Abstract
Anaerobic reductive dehalogenation by Dehalococcoides spp. is an ideal system for studying functional diversity of closely related strains of bacteria. In Dehalococcoides spp., reductive dehalogenases (RDases) are key respiratory enzymes involved in the anaerobic detoxification of halogenated compounds at contaminated sites globally. Although housekeeping genes sequenced from Dehalococcoides spp. are >85% identical at the amino acid level, different strains are capable of dehalogenating diverse ranges of compounds, depending largely on the suite of RDase genes that each strain harbors and expresses. We identified RDase proteins that corresponded to known functions in four characterized cultures and predicted functions in an uncharacterized Dehalococcoides-containing mixed culture. Homologues within RDase subclusters containing PceA, TceA, and VcrA were among the most frequently identified proteins. Several additional proteins, including a formate dehydrogenase-like protein (Fdh), had high coverage in all strains and under all growth conditions.
Comparative genomic studies have revealed that many of the phenotypic differences observed among closely related microbial species in nature are due to genetic islands of diversity that are frequently copied, rearranged, and laterally transferred. Pathogenicity islands, which are mobile genetic elements that confer virulence, are well known in host-associated microorganisms (8). A recent comparative genomic study of the marine photoautotroph Prochlorococcus suggests that natural populations of microorganisms may also contain genetic islands that confer unique phenotypic traits on closely related strains (3). A comparative genomic study of representatives from the Dehalococcoides lineage within the Chloroflexi phylum of bacteria, a group which reductively dehalogenates chlorinated organic pollutants (25), suggests that reductive dehalogenases (RDases) are key enzymes conferring functional differences on closely related strains of Dehalococcoides (14).
Sequenced Dehalococcoides genomes (strains 195, CBDB1, and BAV1 [unfinished]) share a high degree of genomic similarity and synteny in nearly all “housekeeping” genes. There are, however, differences in the total numbers and types of RDases that these strains harbor and in their corresponding substrate ranges (1, 2, 14, 17). In Dehalococcoides ethenogenes (strain 195), RDases are responsible for reductive dechlorination of chlorinated organic compounds, such as the common groundwater contaminants and suspected human carcinogens tetrachloroethene (PCE) and trichloroethene (TCE). In strains 195 and CBDB1, the majority of putative RDase genes are in clusters located near the predicted origin of replication, and while the locations of the clusters are similar, the RDase gene contents are different. The complete genome sequence of strain 195 revealed 19 potential RDase genes, 4 of which were contained within putative integrated mobile genetic elements that may have been acquired recently or are marked for dissemination (23). Thirty-two putative RDases were identified in the complete genome sequence of strain CBDB1, eight of which were located within presumed mobile genetic elements (14). These data suggest that RDases represent genetic islands in Dehalococcoides, the number and types of which determine dehalogenase phenotype.
Although RDase diversity suggests that Dehalococcoides spp. have the potential to degrade a broad range of chlorinated compounds, little or nothing is known about the substrate range of most RDases or about the suite of RDases that are transcribed and translated during reductive dehalogenation. This is largely because relatively few electron acceptors have been found and because cultured Dehalococcoides have complex nutritional requirements, grow to relatively low cell densities, and are not easily amenable to genetic and biochemical studies. Of the >90 RDase gene sequences that have been recovered from Dehalococcoides, only TceA from strain 195 and VcrA from strain VS, which catalyze the reductive dechlorination of TCE to vinyl chloride (VC) and VC to ethene, respectively, have been well characterized (10, 14, 15, 20, 23). As a result, it is of interest to identify specific RDases that are transcribed and translated during reductive dehalogenation.
Some studies have reported patterns of RDase expression and activity under different growth conditions (12, 28; J. Fung, unpublished data). These studies suggest that some RDases may dehalogenate multiple substrates and that multiple RDases may be required to dehalogenate a single polychlorinated substrate. In a preliminary study of oxidoreductase transcript levels and proteins detected in pure and mixed cultures of strain 195, highly expressed RDases, such as PceA, which reduces PCE (J. Magnuson, personal communication), and TceA, which reduces TCE and its daughter products, were detected only in pure cultures (19). Here we improve Dehalococcoides protein enrichments to compare RDases, putative electron-transporting oxidoreductases, and other membrane-associated proteins from five Dehalococcoides-containing cultures, including three mixed cultures. Specifically, we targeted proteins of strain 195 growing on PCE in pure and mixed cultures, proteins of strain CBDB1 growing on 2,3-dichlorophenol (2,3-DCP) in pure culture, and proteins of two Dehalococcoides-containing mixed enrichment cultures that efficiently converted TCE to ethene. These enrichment cultures included KB1, a well-characterized culture (4, 28) used for bioaugmentation at chloroethene-contaminated sites (16), and a culture from the DOE Environmental Management, Soil, and Groundwater Closure Project at the Savannah River National Laboratory (SRNL), in which Dehalococcoides spp. have been identified but not characterized (see Materials and Methods). We identified multiple peptides from subclusters of RDases that corresponded to known functions in characterized cultures and predicted functions in the SRNL enrichment culture. Other putative electron-transporting oxidoreductases, structural proteins, chaperonins, and hypothetic proteins were also identified and had high protein coverage in all cultures and under all growth conditions. Liquid chromatography-tandem mass spectrometry (LC-MS-MS) peptide detection and RDase discrimination in different strains and under pure and mixed growth conditions suggest a comparative proteomics application for the environmentally important process of reductive dehalogenation.
MATERIALS AND METHODS
Strain 195 pure- and mixed-culture growth conditions.
PCE-dechlorinating pure cultures of Dehalococcoides strain 195 were grown in 400-ml amounts in 1-liter bottles sealed with Teflon-coated butyl rubber stoppers as previously described (17). The culture was pelleted and stored at 4°C prior to protein extraction. Direct cell counts obtained using the nucleic acid dye DAPI (4′,6′-diamidino-2-phenylindole) indicated that the concentration of Dehalococcoides cells was approximately 7.5 × 107 cells/ml (unpublished data). A PCE-dechlorinating mixed culture of Dehalococcoides strain 195 with butyrate as the electron donor was maintained as previously described (6, 21). Approximately 400 ml of culture was pelleted and stored at 4°C prior to protein extraction.
CBDB1 growth conditions.
2,3-DCP-dechlorinating cultures of Dehalococcoides CBDB1 were maintained in a basal medium previously described (1, 2). Briefly, the culture inoculum size was 2% (vol/vol) in a 1,000-ml incubation container containing 400 ml of growth medium. Incubation containers were sealed with Teflon-coated butyl rubber stoppers, incubated at 35°C, and given successive doses of 2,3-DCP and H2. Approximately 400 ml of culture was pelleted and stored at 4°C prior to protein extraction. Direct cell counts obtained using DAPI indicated that the concentration of Dehalococcoides cells was approximately 2.5 × 106 cells/ml.
KB1 growth conditions.
TCE-dechlorinating cultures enriched for Dehalococcoides were maintained as previously described (4). Briefly, the culture inoculum size was 20% (vol/vol) in three 120-ml incubation containers containing 100 ml of growth medium (5). Incubation containers were sealed with Teflon-coated butyl rubber stoppers, incubated at 35°C, and fed successive doses of TCE and methanol. Approximately 300 ml of culture was pelleted and stored at 4°C prior to protein extraction.
SRNL enrichment and growth conditions.
The SRNL enrichment culture was developed using sediment from a wetland where a plume of TCE-contaminated groundwater emerges. Field data indicate that complete dechlorination of TCE to ethene and ethane occurs in the wetland. Samples from microcosms that actively dechlorinated cis-DCE and VC exhibited strong positive signals for Dehalococcoides in 16S rRNA-based PCR assays, but little is known about the specific strains present. Samples from the microcosms were transferred into a medium described by Edwards and Grbic-Galic (5), with minor modifications (the phosphate buffer solution was made with 52.5 g K2HPO4 per liter; the bicarbonate solution was prepared with 16 g NaHCO3 per liter, and 50 ml was added per liter; the vitamin solution was replaced with 10 ml of a 5.0-g/liter solution of filter-sterilized yeast extract; and the headspace was purged with a 70% N2-30% CO2 gas mixture). Sodium lactate was used as the electron donor for the enrichment. After consuming repeated additions of TCE, the culture was transferred again and grown in triplicate 2.5-liter bottles. The amounts of TCE and PCE added with each feeding were increased to approximately 40 mg/liter and 8 mg/liter, respectively. The enrichment was maintained at 22 to 24°C under quiescent conditions. Samples of the SRNL enrichment culture have proven effective in a microcosm test of bioaugmentation for a TCE and PCE plume at the site that did not respond to biostimulation alone (unpublished data).
Protein extractions.
Cell pellets were prepared from approximately 300 to 400 ml of culture media by centrifugation for 30 min at 17,000 × g. The supernatant was discarded, and cell pellets were stored at −20°C. Pellets were resuspended in 3 ml of 20 mM Tris buffer, pH 7.4. Large particles and attached cells were removed from mixed cultures to enrich for Dehalococcoides by gravity separation for 10 to 20 min. Crude extracts were prepared by passing the cells through a French pressure cell at 8,000 lb/in2 and subsequent centrifugation for 10 min at 8,000 × g to 10,000 × g. Membrane-enriched material for proteomic analyses was obtained by transfer of 1.5 ml of the lysate fraction to new microcentrifuge tubes and centrifugation for 60 min at 104,000 × g. Membrane-enriched proteins were solubilized in 30 μl of 0.1% dodecylmaltoside in 20 mM Tris, pH 7.4, and approximately 5 to 10 μg of protein from strain 195, KB1, and SRNL cultures or <1 μg of protein from CBDB1 cultures was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were divided into eight (strain 195), six (KB1), or four (CBDB11 and SRNL) sections containing protein bands from 20 to 120 kDa. Individual gel sections were placed in 1.5-ml microcentrifuge tubes each and stored at −20°C. In-gel digestion and tryptic peptide extractions were performed on gel slices following a protocol modified from Shevchenko and colleagues (24). Gel-extracted supernatants were combined and evaporated to dryness by a Speedvac.
Nano-LC-MS-MS.
Peptide samples were reconstituted in 15 μl of 0.1% formic acid with 2% acetonitrile prior to MS analyses. Nano-LC was carried out with an LC Packings UltiMate integrated capillary high-performance liquid chromatography system equipped with a Switchos valve switching unit (Dionex, Sunnyvale, CA). The gel-extracted peptides (6.4 μl) were injected using a Famous auto sampler onto a C18 PepMap100 trap column for on-line desalting and then separated on a PepMap C18 RP nanocolumn and eluted in a 60-min gradient of 5% to 45% acetonitrile in 0.1% formic acid at 250 nl/min. The nano-LC was connected in-line to a hybrid triple quadrupole linear ion trap mass spectrometer, 4000 Q Trap from ABI/MDS Sciex (Framingham, MA), equipped with a micro ion spray head ion source.
MS data acquisition was performed using Analyst 1.4.1 software (Applied Biosystems) in the positive ion mode for information-dependent acquisition (IDA) analysis. The nanospray voltage was 2.0 kV for all experiments in positive ion mode. Nitrogen was used as the curtain (value of 10) and collision gas (set to high) with heated interface on. The declustering potential was set at 50 eV, and Gas1 was 15 lb/in2. In IDA analysis, after each survey scan for m/z 400 to m/z 1,550 and an enhanced resolution scan, the three highest-intensity ions with multiple charge states were selected for MS-MS, with rolling collision energy applied for detected ions based on different charge states and m/z values.
Protein identification.
MS-MS data generated from nano-LC/electrospray ionization-based IDA analyses were interrogated using ProID 1.4 (Applied Biosystems) for database searching against Dehalococcoides strain 195, CBDB1, or composite (195, CBDB1, and sequenced RDases) databases, which were created to interrogate strain KB1 and SRNL mass spectra. One trypsin miscleavage, the carbobamidomethyl modification of cysteine, and a methionine oxidation were used for searching. Protein identification was limited to at least one peptide with a Pro Group confidence score of >95 (ProtScore threshold, 1.3) and at least one additional peptide with a Pro Group confidence score of >20. Protein sequence coverage was calculated as the number of nonoverlapping amino acid residues from peptide fragments divided by the total number of amino acid residues from a corresponding protein. Protein abundance estimates were determined using exponentially modified protein abundance index (emPAI) values (11).
RDase phylogeny.
Eighty-nine complete or nearly complete RDase amino acid sequences from Dehalococcoides and two outgroup RDase sequences from Desulfitobacterium were aligned using ClustalX (version 1.83.1). Fifty of the putative RDases were identified in sequenced strains 195 (n = 18) and CBDB1 (n = 32), 35 were identified in the KB1, BAV1, and FL2 cultures (n = 14, n = 8, and n = 13, respectively), and four were single RDases identified in the VS, PM-VC1, PM-VC2, and YK-TCE1 cultures (10, 14, 20, 23, 28). The complete sequence alignment was 672 amino acids in length, and 253 positions remained after excluding gapped regions. Parsimony phylogenetic analysis methods were used to identify robust phylogenetic relationships within the data set and were performed with the program PAUP* 4.0 beta 10 (26). The tree topology was inferred by analyses of 239 parsimony-informative characters employing a heuristic search, tree bisection-reconnection, and a starting tree obtained by stepwise addition with random sequence addition. Bootstrap proportions from 100 and 1,000 replicate resampled data sets were used to estimate the relative confidence scores in phylogenetic groups and were determined using parsimony and neighbor-joining methods, respectively. The tree was rooted with outgroup RDase sequences from Desulfitobacterium chlororespirans and Desulfitobacterium hafniense.
RESULTS
Proteins detected in pure and mixed cultures of strain 195.
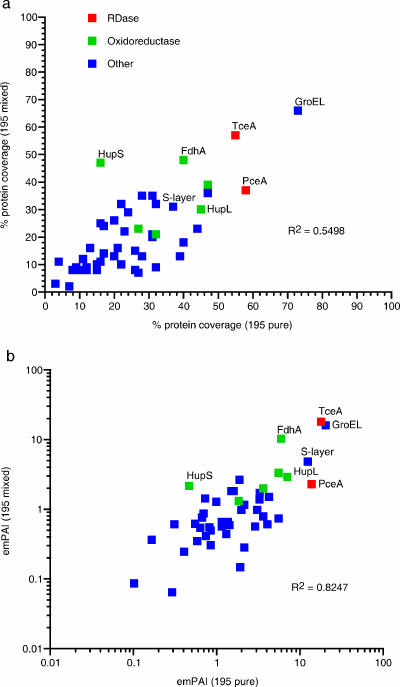
Proteomic analyses of membrane-enriched fractions from pure and mixed cultures of strain 195 were compared to determine the feasibility of extracting and detecting Dehalococcoides RDases and other membrane-associated proteins from mixed communities. Sixty-seven proteins were identified from pure cultures, and 73 proteins were identified from mixed cultures. Seventy percent of the proteins identified in pure cultures were also identified in mixed cultures (Fig. 1), including TceA and PceA RDases, subunits of the predicted Hup [Ni/Fe]-hydrogenase ([Ni/Fe]-H2ase), and gene products annotated as cochaperonin GroEL and formate dehydrogenase (Fig. 1a and b). Complete lists of each protein, plotted by an emPAI and listed by locus tag, are given in Fig. S1 and Table S1 in the supplemental material. These genes were highly expressed in quantitative PCR studies, and/or their products had high percent protein coverage in a previous study of respiratory oxidoreductases (19). Pure- and mixed-culture abundance estimates exhibited a strong linear relationship for both percent coverage and emPAI values (Fig. 1a and b), which suggests that sequence coverage can be used as a general estimate of relative abundance. emPAI values, however, have previously been used to estimate protein concentrations in LC-MS-MS experiments (11). In our experiments, TceA, HupL, and putative S-layer proteins all showed significant increases in estimates of protein abundance relative to those suggested by percent protein coverage alone.
FIG. 1.
Comparison of Dehalococcoides strain 195 proteins detected in both pure- and mixed-culture membrane-enriched cell fractions by percent sequence coverage (a) and protein abundance (b). RDases, putative oxidoreductases, and other frequently identified proteins with high percent sequence coverage and/or high abundance are labeled. Percent protein sequence coverage was determined using unique nonoverlapping tryptic peptide sequences recovered from each culture. Protein abundance (emPAI) was calculated as described by Ishihama and colleagues (11).
Two uncharacterized RDases, DET1545 and DET1559, and several other putative respiratory oxidoreductases were detected only in either pure- or mixed-culture membrane-enriched cell fractions (see Fig. S2a and b in the supplemental material, respectively). Although three [Fe]-H2ase (Hym) subunits and a single NADH ubiquinone oxidoreductase (Nuo) subunit were detected only in cells grown under mixed-culture conditions, inclusion of proteins identified by a single peptide with >95% confidence suggests that the Hym [Fe]-H2ase and Nuo NADH ubiquinone oxidoreductase were also present in pure cultures (see Table S2 in the supplemental material). Low detection may have resulted from a combination of factors, including low protein abundance, differences in the protein compositions of similar but not identical sectioning of gels, interference from non-Dehalococcoides proteins in mixed culture, and strict identification criteria used for these analyses.
CBDB1, KB1, and SRNL protein detection.
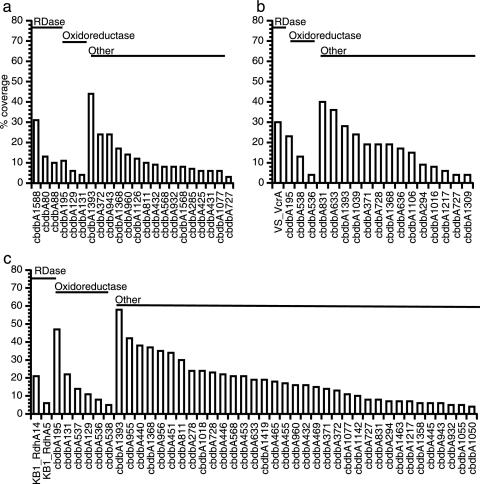
Putative RDases, oxidoreductases, and other frequently detected proteins, including cochaperonin GroEL, Fdh-like protein (Fdh), S-layer, and conserved hypothetical proteins, were also identified in CBDB1, SRNL, and KB1 cultures (Fig. 2). Proteins from KB1 and SRNL cultures, which contained unsequenced Dehalococcoides, were identified using the complete genome sequences of strains CBDB1 and 195 and all RDase sequences currently available in public databases. A comparison based on percent protein sequence coverage of homologues from CBDB1 and 195 indicated that Dehalococcoides proteins from both KB1 and SRNL cultures were more closely related to proteins from strain CBDB1 than to those from strain 195 (Table 1). emPAI estimates based on CBDB1 sequence information were similar to those reported for percent protein sequence coverage (data not shown). A complete list of CBDB1, KB1, and SRNL proteins identified by a single peptide with a confidence score of >95% is given in Table S3 in the supplemental material.
FIG. 2.
Percent protein coverage of Dehalococcoides CBDB1 (a) proteins detected in pure-culture membrane-enriched cell fractions and SRNL (b) and KB1 (c) proteins detected in mixed-culture membrane-enriched cell fractions. RDases, putative oxidoreductases, and other frequently identified proteins are organized by category and decreasing protein sequences coverage, respectively.
TABLE 1.
Percent protein sequence coverage of RDases and other Dehalococcoides proteins detected in three or more cultures
| RDase or protein (locus tag[s]) | % Protein sequence coverage
|
||||
|---|---|---|---|---|---|
| Strain 195
|
CBDB1 (pure culture) | KB1a | SRNLa | ||
| Pure culture | Mixed culture | ||||
| Reductive dehalogenases | |||||
| RDase putative PceA (DET0318, cbdbA1588) | 58 | 37 | 31 | ||
| RDase TceA (DET0079) | 55 | 57 | |||
| RDase (DET1559, cbdbA80) | 8 | 13 | |||
| RDase (DET1545, KB1 Rdha5) | 6 | 6 | |||
| RDase (cbdbA88) | 10 | ||||
| RDase (KB1 Rdha14, VcrA) | 21 | 31, 30 | |||
| Proteins detected in three or more strains | |||||
| Cochaperonin GroEL (DET1428, cbdbA1393) | 73 | 66 | 44 | 35, 58 | 25, 28 |
| Formate dehydrogenase (DET0187, cbdbA195) | 40 | 48 | 11 | 19, 47 | 2, 23 |
| Putative S-layer protein (DET1407, cbdbA1368) | 37 | 31 | 17 | 4, 37 | 4, 19 |
| Hypothetical protein (DET0754, cbdbA727) | 27 | 7 | 3 | 6, 8 | 0, 4 |
| Ketol acid reductoisomerase (DET0831, cbdbA811) | 47 | 36 | 10 | 17, 30 | |
| [Ni/Fe]-hydrogenase (DET0110, cbdbA129) | 45 | 30 | 6 | 3, 11 | |
| Myo-inositol-1-phosphate synthase (DET0979, cbdbA943) | 40 | 13 | 24 | 3, 6 | |
| Translation elongation factor (DET0997, cbdbA960) | 31 | 20 | 14 | 13, 16 | |
| GTPase domain protein (DET0589, cbdbA568) | 23 | 22 | 8 | 18, 21 | |
| Phospho-2-dehydro-3-deoxyheptonate aldolase (DET0468, cbdbA432) | 20 | 13 | 9 | 16, 16 | |
| Polyribonucleotide nucleotidyltransferase (DET0970, cbdbA932) | 9 | 8 | 4, 5 | ||
| ATP synthase F1, beta subunit (DET0564, cbdbA538) | 47 | 39 | 2, 5 | 10, 13 | |
| ATP synthase F1, alpha subunit (DET0562, cbdbA536) | 32 | 22 | 4, 8 | 2, 4 | |
| Hypothetical protein (DET0755, cbdbA728) | 31 | 21 | 10, 23 | 3, 19 | |
| SPFH domain protein (DET0848, cbdbA831) | 28 | 35 | 8, 8 | 33, 40 | |
| Serine protease (DET1285, cbdbA1217) | 26 | 8 | 3, 7 | 6, 6 | |
| Amino acid ABC transporter (DET0419, cbdbA371) | 22 | 10 | 0, 14 | 0, 19 | |
| ABC-type cobalamin/Fe3+ siderophore (DET0650, cbdbA633) | 22 | 4, 19 | 4, 36 | ||
| Hypothetical protein (DET0352, cbdbA294) | 14 | 0, 7 | 0, 9 | ||
Two values in one column entry indicate different coverages of locus tags provided at left, e.g., 31% coverage of KB1 Rdha14 and 30% coverage of VcrA in the SRNL mixed culture.
Twenty-two proteins were detected in membrane-enriched cell fractions from strain CBDB1 and were extracted from an order of magnitude fewer cells (∼1 × 109) than from similar cultures of strain 195 (∼1 × 1010), and 18 Dehalococcoides proteins were identified in similar cell fractions from the SRNL culture, which contained a Dehalococcoides strain or strains for which no sequence information was available (Fig. 2a and b, respectively). A putative PceA RDase homologue (cbdbA1588) was the second most frequently identified protein in strain CBDB1 (31% protein sequence coverage), and a homolog of RdhA14 from KB1 (28) was the third most frequently detected protein in the SRNL enrichment cultures (31% and 30% protein sequence coverage for KB1 Rdha14 and VcrA, respectively). Forty-three proteins were detected in strain KB1 membrane-enriched cell fractions, including two RDases (see below), the Hup [Ni/Fe]-H2ase, and the putative Fdh oxidoreductase (Fig. 2c).
RDase diversity.
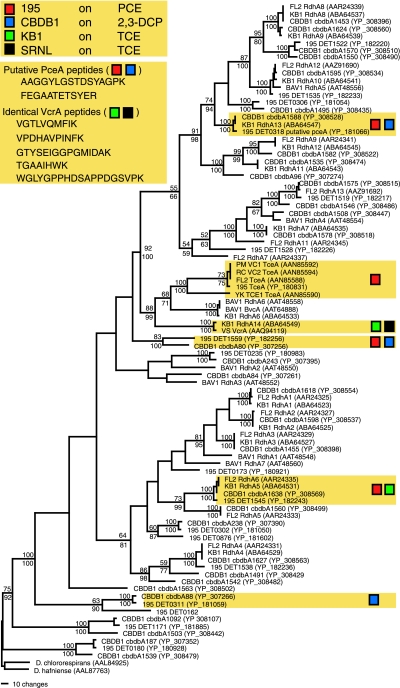
Eighty-nine complete or nearly complete RDase protein sequences from Dehalococcoides isolates or enrichment cultures containing Dehalococcoides were obtained from public databases and aligned to identify clusters of similar RDases detected by proteomic analyses (Fig. 3). Despite the extensive diversity and broad distribution observed among RDases in cultured strains, relatively few RDases were identified by LC-MS-MS proteomic analyses of these cultures (Fig. 3; Table 1). However, high protein sequence coverage was obtained for putative TceA, PceA, and VcrA homologues. TceA was detected only in pure and mixed cultures of strain 195. High sequence coverage of putative PceA homologues was found with PCE-grown pure cultures of strain 195 (DET0318, 58%) and 2,3-DCP-grown pure cultures of strain CBDB1 (cbdbA1588, 31%). Coverage of VcrA or its close homologue in KB1 (RdhA14) was high in both KB1 and the SRNL mixed cultures grown on TCE (21 and 30/31%, respectively) (Table 1). Multiple peptides from PceA and VcrA subclusters were identified in more than one strain, suggesting that these peptides may serve as RDase-specific indicators of activity (Fig. 3).
FIG. 3.
Parsimony amino acid tree showing relationships among previously described RDases from strains 195, CBDB1, KB1, BAV1, FL2, and VS and three individual Dehalococcoides RDases from VC- and TCE-dechlorinating strains. Shaded boxes indicate groups identified by proteomic analyses (in tree) and identical putative PceA and VcrA peptides detected in two or more cultures. Bootstrap proportions used to estimate the relative confidence scores in phylogenetic groups were determined using parsimony (values above nodes) and neighbor-joining (values below nodes) methods.
Protein sequence coverage was lower for RDase subclusters lacking characterized representatives. However, multiple peptides were detected from two additional RDases in strain 195 (DET1545 and DET1559) and two RDases in strain CBDB1 (cbdbA80 and cbdbA88). Phylogenetic analyses indicate that DET1559 and cbdbA80 are closely related (Fig. 3). RDase proteins from organisms other than Dehalococcoides were not identified in any of the enrichment cultures.
Comparative proteomics of “FdhA.”
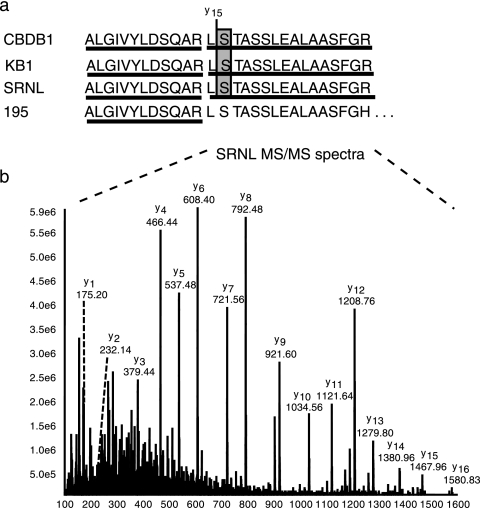
“FdhA” in strain 195 is predicted from its gene sequence to contain a serine residue at a key position in the active site (Fig. 4); all known FdhA homologues in other organisms code for either cysteine or selenocysteine at this position (19). Interestingly, expression studies indicate that “FdhA” in Dehalococcoides strain 195 is more highly expressed than RDases and H2-ases; yet, our studies indicated that formate does not donate electrons for reductive dechlorination and that cells lack formate dehydrogenase activity (19). In strain 195, the critical residue is located in a large tryptic peptide containing 56 amino acid residues with a predicted molecular weight of 5,822.4, outside the range used for these studies. However, in the predicted CBDB1 sequence for this protein, a histidine residue is replaced by an arginine, leading to a 16-amino-acid tryptic peptide (Fig. 4a) that was readily identified in this culture as well as in the KB1 culture and the uncharacterized SRNL samples (Fig. 4b), thereby confirming the identity of the serine residue in the final protein.
FIG. 4.
Putative formate dehydrogenase protein fragments and unique serine-containing tryptic peptides detected in membrane-enriched cell fractions from CBDB1, KB1, and SRNL cultures (a) and a representative MS-MS spectrum from uncharacterized Dehalococcoides in the SRNL culture (b). The molecular weight difference between product ions y15 and y14 is 87, demonstrating a serine residue in the corresponding sequence.
DISCUSSION
In this study, we identified key enzymes from an uncharacterized strain of Dehalococcoides in a mixed community by focusing on highly conserved and presumably abundant membrane-associated proteins, several of which confer dehalogenase phenotype. Although MS-MS proteomic applications are typically limited by contaminating proteins from diverse taxa present in mixed microbial communities, recent environmental proteomic studies have addressed this issue by focusing on less complex systems, such as an acid mine drainage community (22, 27), or by targeting membrane-associated proteins from abundant organisms, such as SAR11 (7, 18). Here we improved our detection of proteins by first physically separating Dehalococcoides cells from mixed cultures and then fractionating membrane proteins. We identified closely related categories of membrane-associated RDases that have been reported previously in studies of RDase diversity and expression (10, 12, 28). RDase and other proteomic data reported here indicate that key functional enzymes can be identified from uncharacterized Dehalococcoides populations present in mixed communities, that differences in Dehalococcoides RDases correspond to functional differences observed in dechlorination, and that unique peptides may serve as strain-specific indicators of Dehalococcoides activity.
In our previous studies of strain 195 (19), we were unable to verify that “FdhA” had a serine instead of cysteine or selenocysteine at a critical position as predicted by its coding sequence. A fortuitous arginine substitution in “FdhA” homologues from other cultures allowed us to unambiguously identify the serine in those cultures, demonstrating the power of comparative proteomics. The lack of formate metabolism in strain 195 cells (19) and the presence of a serine rather than cysteine or selenocysteine at the active site argue against Fdh being a formate dehydrogenase. Fdh is predicted to be periplasmic and part of a three-protein complex similar to many catabolic formate dehydrogenases, which serve as quinone reductases (23). Interestingly, it was shown recently that Dehalococcoides membranes contain a high content of ubiquinones and menaquinones, which may function as radical quenchers (29). The Fdh in Dehalococcoides may play a role in poising the oxidation/reduction state of these quinones. Regardless of their function, these highly expressed, abundant, and ubiquitous proteins may serve as excellent Dehalococcoides-specific indicators of activity.
RDases, however, represent more specific indicators of reductive dehalogenation. High coverage of the PceA and TceA RDases was observed with pure and mixed cultures of strain 195. In strain CBDB1 grown on 2,3-DCP, the highest coverage was for cbdbA1588, a close relative of PceA in strain 195. In other studies (J. M. Fung et al., unpublished), we have garnered evidence that PceA also serves to reduce 2,3-DCP in strain 195. The presence of the close homologues DET1559 in PCE-grown strain 195 and cbdbA80 in 2,3-DCP-grown CBDB1 (Fig. 3) suggests that this RDase cluster may play a role in dechlorination of these substrates, that they are coregulated with the PceA homologues, or both.
The KB1 culture contains two Dehalococcoides strains with 16S rRNA gene sequences closely related to that of CBDB1 (4), in what is called the “Pinellas” cluster (9). We detected peptides corresponding to RdhA14 and RdhA5. A survey of Dehalococcoides RDase genes in KB1 using degenerate PCR primers detected 14 RDases, although no homologues of tceA were found (28). Expression studies of cultures grown on TCE detected transcripts of rdhA14, which encodes a homologue of the ethene-producing VC dehalogenase VcrA from Dehalococcoides strain VS (14); rdhA5, a homologue of DET1545; and rdhA6, which encodes a homologue of BvcA, a VC dehalogenase from Dehalococcoides strain BAV1 (13). Detection of a single VC RDase suggests that KB1 populations may have shifted, that expression is more variable for rdhA6, or that the transcript is not translated in protein. Alternatively, rdhA6 was present but not detected.
The proteomic data reported here demonstrate that peptides from functional enzymes responsible for determining phenotype may be used to differentiate closely related strains of bacteria. Proteomic differences identified in Dehalococcoides suggested novel roles for some reductive dehalogenases and other putative respiratory enzymes and demonstrated that strain-specific functions can be detected in mixed cultures containing uncharacterized Dehalococcoides.
Supplementary Material
Acknowledgments
This study was supported by a Cornell University postdoctoral research fellowship for R. M. Morris and in part by an NSF Metabolic Biochemistry grant (MCB-0236044) to S. H. Zinder and the U.S. Department of Energy Office of Cleanup Technologies administered by the Savannah River Operations Office (contract no. DE-AC09-96SR18500).
We also thank Elizabeth Edwards and Lorenz Adrian for providing initial KB1 and CBDB1 cultures, respectively, and acknowledge the services provided by the Cornell Proteomics and Mass Spectrometry core facility.
Footnotes
Published ahead of print on 10 November 2006.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adrian, L., U. Szewzyk, J. Wecke, and H. Gorisch. 2000. Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580-583. [DOI] [PubMed] [Google Scholar]
- 2.Bunge, M., L. Adrian, A. Kraus, M. Opel, W. G. Lorenz, J. R. Andreesen, H. Gorisch, and U. Lechner. 2003. Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature 421:357-360. [DOI] [PubMed] [Google Scholar]
- 3.Coleman, M. L., M. B. Sullivan, A. C. Martiny, C. Steglich, K. Barry, E. F. DeLong, and S. W. Chisholm. 2006. Genomic islands and the ecology and evolution of Prochlorococcus. Science 311:1768-1770. [DOI] [PubMed] [Google Scholar]
- 4.Duhamel, M., K. Mo, and E. A. Edwards. 2004. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl. Environ. Microbiol. 70:5538-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards, E. A., and D. Grbic-Galic. 1994. Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl. Environ. Microbiol. 60:313-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fennell, D. E., and J. M. Gossett. 1998. Modeling the production of and competition for hydrogen in a dechlorinating culture. Environ. Sci. Technol. 32:2450-2460. [Google Scholar]
- 7.Giovannoni, S. J., L. Bibbs, J. C. Cho, M. D. Stapels, R. Desiderio, K. L. Vergin, M. S. Rappe, S. Laney, L. J. Wilhelm, H. J. Tripp, E. J. Mathur, and D. F. Barofsky. 2005. Proteorhodopsin in the ubiquitous marine bacterium SAR11. Nature 438:82-85. [DOI] [PubMed] [Google Scholar]
- 8.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 9.Hendrickson, E. R., J. A. Payne, R. M. Young, M. G. Starr, M. P. Perry, S. Fahnestock, D. E. Ellis, and R. C. Ebersole. 2002. Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl. Environ. Microbiol. 68:485-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holscher, T., R. Krajmalnik-Brown, K. M. Ritalahti, F. Von Wintzingerode, H. Gorisch, F. E. Loffler, and L. Adrian. 2004. Multiple nonidentical reductive-dehalogenase-homologous genes are common in Dehalococcoides. Appl. Environ. Microbiol. 70:5290-5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihama, Y., Y. Oda, T. Tabata, T. Sato, T. Nagasu, J. Rappsilber, and M. Mann. 2005. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell. Proteomics 4:1265-1272. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, D. R., P. K. Lee, V. F. Holmes, A. C. Fortin, and L. Alvarez-Cohen. 2005. Transcriptional expression of the tceA gene in a Dehalococcoides-containing microbial enrichment. Appl. Environ. Microbiol. 71:7145-7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajmalnik-Brown, R., T. Holscher, I. N. Thomson, F. M. Saunders, K. M. Ritalahti, and F. E. Loffler. 2004. Genetic identification of a putative vinyl chloride reductase in Dehalococcoides sp. strain BAV1. Appl. Environ. Microbiol. 70:6347-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kube, M., A. Beck, S. H. Zinder, H. Kuhl, R. Reinhardt, and L. Adrian. 2005. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat. Biotechnol. 23:1269-1273. [DOI] [PubMed] [Google Scholar]
- 15.Magnuson, J. K., M. F. Romine, D. R. Burris, and M. T. Kingsley. 2000. Trichloroethene reductive dehalogenase from Dehalococcoides ethenogenes: sequence of tceA and substrate range characterization. Appl. Environ. Microbiol. 66:5141-5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major, D. W., M. L. McMaster, E. E. Cox, E. A. Edwards, S. M. Dworatzek, E. R. Hendrickson, M. G. Starr, J. A. Payne, and L. W. Buonamici. 2002. Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ. Sci. Technol. 36:5106-5116. [DOI] [PubMed] [Google Scholar]
- 17.Maymo-Gatell, X., Y. Chien, J. M. Gossett, and S. H. Zinder. 1997. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568-1571. [DOI] [PubMed] [Google Scholar]
- 18.Morris, R. M., M. S. Rappe, S. A. Connon, K. L. Vergin, W. A. Siebold, C. A. Carlson, and S. J. Giovannoni. 2002. SAR11 clade dominates ocean surface bacterioplankton communities. Nature 420:806-810. [DOI] [PubMed] [Google Scholar]
- 19.Morris, R. M., S. Sowell, D. Barofsky, S. Zinder, and R. Richardson. 2006. Transcription and mass-spectroscopic proteomic studies of electron transport oxidoreductases in Dehalococcoides ethenogenes. Environ. Microbiol. 8:1499-1509. [DOI] [PubMed] [Google Scholar]
- 20.Muller, J. A., B. M. Rosner, G. Von Abendroth, G. Meshulam-Simon, P. L. McCarty, and A. M. Spormann. 2004. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp. strain VS and its environmental distribution. Appl. Environ. Microbiol. 70:4880-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahm, B. G., R. M. Morris, and R. E. Richardson. 2006. Temporal expression of respiratory genes in an enrichment culture containing Dehalococcoides ethenogenes. Appl. Environ. Microbiol. 72:5486-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram, R. J., N. C. VerBerkmoes, M. P. Thelen, G. W. Tyson, B. J. Baker, R. C. Blake, M. Shah, R. L. Hettich, and J. F. Banfield. 2005. Community proteomics of a natural microbial biofilm. Science 308:1915-1920. [PubMed] [Google Scholar]
- 23.Seshadri, R., L. Adrian, D. E. Fouts, J. A. Eisen, A. M. Phillippy, B. A. Methe, N. L. Ward, W. C. Nelson, R. T. Deboy, H. M. Khouri, J. F. Kolonay, R. J. Dodson, S. C. Daugherty, L. M. Brinkac, S. A. Sullivan, R. Madupu, K. E. Nelson, K. H. Kang, M. Impraim, K. Tran, J. M. Robinson, H. A. Forberger, C. M. Fraser, S. H. Zinder, and J. F. Heidelberg. 2005. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science 307:105-108. [DOI] [PubMed] [Google Scholar]
- 24.Shevchenko, A., O. N. Jensen, A. V. Podtelejnikov, F. Sagliocco, M. Wilm, O. Vorm, P. Mortensen, H. Boucherie, and M. Mann. 1996. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 93:14440-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smidt, H., and W. M. de Vos. 2004. Anaerobic microbial dehalogenation. Annu. Rev. Microbiol. 58:43-73. [DOI] [PubMed] [Google Scholar]
- 26.Swofford, D. L. 2002. PAUP. Phylogenetic analysis using parsimony (*and other methods), 4th ed. Sinauer Associates, Sunderland, MA.
- 27.Tyson, G. W., J. Chapman, P. Hugenholtz, E. E. Allen, R. J. Ram, P. M. Richardson, V. V. Solovyev, E. M. Rubin, D. S. Rokhsar, and J. F. Banfield. 2004. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428:37-43. [DOI] [PubMed] [Google Scholar]
- 28.Waller, A. S., R. Krajmalnik-Brown, F. E. Loffler, and E. A. Edwards. 2005. Multiple reductive-dehalogenase-homologous genes are simultaneously transcribed during dechlorination by Dehalococcoides-containing cultures. Appl. Environ. Microbiol. 71:8257-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White, D. C., R. Geyer, A. D. Peacock, D. B. Hedrick, S. S. Koenigsberg, Y. Sung, J. He, and F. E. Loffler. 2005. Phospholipid furan fatty acids and ubiquinone-8: lipid biomarkers that may protect Dehalococcoides strains from free radicals. Appl. Environ. Microbiol. 71:8426-8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.