et al, 2000). As discussed earlier, the phenotype of fis mutants might be caused by the de-repression of maternally silenced genes. If paternally silenced genes are reactivated in hypomethylated pollen, the paternal excess phenotype in fie mutants might be alleviated. On the basis of these results, it is possible that imprinting in the endosperm involves DNA methylation. Whether the regulation of imprinted loci also involves histone methylation remains to be determined. In mammals, mutants deficient for EED—the mammalian FIE homologue—show alterations of the DNA methylation status at several imprinted loci that are associated with a loss of imprinting (Mager et al, 2003). Given that EZH2—the interaction partner of EED—interacts with DNA methyltransferases and is required for targeting methyltransferases to EZH2-regulated promoters (Vire et al, 2006), there might be a direct link between PcG-mediated imprinting and DNA methylation.
Figure 2.
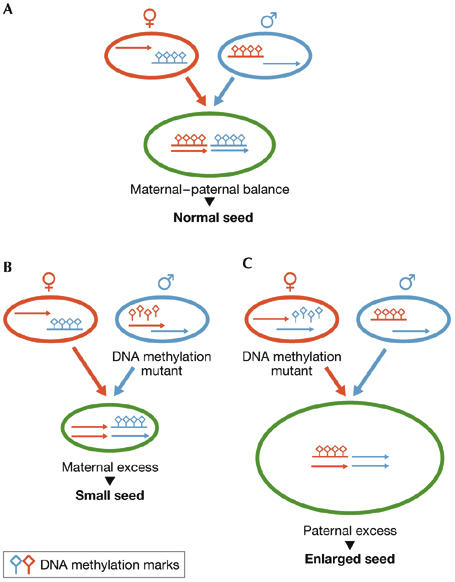
Mutations in the DNA methylation maintenance system affect the maternal–paternal genome balance and seed size. (A) In female gametes (red), maternally expressed genes (red arrow), which often restrict seed growth, are hypomethylated and active whereas paternally expressed genes (blue), which often promote seed growth, are methylated and silenced. Reciprocally, in male gametes (blue), growth-promoting paternally expressed genes (blue arrow) are hypomethylated and active, whereas growth-restricting maternally expressed genes (red) are methylated and silenced. After fertilization, differential allele-specific gene expression persists in seeds (green), resulting in balanced maternal and paternal gene expression and normal-sized seeds. (B) If DNA methylation is impaired in male gametes, genes that are normally paternally silenced become active, causing excess expression of growth-restricting genes in seeds and decreased seed size. (C) If DNA methylation is impaired in female gametes, genes that are normally maternally silenced become active, causing excess expression of growth-promoting genes in seeds and increased seed size.
Concluding remarks and open questions
Results that have accumulated in recent years strongly support the idea that imprinted genes regulate endosperm development in flowering plants, and that imprinting in the endosperm is regulated by the FIS PcG complex and DNA methylation. How these processes are interconnected is still unknown and identifying the targeting information for PcG proteins and DNA methyltransferases will be the subject of future investigations. Finally, to understand why imprinting has evolved in flowering plants, it will be necessary to identify more genes that are regulated by imprinting and to uncover whether these genes regulate endosperm development.

Claudia Köhler

Grigory Makarevich
Acknowledgments
Due to space constraints, much worthwhile work could not be cited. Research in the Köhler lab is supported by grants from the Swiss National Science Foundation, the Swiss Federal Institute of Technology Zurich (ETH Zurich) and the EMBO Young Investigator Programme.
References
- Adams S, Vinkenoog R, Spielman M, Dickinson HG, Scott RJ (2000) Parent-of-origin effects on seed development in Arabidopsis thaliana require DNA methylation. Development 127: 2493–2502 [DOI] [PubMed] [Google Scholar]
- Bantignies F, Cavalli G (2006) Cellular memory and dynamic regulation of Polycomb group proteins. Curr Opin Cell Biol 18: 275–283 [DOI] [PubMed] [Google Scholar]
- Baroux C, Gagliardini V, Page DR, Grossniklaus U (2006) Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20: 1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Zhang Y (2004) The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev 14: 155–164 [DOI] [PubMed] [Google Scholar]
- Chanvivattana Y, Bishopp A, Schubert D, Stock C, Moon YH, Sung ZR, Goodrich J (2004) Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131: 5263–5276 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 94: 4223–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Gehring M, Johnson L, Hannon M, Harada JJ, Goldberg RB, Jacobsen SE, Fischer RL (2002) DEMETER, a DNA glycosylase domain protein, is required for endosperm gene imprinting and seed viability in Arabidopsis. Cell 110: 33–42 [DOI] [PubMed] [Google Scholar]
- Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL (2006) DEMETER DNA glycosylase establishes MEDEA Polycomb gene self-imprinting by allele-specific demethylation. Cell 124: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus U, Vielle-Calzada JP, Hoeppner MA, Gagliano WB (1998) Maternal control of embryogenesis by MEDEA, a Polycomb group gene in Arabidopsis. Science 280: 446–450 [DOI] [PubMed] [Google Scholar]
- Guitton AE, Berger F (2005) Loss of function of MULTICOPY SUPPRESSOR OF IRA 1 produces nonviable parthenogenetic embryos in Arabidopsis. Curr Biol 15: 750–754 [DOI] [PubMed] [Google Scholar]
- Guitton AE, Berger F (2005) Control of reproduction by Polycomb group complexes in animals and plants. Int J Dev Biol 49: 707–716 [DOI] [PubMed] [Google Scholar]
- Guitton AE, Page DR, Chambrier P, Lionnet C, Faure JE, Grossniklaus U, Berger F (2004) Identification of new members of FERTILIZATION INDEPENDENT SEED Polycomb group pathway involved in the control of seed development in Arabidopsis thaliana. Development 131: 2971–2981 [DOI] [PubMed] [Google Scholar]
- Haig D, Westoby M (1989) Parent specific gene expression and the triploid endosperm. Am Nature 134: 147–155 [Google Scholar]
- Ingouff M, Haseloff J, Berger F (2005) Polycomb group genes control developmental timing of endosperm. Plant J 42: 663–674 [DOI] [PubMed] [Google Scholar]
- Jullien PE, Kinoshita T, Ohad N, Berger F (2005) Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18: 1360–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien PE, Katz A, Oliva M, Ohad N, Berger F (2005) Polycomb group complexes self-regulate imprinting of the Polycomb group gene MEDEA in Arabidopsis. Curr Biol 16: 486–492 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Yadegari R, Harada JJ, Goldberg RB, Fischer RL (1999) Imprinting of the MEDEA Polycomb gene in the Arabidopsis endosperm. Plant Cell 11: 1945–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Miura A, Choi Y, Kinoshita Y, Cao X, Jacobsen SE, Fischer RL, Kakutani T (2004) One-way control of FWA imprinting in Arabidopsis endosperm by DNA methylation. Science 303: 521–523 [DOI] [PubMed] [Google Scholar]
- Kiyosue T et al. (1999) Control of fertilization-independent endosperm development by the MEDEA Polycomb gene in Arabidopsis. Proc Natl Acad Sci USA 96: 4186–4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Grossniklaus U (2005) Seed development and genomic imprinting in plants. Prog Mol Subcell Biol 38: 237–262 [DOI] [PubMed] [Google Scholar]
- Köhler C, Hennig L, Bouveret R, Gheyselinck J, Grossniklaus U, Gruissem W (2003) Arabidopsis MSI1 is a component of the MEA/FIE Polycomb group complex and required for seed development. EMBO J 22: 4804–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Hennig L, Spillane C, Pien S, Gruissem W, Grossniklaus U (2003) The Polycomb-group protein MEDEA regulates seed development by controlling expression of the MADS-box gene PHERES1. Genes Dev 17: 1540–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Page DR, Gagliardini V, Grossniklaus U (2005) The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37: 28–30 [DOI] [PubMed] [Google Scholar]
- Koltunow AM (1993) Apomixis: embryo sacs and embryos formed without meiosis or fertilization in ovules. Plant Cell 5: 1425–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Mitsuya K, Umlauf D, Smith P, Dean W, Walter J, Higgins M, Feil R, Reik W (2004) Imprinting on distal chromosome 7 in the placenta involves repressive histone methylation independent of DNA methylation. Nat Genet 36: 1291–1295 [DOI] [PubMed] [Google Scholar]
- Lund AH, van Lohuizen M (2004) Polycomb complexes and silencing mechanisms. Curr Opin Cell Biol 16: 239–246 [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Koltunow A, Dennis ES, Peacock WJ, Chaudhury AM (1999) Genes controlling fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 96: 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A (2000) Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci USA 97: 10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager J, Montgomery ND, de Villena FP, Magnuson T (2003) Genome imprinting regulated by the mouse Polycomb group protein EED. Nat Genet 33: 502–507 [DOI] [PubMed] [Google Scholar]
- Makarevich G, Leroy O, Akinci U, Schubert D, Clarenz O, Goodrich J, Grossniklaus U, Köhler C (2006) Different Polycomb group complexes regulate common target genes in Arabidopsis. EMBO Rep 7: 947–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N, Margossian L, Hsu Y-C, Williams C, Repetti P, Fischer RL (1996) A mutation that allows endosperm development without fertilization. Proc Natl Acad Sci USA 93: 5319–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad N, Yadegari R, Margossian L, Hannon M, Michaeli D, Harada JJ, Goldberg RB, Fischer RL (1999) Mutations in FIE, a WD Polycomb group gene, allow endosperm development without fertilization. Plant Cell 11: 407–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RJ, Spielman M, Bailey J, Dickinson HG (1998) Parent-of-origin effects on seed development in Arabidopsis thaliana. Development 125: 3329–3341 [DOI] [PubMed] [Google Scholar]
- Silva J, Mak W, Zvetkova I, Appanah R, Nesterova TB, Webster Z, Peters AH, Jenuwein T, Otte AP, Brockdorff N (2003) Establishment of histone H3 methylation on the inactive X chromosome requires transient recruitment of Eed–Enx1 Polycomb group complexes. Dev Cell 4: 481–495 [DOI] [PubMed] [Google Scholar]
- Tilghman SM (1999) The sins of the fathers and mothers: genomic imprinting in mammalian development. Cell 96: 185–193 [DOI] [PubMed] [Google Scholar]
- Umlauf D, Goto Y, Cao R, Cerqueira F, Wagschal A, Zhang Y, Feil R (2004) Imprinting along the Kcnq1 domain on mouse chromosome 7 involves repressive histone methylation and recruitment of Polycomb group complexes. Nat Genet 36: 1296–1300 [DOI] [PubMed] [Google Scholar]
- Vielle-Calzada JP, Thomas J, Spillane C, Coluccio A, Hoeppner MA, Grossniklaus U (1999) Maintenance of genomic imprinting at the Arabidopsis MEDEA locus requires zygotic DDM1 activity. Genes Dev 13: 2971–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkenoog R, Spielman M, Adams S, Fischer RL, Dickinson HG, Scott RJ (2000) Hypomethylation promotes autonomous endosperm development and rescues postfertilization lethality in fie mutants. Plant Cell 12: 2271–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vire E et al. (2006) The Polycomb group protein EZH2 directly controls DNA methylation. Nature 439: 871–874 [DOI] [PubMed] [Google Scholar]
- Wang J, Mager J, Chen Y, Schneider E, Cross JC, Nagy A, Magnuson T (2001) Imprinted X inactivation maintained by a mouse Polycomb group gene. Nat Genet 28: 371–375 [DOI] [PubMed] [Google Scholar]


