Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) contains several open reading frames (ORFs) encoding proteins capable of initiating signal transduction pathways. Among them is the K15 ORF, which consists of eight exons encoding a protein with 12 predicted transmembrane domains and a cytoplasmic C terminus. When transiently expressed, the 8-exon K15 transcript gives rise to a protein with an apparent molecular mass of 45 kDa. K15 interacts with cellular proteins, TRAF (tumor necrosis factor receptor-associated factor) and Src kinases, and activates AP-1, NF-κB, and the mitogen-activated protein kinases (MAPKs) c-jun-N-terminal kinase and extracellular signal-regulated kinase. This signaling activity of K15 is related to phosphorylation of Y481 of the K15 SH2-B motif Y481EEV. In this study we demonstrate the expression of an endogenous 45-kDa K15 protein in KSHV BAC36-infected epithelial cells. This endogenous K15 protein shows the same intracellular localization as transiently expressed K15, and expression kinetic studies suggest it to be a lytic gene. We have further determined the downstream target genes of K15 signaling using DNA oligonucleotide microarrays. We demonstrate that K15 is capable of inducing expression of multiple cytokines and chemokines, including interleukin-8 (IL-8), IL-6, CCL20, CCL2, CXCL3, and IL-1α/β, as well as expression of Dscr1 and Cox-2. In epithelial cells, K15-induced upregulation of most genes was dependent on phosphorylation of Y481, whereas in endothelial cells mutation of Y481 did not result in a complete loss of Dscr1 and Cox-2 expression and NFAT-activity. Our study establishes K15 as one of the KSHV lytic genes that are inducing expression of multiple cytokines, which have been shown to play an important role in KSHV-associated pathogenesis.
The eighth human herpesvirus, Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8), was originally identified in Kaposi's sarcoma (KS) tissues by representational difference analysis (21). KSHV plays an essential role in the pathogenesis of three human neoplastic disorders, Kaposi's sarcoma (81, 114), a very rare form of B-cell lymphoma called body cavity-based lymphoma (BCBL) or primary effusion lymphoma (PEL) (19), and the plasma cell variant of multicentric Castleman's disease (120).
KS is a multicentric vascular neoplasm involving the skin and mucosal surfaces and in aggressive cases may involve visceral organs and lymph nodes. KS lesions contain distinctive proliferating spindle cells, activated endothelial cells, fibroblasts, smooth muscle cells, and infiltrating inflammatory cells (82, 103, 104). Endothelial cell-derived spindle cells represent the neoplastic component of the KS tumor and are infected by KSHV (10, 32, 60, 94, 101, 115). In these cells, four latent viral genes are expressed: open reading frame (ORF)73/Lana-1, ORF K12/kaposin, ORF K13/vFLIP, ORF72/vCyclin. In some spindle cells KSHV is not strictly latent but undergoes lytic replication (60, 94, 122). Similarly, in cell lines derived from PEL (9, 20), only latent genes are expressed in the majority of cells, but in some PEL cell lines a minority of KSHV-infected cells will spontaneously switch into the lytic cycle.
The lytic viral replication cycle of KSHV can be experimentally induced in PEL cells by addition of the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) or n-butyrate (78, 107). Virus harvested from induced PEL cell lines can infect the epithelial cell line 293 (39, 106), primary endothelial cell cultures (16, 24, 38), immortalized endothelial cell lines (64, 82), human and murine fibroblasts, HeLa cells, and the endothelial SLK cell line (7). Infectious KSHV can also be produced from latently infected 293 and TIME cells after induction of the lytic cycle following transfection or transduction of the viral lytic transactivator regulator of transcriptional activation (RTA/ORF50) (7). Viral particles generated from 293 cells stably transfected with a recombinant KSHV genome in a bacterial artificial chromosome (BAC) can infect 293, HeLa, and human endothelial cells (135).
Several studies have demonstrated the importance of inflammatory cytokines in KSHV-associated pathogenesis (34). KSHV encodes various genes capable of regulating cellular cytokine expression such as the viral G-protein-coupled receptor (vGPCR) (99, 117), K1 (67), kaposin B (75), and homologues of human interleukin-6 (IL-6) and cellular CC chemokines (87). The lytic KSHV membrane proteins vGPCR and K1 are signaling proteins capable of activating multiple cellular signaling cascades such as mitogen-activated protein (MAP) kinase pathways and several transcription factors implicated in inflammatory responses (14, 88).
Another KSHV-encoded membrane protein capable of eliciting cellular signal transduction pathways is K15 (14). The K15 ORF is located between the viral terminal repeat region and ORF75 of the KSHV genome (23, 44, 100). Multiple alternatively spliced transcripts are generated from the K15 gene, with the most prominent transcript encompassing eight exons. The sequences of all K15 cDNA clones isolated so far are predicted to contain a common C-terminal cytoplasmic region (encoded by exon 8) linked to a variable number of transmembrane domains, with the full-length transcript (exons 1 to 8) coding for a protein with up to 12 transmembrane domains.
Expression of K15 transcripts was identified in unstimulated KSHV-positive PEL cells and was shown to be upregulated upon lytic cycle induction (23, 44, 100). Gene array studies indicate that K15 is predominantly expressed during the lytic cycle in PEL cell lines (57, 85, 98). In the PEL cell line BCBL-1, ectopic RTA/ORF50 expression, and TPA induction were shown to mediate K15 promoter transactivation (130). Taken together, these findings suggest that K15 is expressed after activation of the lytic cycle in PEL cells.
Cloned into mammalian expression vectors, the eight-exon K15 isoform is translated into a protein with an apparent mass of ∼45 kDa that associates with lipid rafts and forms patches on the plasma and cytoplasmic membranes in 293 and Cos-1 cells with a mainly perinuclear localization (13, 23, 44, 116). However, in uninduced PEL cell lines, a protein of 23 kDa was detected with a monoclonal antibody raised to the C-terminal domain of K15, and immunofluorescence studies showed a cytoplasmic staining in the majority of these cells (116). Upon induction of the lytic cycle, expression of this 23-kDa protein decreased over time, suggesting latent kinetics for this protein. By immunostaining with a monoclonal K15 antibody, K15 expression has so far been observed in multicentric Castleman's disease plasmablasts but not in KS tumors (116).
The cytoplasmic domain of K15 contains several signaling motifs: a proline-rich motif that could potentially serve as an SH3-binding (SH3-B) motif (PP387PLPP), a motif reminiscent of a tumor necrosis factor receptor-associated factor (TRAF) binding site (ATQ475PTDD), and two potential SH2-binding (SH2-B) sites (VFGY431ASI and DDLY481EEV). K15 shows structural and functional similarities to Epstein-Barr virus (EBV) latent membrane proteins LMP1 and LMP2A (14). Like LMP1, K15 interacts with TRAF-1, TRAF-2, and TRAF-3, activates the MAP kinase c-jun-N-terminal kinase (JNK) 1, and the transcription factors NF-κB and AP-1 (13, 44). Reminiscent of LMP2A, K15 interacts with members of the Src family of protein tyrosine kinases via its C-terminal domain and is phosphorylated in vitro at Y481 by these kinases (13). A chimera of the CD8 molecule fused to the K15 cytoplasmic domain is constitutively phosphorylated at Y481 of the Y481EEV motif in vivo (23). The CD8-K15 chimera was shown to downregulate B-cell receptor signaling in B cells, which seems to be mediated via the putative SH3-B and the SH2-B motif Y481EEV (23). Further, K15 can induce activation of the MAP kinase Erk2 via the classical Ras/Raf/MEK pathway (13).
Here, we show the expression of a 45-kDa K15 protein in the epithelial cell line 293 stably transfected with the entire KSHV genome cloned as a bacterial artificial chromosome (KSHV BAC36). This 45-kDa K15 protein expressed in the context of the complete KSHV genome was significantly induced upon lytic cycle induction and shows a similar intracellular localization as the transfected eight-exon cDNA of K15. Employing two types of DNA oligonucleotide microarrays, we identified several cytokines, chemokines, and cellular genes such as Down syndrome critical region 1 (Dscr1), cyclooxygenase 2 (Cox-2), and matrix metalloproteases as downstream targets of K15. With few exceptions, K15-induced activation of gene expression in epithelial cells was dependent on Y481 of the SH2-B motif of K15. In contrast, in SLK endothelial cells the K15 mutant K15 F481 was still able to induce expression of K15-downstream targets Dscr1 and Cox-2 and NFAT activity, indicating host-cell-dependent functions of Y481. These results establish K15 as one of the KSHV-encoded proteins that on their own have the capacity to modulate the host cell response toward an inflammatory phenotype and may therefore be involved in KSHV-associated pathogenesis.
MATERIALS AND METHODS
Cell lines, transfections, and lytic cycle induction.
The cell lines HEK (human embryonic kidney) 293-T, HEK 293, Cos-7, and human SLK endothelial cells were cultured in Dulbecco's modified Eagle medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS), 50 IU/ml penicillin, and 50 μg/ml streptomycin at 37°C in humidified air with 5% CO2. The endothelial SLK cell line was established from a biopsy of an oral KS tumor of an iatrogenically immunosuppressed human immunodeficiency virus-negative patient and has since lost the KSHV genome (49). The human cervix epithelial HeLa cell line and the epithelial kidney cell line PtK2 (isolated from Potorous tridactylis) were cultured in minimal essential medium (Cytogen) supplemented as above. Sf9 insect cells were cultured in Grace's insect medium (Gibco) with 10% FCS, 50 IU/ml penicillin, and 50 μg/ml streptomycin at 27°C. For transfections, cells were grown to subconfluence in six-well plates. 293-T cells, Cos-7, PtK2, and HeLa cells were transfected with FuGENE transfection reagent (Roche) (FuGENE:DNA ratio of 3 μl:1 μg). SLK cells were transfected with Lipofectamine 2000 according to the manufacturer's instructions with a Lipofectamine:DNA ratio of 4 μl:4 μg (Invitrogen). For immunofluorescence studies, SLK cells were transfected with 2 μg of DNA. The KSHV BAC clone 36 (135) was kindly provided by S. J. Gao. KSHV BAC36 DNA for transfection of 293 cells was prepared from Escherichia coli strain DH10B with the Maxi-BAC Kit (Machery and Nagel). For transfection, 293 cells were plated at 3 × 105 cells per well of a six-well plate and transfected 2 days later at 70% confluence with Lipofectamine 2000, according to the manufacturer's instructions (Invitrogen) (DNA:Lipofectamine ratio of 4 μg:10 μl). The transfection efficiency could be monitored by green fluorescent protein expression. The medium was changed the following day, and 48 h posttransfection cells were trypsinized and split 1:2. Eight days posttransfection, cells were trypsinized and split 1:3 in Dulbecco's modified Eagle medium containing HygromycinB (150 μg/ml). The medium was changed every 3 days and when foci developed, individual foci were picked and transferred into a 96-well plate under selection with HygromycinB.
The lytic viral life cycle was induced 1 or 2 days after seeding 293 KSHV BAC36 cells by addition of 1 mM sodium butyrate (Sigma) or infection with baculovirus coding for KSHV ORF50/RTA or both. At different time points postinduction, cells were lysed in 250 μl of TBS-T (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitors phenylmethylsulfonyl fluoride [1 mM], leupeptin [50 μM], aprotinin [100 U/ml], benzamidine [200 μM], pepstatin A [1 μM]) per well of a six-well plate. The generation of the recombinant baculovirus expressing KSHV ORF50/RTA was described elsewhere (128). For infection of mammalian cells, RTA baculovirus containing Sf9 insect cell culture supernatant was cleared by centrifugation (500 × g for 15 min), and 200 μl of supernatant was added per well of a six-well plate.
DNA constructs.
The full-length K15 cDNA clone (K15 amino acids [aa] 1 to 489, exons 1 to 8) and the point mutant K15 Y481F used in this study were generated as follows. The full-length K15 wild-type (WT) construct containing an artificial Kozak sequence (bold) 5′ of the ATG in exon 1 (underlined) was generated by PCR on clone MBK15 using the primers K15EcoRIKozakfor (5′-TATGAATTCGCCACCATGAAGACACTCATATTCTTCTGG-3′) and FlagLAMPa3′ binding to exon 8 (5′-TATGAATTCCTAGTTCCTGGGAAATAAAAC-3′) (underlining indicates the stop codon in exon 8). The PCR fragment was cloned EcoRI in pFJ-EA. The K15 point mutant K15 Y481F was generated as the K15 WT construct but with reverse primer LAMPaY:Frev (5′-TATGAATTCCTAGTTCCTGGGAAATAAAACCTCCTCAAACAGGTC-3′) introducing the amino acid substitution Y481→F481 (bold). The generation of the full-length K15 P-type cDNA clone MBK15 (K15 aa 1 to 489) from the BCP-1 KSHV isolate (primary effusion cell line infected with KSHV) has already been described (13).
The reporter plasmids Dscr1 (in pGL3b) and Cox-2 (in pGL3b) contain the promoter regions of the dscr1 and cox-2 genes cloned upstream of the luciferase gene of the pGL3b vector, respectively, and were kindly provided by Elia J. Duh (132). The promoterless pGL3b reporter (Promega) served as negative control. The pTA-Luc vector (Clontech) contains the minimal TA promoter upstream of the luciferase reporter gene. The pNFAT-TA-Luc vector (Clontech) additionally contains three tandem copies of the NFAT consensus sequence upstream of the minimal TA promoter and the luciferase reporter gene.
siRNA design and transfection.
The K15 small interfering RNA (siRNA) was targeted to exon 8 of K15 (CAACCACCUUGGCAAUAAU) and was purchased from Dharmacon. HeLa and 293-T cells (in six-well plates) were transfected with 160 pmol of K15 siRNA (diluted in 250 μl of Opti-MEM I medium [Gibco]) and 5 μl of Lipofectamine 2000 (Invitrogen) (diluted in 250 μl of Opti-MEM I) at 30 to 50% confluence according to the manufacturer's instructions. Six hours later, the medium was replaced and cells were transfected with 1 μg of K15 expression construct (FuGENE).
293 KSHV BAC36 cells were plated at a cell density of 6 × 105 cells/well of a six-well plate and transfected the following day with K15 siRNA as described above. Six hours later, cells were washed, and fresh medium was added. Twenty hours after siRNA transfection, cells were either left untreated or stimulated with 1 mM butyrate or baculovirus coding for KSHV ORF50/RTA or both and lysed 24 h postinduction.
Oligonucleotide DNA microarray experiments.
HeLa cells were seeded in six-well plates at a density of 8 × 104 cells per well and transfected 24 h later with 1 μg of DNA per well using the FuGENE transfection reagent. Thirty-two hours posttransfection, cells were lysed for RNA extraction according to the manufacturer's instructions (RNeasy kit; QIAGEN). For controlling protein expression levels by Western blotting, cells were lysed 32 h posttransfection for 10 min on ice with 250 μl of radioimmunoprecipitation assay (RIPA) 100 buffer (20 mM Tris, pH 7.5, 1 mM EDTA, 100 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 50 μM leupeptin, 100 U/ml aprotinin, 200 μM benzamidine, 1 μM pepstatin A) per well. Lysates were cleared by centrifugation (10,000 × g for 10 min at 4°C) and analyzed by Western blotting. To control transfection efficiency, HeLa cells were seeded onto glass coverslips and analyzed by immunofluorescence using a polyclonal antibody to K15 (13) 32 h posttransfection.
For microarray experiments the Human 40K:A Array and the Human Inflammation Array (MWG Biotech) were used (now distributed by Ocimum Biosolutions as Human 40K A OciChipTM and Human Inflammation OciChipTM, respectively). The Human Inflammation Array contains 155 validated oligonucleotide probes for 136 inflammatory and 19 housekeeping genes. The Human 40K:A Array contains approximately 20,000 probes covering mainly well-characterized human transcripts.
Total RNA from cells transfected as described above was purified with the RNeasy kit followed by on-column DNase I digestion (QIAGEN). RNA was used to prepare Cy3- or Cy5-labeled cRNA by oligo(dT)-T7-primed double-stranded cDNA synthesis (cDNA synthesis system; Roche), followed by in vitro transcription with T7-polymerase (MEGAscript T7 kit; Ambion) as directed by the manufacturers. cRNA yields were determined photometrically.
For low-density microarray experiments, 16 μg of Cy3-labeled cRNA derived from approximately 2.5 μg of total RNA was hybridized individually to microarrays (one sample per array). For high-density microarray experiments, 40 μg of Cy3- and Cy5-labeled cRNA populations, derived from approximately 5 μg of total RNA, representing two different experimental conditions, were combined and cohybridized onto the same microarray (two-color experiment).
cRNAs were fragmented, repurified, and hybridized to microarrays in preprepared hybridization solution (MWG Biotech) at 42°C overnight and then washed sequentially in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% SDS, 1× SSC, and 0.5× SSC. Hybridized arrays were scanned on an Affymetrix 428 scanner at variable photomultiplier tube voltage settings. Fluorescence intensity values were processed using Imagene 4.2 software (Biodiscovery). In order to obtain maximal signal intensities without saturation effects, intensity values from TIFF images were integrated into one value per probe by the MAVI software (version Pro 2.5.1; MWG Biotech). Data were filtered for flagged spots and low intensity values; remaining data were used to calculate ratios of gene expression using Excel macros. Additional information on the microarrays and methodology used can be obtained at http://www.mh-hannover.de/forschung/sfb566/microarray/index.phtml.
Real-time PCR.
The same cDNAs that were used for high-density microarray experiments were used to quantify the amounts of mRNA of actb (Hs99999903_m1), cxcl8 (Hs00174103_m1), and vegf (Hs00173626_m1) by the indicated assays on demand (Applied Biosystems). Real-time reactions were performed in 25-μl mixtures using an ABI7500 instrument, and cycle threshold (CT) values for each PCR product were calculated by the 7500 Fast System software (version 1.3.0). CT values obtained for cxcl8 and vegf were normalized by subtracting the CT values obtained for β-actin (actb) as a housekeeping reference. The resulting ΔCT values were then used to calculate relative changes of mRNA expression as the ratio (R) of mRNA expression of transfected/mock-transfected cells according to the equation: R = 2−(ΔCt(transfected) − ΔCt(mock-transfected)).
Reverse transcription-PCR (RT-PCR).
SLK cells were plated at 3 × 105 cells/well of a six-well plate and transfected with Lipofectamine 2000. The medium was changed 6 h posttransfection. At 32 h posttransfection, cells were lysed for RNA extraction according to the manufacturer's instructions (RNeasy kit; QIAGEN). cDNA was prepared as described for DNA microarray experiments. For the subsequent PCR, 7 ng of HeLa or SLK cDNA was used per PCR with primers spanning the intron between exons 5 and 6 of the dscr1 gene (dscr1 347-365-for, 5′-CCTTCTCCGCAGCAGATGC-3′; dscr1 543-524-rev, 5′-GACTGGGGTCGCATCTTCCAC-3′). This primer combination detects the same dscr1 isoforms as the inflammation DNA microarray and amplifies a spliced transcript of 196 bp and would amplify a genomic fragment of 554 bp if genomic traces of DNA were present. The PCR was performed with the Hot Star Taq kit (QIAGEN) with the following PCR conditions: 15 min at 95°C followed by 22 to 24 cycles of 30 s at 94°C, 30 s at 57°C, 20 s at 72°C, and 7-min final extension at 72°C. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified with primers GAPDHfor (5′-ACCACAGTCCATGCCATCAC-3′) and GAPDHrev (5′-TCCACCACCCTGTTGGTGTA-3′) and the following PCR conditions: 15 min at 95°C followed by 22 to 24 cycles of 30 s at 94°C, 30 s at 60°C, 30 s at 72°C, and 7-min final extension at 72°C. A total of 5 pmol of each primer was used for one PCR of 25 μl. PCR products were separated on 1.5% agarose gels and visualized by ethidium bromide staining. To control equal expression levels of the K15 proteins, SLK cell lysates were analyzed by Western blotting as described for HeLa cells (DNA microarray).
ELISA.
HeLa cells were transfected exactly as described for the DNA microarrays. At 32 h posttransfection, the conditioned medium was collected and centrifuged for 5 min at full speed in a table-top centrifuge, and supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (Immunotools). The cells were lysed in RIPA 100 buffer to control K15 protein expression by Western Blotting as described for the DNA microarrays.
Cytokine antibody array.
HeLa cells were transfected exactly as described for the DNA microarrays. At 32 h posttransfection, the conditioned medium was collected and centrifuged for 5 min at full speed in a table-top centrifuge, and supernatants were analyzed with the Cytokinearray III encompassing 42 different human cytokines according to the manufacturer's instructions (RayBiotech). The captured cytokines were detected with a mixture of biotin-labeled anticytokine antibodies, followed by streptavidin-horseradish peroxidase and chemiluminescence. Quantification of the spots was performed with the Kodak 1D imaging software. The cells were lysed in RIPA 100 buffer to control K15 protein expression by Western blotting as described for DNA microarrays.
Luciferase-based reporter assays.
293-T cells were plated at a cell density of 5 × 105 cells per well of a six-well plate and transiently cotransfected the following day with 100 ng of the reporter plasmid Dscr1 or Cox-2 or the control reporter plasmid pGL3b and 1 μg of K15 expression constructs or empty vector (pFJ-EA) per well of a six-well plate. At 20 h posttransfection, the medium was replaced with medium containing 1% FCS. At 29 h posttransfection, cells were washed once with phosphate-buffered saline (PBS) and lysed in reporter lysis buffer (Promega). Luciferase activities were measured in cleared lysates using a luciferase assay system according to the manufacturer's instructions (Promega). Luciferase activity was calculated as relative induction (n-fold) compared to mock (empty expression vector)-transfected controls. The reporter assays with the pTA (negative control) or pNFAT-TA luciferase reporter plasmids were performed as described above, with the exception that the serum was reduced to 0% FCS 20 h posttransfection. As a positive control for NFAT activation, transfected 293-T cells were stimulated with TPA (200 ng/ml; Sigma) and calcium ionophore (5 μM; Sigma) or as negative control with dimethyl sulfoxide (DMSO) 4 h before cells were lysed. SLK cells were seeded at a cell density of 3 × 105 cells per well of a six-well plate and cotransfected the following day with 3 μg of K15 expression constructs or empty vector together with 1 μg of the reporter vectors pGL3b, pTA (negative controls), Dscr1, Cox-2, or pNFAT-TA using the Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. Medium was changed 6 h posttransfection. At 21 h posttransfection, medium was replaced with medium containing 0% FCS, and cells were lysed in reporter lysis buffer 29 h posttransfection.
Immunoblotting.
For detection of proteins by Western blotting, cleared cell lysates containing K15 proteins were not boiled prior to SDS-polyacrylamide gel electrophoresis. As indicated, the following primary antibodies were used for immunostaining of immunoblots: mouse anti-actin (Chemikon), mouse anti-vimentin (Chemikon), and rabbit anti-K15 (13) antibodies. Immunoblots were analyzed using horseradish peroxidase-coupled secondary antibodies (Dako) and a standard enhanced chemiluminescence reaction.
Immunofluorescence.
HeLa, SLK, and PtK2 cells were washed once with PBS and fixed for 20 min with paraformaldehyde (3% in PBS) and then washed three times with PBS and incubated with NH4CL (50 mM) in PBS for 10 min. Cells were then permeabilized for 5 min with 0.1 to 0.2% Triton X-100 in PBS, washed three times with PBS, and blocked for 1 h in 10% FCS in PBS at room temperature. The first antibody (diluted in 2% FCS in PBS) was applied for 1 h at room temperature, and cells were washed three times with PBS and then incubated for 30 min at room temperature with the secondary antibody (diluted in 2% FCS in PBS). After three washes with PBS, cell nuclei were stained with Hoechst, washed with PBS, and embedded in MOWIOL with Dabco (25 mg/ml, Sigma) and analyzed by fluorescence microscopy. The primary K15 polyclonal antibody was diluted 1:200. Secondary antibodies were anti-rabbit Cy3 (1:200; Jackson ImmunoResearch) or anti-rabbit fluorescein isothiocyanate (1:40) (Dako).
293 KSHV BAC36 cells were plated on coverslips at a cell density of 4 × 105 to 5 × 105 cells per well of a six-well plate coated with 0.1% gelatin in PBS. The lytic viral life cycle was induced the following day by the addition of sodium butyrate (1 mM final concentration; Sigma) or baculovirus encoding the lytic switch protein KSHV RTA/ORF50. At 23 to 38 h postinduction, cells were analyzed by fluorescence microscopy as described above.
RESULTS
A K15 protein of 45 kDa is expressed in the 293 KSHV BAC36 cell line.
When transiently expressed in cell lines such as 293-T or HeLa, the eight-exon K15 transcript is translated into a membrane protein of ∼45 kDa (13, 23, 116). Until now, full-length K15 transcripts (exons 1 to 8) have been detected in KSHV-positive PEL cell lines by RT-PCR (23, 44), but a K15 protein of 45 kDa has, until now, not been observed in any KSHV-positive cell line analyzed.
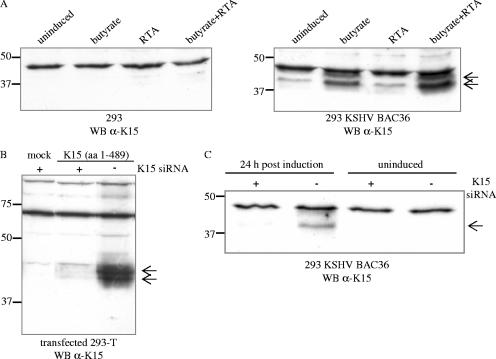
We detected a K15 protein of 45 kDa in 293 cells that were stably transfected with the complete KSHV genome derived from the PEL cell line BCBL-1 and which had previously been cloned into a bacterial artificial chromosome, KSHV BAC clone 36 (135). As shown in Fig. 1A (right blot), this 45-kDa K15 protein was weakly expressed in uninduced 293 KSHV BAC36 cells, and its expression increased upon induction of the lytic viral life cycle with sodium butyrate. Whereas induction of the lytic viral life cycle by infection with a baculovirus coding for the KSHV lytic switch protein RTA/ORF50 did not increase K15 expression, the addition of both compounds (sodium butyrate and baculovirus RTA/ORF50) was most effective in the upregulation of K15 expression (Fig. 1A, right blot). The K15 protein expressed from the complete KSHV genome migrates as a doublet (Fig. 1A, right blot) similar to the K15 protein expressed from a cDNA expression vector (exons 1 to 8) in transiently transfected 293-T cells (Fig. 1B), suggesting posttranslational modifications.
FIG. 1.
A K15 protein of 45 kDa is expressed in 293 cells stably transfected with KSHV BAC36. (A) In 293 cells stably transfected with KSHV BAC36, a 45-kDa protein was detected with the K15 polyclonal antibody (right blot, indicated by arrows), which was not present in 293 cells (left blot). The K15 polyclonal antibody detects an unspecific band of approximately 48 kDa in lysates of 293 and 293 KSHV BAC36. For induction of the lytic viral life cycle, cells were either treated with butyrate or infected with baculovirus coding for the KSHV RTA/ORF50 (regulator of transcriptional activation) protein, or both. At 48 h postinduction, cells were lysed and analyzed for K15 protein expression by Western blotting with the K15 polyclonal antibody. 293 cells were treated in a similar manner. (B) 293-T cells were transiently transfected with empty expression vector pFJ-EA (mock) or a full-length K15 cDNA construct encompassing exons 1 to 8 (aa 1 to 489). At 6 h prior to DNA transfection, cells were transfected with a K15 siRNA directed against exon 8, which codes for the C-terminal cytoplasmic domain of K15 (+, K15 siRNA transfected; −, not K15 siRNA transfected). At 36 h posttransfection, cells were lysed and analyzed by Western blotting with the polyclonal K15 antibody. (C) The 293 KSHV BAC36 cell line was transfected with K15 siRNA as described in Material and Methods. Twenty hours after siRNA transfection, either cells were left untreated (uninduced) or the lytic viral life cycle was induced by addition of butyrate and baculovirus RTA/ORF50. Cells were lysed 24 h postinduction, and lysates were analyzed by Western blotting with the polyclonal K15 antibody. WB, Western blotting.
To verify that the 45-kDa protein detected by our polyclonal K15 antibody in the 293 KSHV BAC36 cell line was indeed derived from ORF K15, an siRNA directed against exon 8 of K15 was first validated in a cotransfection experiment in 293-T cells with the K15 expression construct comprised of exons 1 to 8 (Fig. 1B). After transfection into the 293 KSHV BAC36 cell line, this K15 siRNA was able to significantly reduce the expression of the 45-kDa protein detected by the polyclonal K15 antibody in sodium butyrate and RTA/ORF50-induced 293 KSHV BAC36 cells (Fig. 1C), indicating that the latter is in fact derived from ORF K15.
Intracellular localization of endogenously and transiently expressed K15 protein.
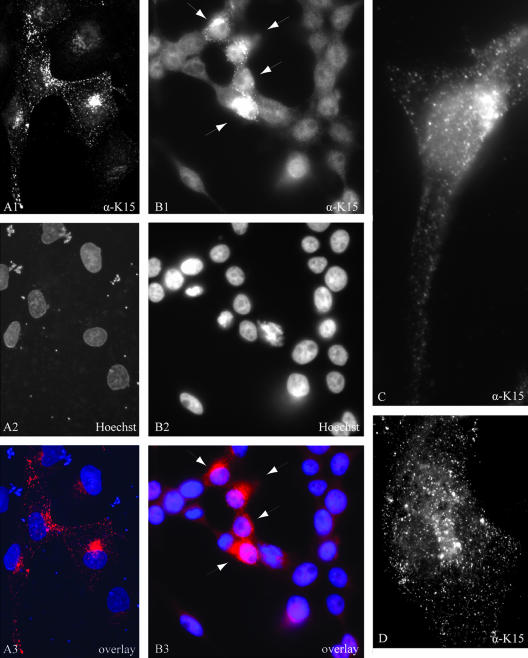
Different intracellular localization patterns of the transiently expressed K15 protein have been reported which range from endoplasmic reticulum localization in 293 and HeLa cells (116) to the formation of large patches on external and cytoplasmic membranes in 293 cells (44), and cytoplasmic, plasma membrane, and perinuclear localization in Cos-1 and 293 cells (23, 116). In the epithelial cell line PtK2 and the endothelial cell line SLK, the transfected eight-exon K15 expression construct produced a distinct punctuate expression pattern evenly distributed over the entire cell and concentrated in the perinuclear region, which was visualized with either a monoclonal K15 antibody directed against amino acids encoded in exon 8 (data not shown) or the polyclonal K15 antibody (Fig. 2; PtK2 in image A1 and SLK in image D). In K15-expressing PtK2 cells, the majority of K15 did not colocalize with the endoplasmic reticulum marker calnexin (data not shown).
FIG. 2.
Subcellular localization of K15 in 293 KSHV BAC36 cells and in transiently transfected PtK2 and SLK cells. (A) PtK2 cells were transiently transfected with the eight-exon K15 expression construct. At 48 h posttransfection, cells were fixed and labeled with the K15 polyclonal antibody and anti-rabbit Cy3 as secondary antibody (A1) as described in Material and Methods. Nuclei were stained with Hoechst 33258 (A2). A3 shows an overlay of images A1 and A2. (B) 293 cells stably transfected with KSHV BAC36 were treated with 1 mM butyrate for 23 h, fixed, and labeled with the K15 polyclonal antibody (B1) as described in Material and Methods. The arrows indicate cells expressing K15 protein. Nuclei were stained with Hoechst 33258 (B2). B3 shows an overlay of images B1 and B2. (C) Image showing a single 293 KSHV BAC36 cell expressing endogenous K15. The lytic cycle was induced in 293 KSHV BAC36 cells by adding 1 mM butyrate and baculovirus coding for KSHV RTA/ORF50 to the cell culture medium. At 38 h postinduction, cells were fixed and labeled with the K15 polyclonal antibody as described in Material and Methods. (D) SLK endothelial cells were transiently transfected with the eight-exon K15 cDNA expression construct, fixed, labeled with the K15 polyclonal antibody, and analyzed by fluorescence microscopy.
We next examined the 293 KSHV BAC36 cell line, after treatment with butyrate, for the expression of endogenous K15 protein by immunofluorescence with the K15 polyclonal antibody. As observed for transiently expressed K15 protein in SLK and PtK2 cells, endogenous K15 expression was concentrated in the perinuclear region (Fig. 2, image B1). Image C (Fig. 2), which shows a higher magnification of a K15-expressing 293 KSHV BAC36 cell, illustrates that the K15 protein expressed in the context of the entire KSHV genome shows a punctuate localization similar to that observed in PtK2 or SLK cells transiently transfected with K15 (Fig. 2, images A1 and D). In uninduced 293 KSHV BAC36 cells only very few cells were positive for the K15 protein (data not shown). We observed that a small percentage of 293 KSHV BAC36 cells expressed the late lytic glycoprotein K8.1 A/B and the early lytic protein ORF59 without induction of the lytic cycle (data not shown). It is therefore likely that the K15 expression observed in uninduced 293 KSHV BAC36 cells occurs in a subset of cells that have spontaneously switched on the lytic cycle.
Genome-wide investigation of K15-regulated cellular genes reveals several genes related to inflammation.
The K15 membrane protein activates the MAP kinases extracellular signal-regulated kinase (ERK) and JNK and the transcription factors AP-1 and NF-κB (13). K15 is constitutively tyrosine phosphorylated at Y481 in vivo (23), and we have shown that members of the Src family of protein tyrosine kinases phosphorylate Y481 in vitro (13). All signaling activities of K15 described so far are significantly impaired when Y481 is mutated to F481 (13).
In order to explore the downstream targets of K15 signaling, we performed high-density DNA microarrays on K15-transfected cells. Since the 293 KSHV BAC36 epithelial cell line was the first cell system in which we could detect the expression of the 45-kDa K15 protein in the context of the complete viral genome, we chose epithelial cells for further experiments. HeLa cells were transiently transfected with either pFJ-EA (empty vector control; mock) or K15 expression constructs K15 WT or the K15 F481 mutant. At 32 h posttransfection, cells were lysed for RNA extraction, and cDNA was prepared as described in Material and Methods and the notes to Table 1. In these experiments, the transfection efficiency of HeLa cells was about 50% for both K15 WT and K15 F481, and both proteins were expressed at equal levels as judged by immunofluorescence and Western blotting (data not shown).
TABLE 1.
Identification of K15-regulated genesa
| Identifier | Gene description and/or abbreviation | Gene expression (mean ratio)
|
|||||
|---|---|---|---|---|---|---|---|
| HD arrayb
|
Inflammation array (expt. 1)c
|
Inflammation array (expt. 2)c
|
|||||
| K15 WT/control | K15 F481/control | K15 WT/control | K15 F481/control | K15 WT/control | K15 F481/control | ||
| NM_000584 | Il-8 | 6.99 | 1.42 | 58.1 | 2.7 | 22.6 | 4.2 |
| AY063515 | ATP-binding cassette protein c13 variant b | 3.72 | 2.39 | NA | NA | NA | NA |
| NM_000600 | Il-6 (interferon beta 2) | 3.32 | 0.96 | 3.8 | 0.9 | 4.7 | 2.8 |
| NM_014326 | Death-associated protein kinase 2; dapk2 | 3.23 | 2.43 | NA | NA | NA | NA |
| NM_004414 | dscr1 | 3.22 | 1.34 | 5.9 | 1.7 | 5.3 | 1.9 |
| NM_058178 | Neuronal pentraxin receptor, isoform 2; nptxr | 3.16 | 2.96 | NA | NA | NA | NA |
| NM_018968 | Syntrophin gamma 2; sntg2 | 3.07 | 3.41 | NA | NA | NA | NA |
| NM_002090d | Chemokine (c-x-c) ligand 3; cxcl3, gro3, groγ | 2.97 | 1.03 | 2.7 | 0.8 | 2.0 | 1.1 |
| NM_004864 | Growth differentiation factor 15 (GDF 15) | 2.97 | 2.38 | NA | NA | NA | NA |
| NM_005347 | Heat shock 70-kDa protein 5; hspa5 | 2.95 | 1.04 | NA | NA | NA | NA |
| NM_015980 | Hmp 19 protein; loc51617 | 2.76 | 2.22 | NA | NA | NA | NA |
| NM_013409 | Follistatin isoform fst344 precursor; fst | 2.76 | 1.11 | NA | NA | NA | NA |
| NM_004113 | Fibroblast growth factor 12, isoform 2; fgf 12 | 2.70 | 2.70 | NA | NA | NA | NA |
| NM_000636 | Superoxide dismutase 2; sod2 | 2.64 | 1.27 | 3.0 | 1.1 | 3.5 | 2.5 |
| NM_002526 | 5′ nucleotidase, ecto; nt5e | 2.53 | 1.53 | NA | NA | NA | NA |
| BC016341 | Similar to interferon-stimulated gene; 20 kDa | 2.49 | 1.22 | NA | NA | NA | NA |
| NM_138973 | Beta-site app-cleaving enzyme 1; bace | 2.35 | 1.72 | NA | NA | NA | NA |
| AC005058 | Homo sapiens BAC clone CTB-54D4 from 7q31 | 2.35 | 0.94 | NA | NA | NA | NA |
| NM_000575 | Il-1α | 2.19 | 0.98 | 7.0 | 0.9 | 5.4 | 1.8 |
| NM_006010 | Arginine-rich protein; armet | 2.18 | 0.93 | NA | NA | NA | NA |
| NM_001855 | Alpha 1 typexycollagen precursor; col15a1 | 2.17 | 1.00 | NA | NA | NA | NA |
| NM_004050 | Bcl2-like 2 protein; bcl2l2 | 2.13 | 0.63 | NA | NA | NA | NA |
| NM_018677 | Acetyl coenzyme A synthetase; loc55902 | 2.12 | 1.02 | NA | NA | NA | NA |
| NM_006290 | Tumor necrosis factor α - induced protein; tnfaip3 | 2.11 | 1.05 | 3.6 | 1.4 | 1.9 | 2.0 |
| NM_002970 | Sperimidine/spermine n1-acetyltransferase; sat | 2.09 | 0.85 | NA | NA | NA | NA |
| NM_003711 | Phosphatidic acid phosphatase 2a; ppap2a | 2.07 | 1.62 | NA | NA | NA | NA |
| XM_029384 | Kinesin family member 13a; kif13a | 2.02 | 1.62 | NA | NA | NA | NA |
| NM_002982 | Monocyte chemotactic protein 1; mcp 2; ccl 2 | 1.97 | 1.04 | 2.7 | 1.0 | 2.0 | 1.0 |
| D14826 | Herem 2beta-b protein; herem-2 | 1.94 | 1.20 | NA | NA | NA | NA |
| AK000917 | cDna clone unnamed protein product | 1.94 | 1.17 | NA | NA | NA | NA |
| D14825 | herem-1 | 1.94 | 1.29 | NA | NA | NA | NA |
| NM_013372 | Cysteine knot superfamily 1; cktsf1b1 | 1.93 | 1.21 | NA | NA | NA | NA |
| NM_000710 | Bradykinin receptor b1; bdkrb1 | 1.90 | 0.89 | NA | NA | NA | NA |
| NM_052815 | Immediate-early response 3; ier3 | 1.86 | 0.94 | NA | NA | NA | NA |
| NM_002135 | Nuclear receptor subfamily 4; nr4a1 | 1.84 | 1.24 | NA | NA | NA | NA |
| BC002894 | Unknown (protein for mgc:11256) | 1.84 | 1.02 | NA | NA | NA | NA |
| NM_002257 | Kallikrein 1 preproprotein; klk1 | 1.80 | 1.91 | NA | NA | NA | NA |
| NM_002727 | Proteoglycan 1, secretory granule; prg1 | 1.80 | 0.91 | 2.4 | 0.9 | 2.8 | 1.5 |
| AB023182 | Kiaa0965 protein; kiaa0965 | 1.80 | 1.20 | NA | NA | NA | NA |
| NM_001549 | Interferon-induced protein; ifit4 | 1.75 | 1.41 | NA | NA | NA | NA |
| NM_014220 | Transmembrane 4 superfamily member 1; tm4sf1 | 1.75 | 0.85 | NA | NA | NA | NA |
| NM_004482 | Polypeptide N-acetylgalactosaminyltransferase 3; galnt3 | 1.72 | 0.97 | NA | NA | NA | NA |
| NM_003299 | Tumor rejection antigen (gp96) 1; tra1 | 1.70 | 1.11 | NA | NA | NA | NA |
| NM_021013 | Type I hair keratin; krth4 | 1.69 | 0.93 | NA | NA | NA | NA |
| NM_003020 | Secretory granule; neuroendocrine protein 1 (7b2 protein); sgne 1 | 1.69 | 1.02 | NA | NA | NA | NA |
| AB002377 | Kiaa0379 protein; kiaa0379 | 1.67 | 1.05 | NA | NA | NA | NA |
| AK023410 | cDna clone weakly similar to sodium-independent organic anion transporter 2 | 1.65 | 1.03 | NA | NA | NA | NA |
| NM_004184 | Tryptophanyl-trna synthetase; wars | 1.65 | 1.02 | NA | NA | NA | NA |
| NM_005168 | Ras homolog gene family, member e; arhe | 1.64 | 0.93 | NA | NA | NA | NA |
| NM_016306 | Dnai (hsp40) homolog, b11; dnajb11 | 1.63 | 1.03 | NA | NA | NA | NA |
| NM_145918 | Cathepsin 1 preproprotein; cts1 | 1.61 | 1.03 | NA | NA | NA | NA |
| NM_001172 | Arginase, type II precursor; arg2 | 1.58 | 1.05 | NA | NA | NA | NA |
| NM_021127 | PMA-induced protein; pmaip1 | 1.58 | 0.85 | NA | NA | NA | NA |
| XM_059160 | Similar to dj1174n9.1; loc 127544 | 1.58 | 1.02 | NA | NA | NA | NA |
| XM_170564 | Similar to keratin 17; loc 254207 | 1.56 | 1.07 | NA | NA | NA | NA |
| XM_011184 | Sec24-relatedprotein d; sec24d | 1.56 | 1.16 | NA | NA | NA | NA |
| NM_004591 | Chemokine (c-c motif) ligand 20; ccl20 | NS | NS | 7.2 | 0.9 | 7.7 | 1.1 |
| NM_000963 | Cyclooxygenase 2; ptgs2 | NS | NS. | 5.2 | 1.6 | 6.4 | 2.9 |
| NM_002421 | Matrixmetalloproteinase 1; mmp1 | NS | NS | 3.2 | 0.8 | 4.7 | 2.2 |
| NM_002422 | Matrixmetalloproteinase 3; mmp3 | NS | NS | 4.6 | 1.0 | 3.1 | 1.5 |
| NM_000576 | Il-1β | NS | NS | 3.7 | 1.1 | 3.4 | 1.7 |
| NM_001548 | Interferon-induced protein; ifit1 | NS | NS | 1.8 | 0.8 | 4.2 | 5.0 |
| NM_002852 | Pentaxin-related gene, Il-1β induced; ptx3 | NS | NS | 3.0 | 0.5 | 2.2 | 0.4 |
| NM_001710 | Complement factor b preproprotein; bf | NS | NS | 2.3 | 1.2 | 2.8 | 2.2 |
| NM_001165 | Baculoviral iap repeat containing protein 3; birc3 | NS | NS | 2.6 | 0.8 | 2.3 | 1.2 |
| NM_002923 | Regulator of g-protein signaling 2; rgs2 | NS | NS | 2.1 | 1.4 | 2.2 | 1.5 |
| NM_002985 | Small inducible cytokine a5 precursor; ccl5 | NS | NS | 2.3 | 1.3 | 2.0 | 1.6 |
| NM_004049 | Bcl2-related protein a1; bcl2a1 | NS | NS | 1.6 | 0.9 | 2.5 | 1.6 |
| NM_002133 | Heme oxygenase (decyclizing) 1; hmox1 | NS | NS | 1.6 | 1.4 | 2.3 | 1.9 |
| NM_002462 | Myxovirus resistance protein 1; Mx1 | NS | NS | 1.6 | 1.2 | 2.4 | 3.9 |
| NM_022168 | Melanoma differentiation ass. protein-5; mda5 | NS | NS | 1.5 | 0.9 | 2.4 | 4.2 |
| NM_001166 | Baculoviral iap repeat-containing protein 2; birc2 | NS | NS | 2.0 | 1.1 | 1.6 | 1.1 |
| NM_020529 | NFkB inhibitor alpha; nfkbia | NS | NS | 1.7 | 0.7 | 1.5 | 1.1 |
| NM_001993 | Coagulation factor iii precursor; f3 | NS | NS | 1.5 | 0.7 | 1.6 | 1.1 |
| AF119859 | Pro1873 | 0.62 | 1.18 | NA | NA | NA | NA |
| NM_016644 | Mesenchymal stem cell protein dsc54; loc51334 | 0.62 | 0.81 | NA | NA | NA | NA |
| NM_014502 | Nuclear matrix protein mmp200 related to splicing factor prp19; nmp200 | 0.57 | 1.00 | NA | NA | NA | NA |
HeLa cells were transiently transfected with the K15 expression constructs K15 WT (exons 1 to 8) or the K15 mutant K15 F481 or empty vector pFJ-EA (mock, or control). At 32 h posttransfection, RNA was harvested. Additional information on the methodology of the microarrays is available at http://www.mh-hannover.de/forschung/sfb566/microarray/index.phtml. The complete data set is available on request.
High density (HD) microarray experiments were performed as “two-color hybridizations”. The vector control cRNA population was cohybridized with cRNAs generated from transient transfections with K15 WT or K15 F481 expression vectors. For each of the two resulting comparisons (K15 WT/control and K15 F481/control) an additional “dye-swap hybridization” was carried out. Thus, four high-density microarray hybridizations were performed altogether. After hybridization and data extraction (as described in Material and Methods), the whole data set was filtered for genes that were regulated at least 1.5-fold by the K15 WT construct in each of the two corresponding “dye swap arrays”. Listed are the mean ratios of gene expression for 59 cellular genes represented on the HD microarray. Genes that are upregulated by both K15 WT and K15 F481 are shown in bold. NS, not significant.
For the customized inflammation array, 27 genes were found to be regulated by K15. Shown are the values comparing gene expression of K15 WT or K15 F481 with empty vector-transfected cells (control) from two independent experiments. Only genes that were regulated by at least 1.5-fold are shown. NA, not available.
The oligonucleotide probe detecting NM_002090 gro3 (cxcl3 and groγ) also detects gro1 (cxcl1 and groα) and gro2 (cxcl2 and groβ).
As depicted in Table 1, by the filter criteria applied, 56 genes were consistently upregulated more than 1.5-fold by K15, and 3 genes were downregulated. Cellular genes induced by K15 included several involved in inflammation, such as the chemokines interleukin-8 (cxcl8), gro1 (cxcl1/groα), and monocyte chemotactic protein 1 (mcp-1; ccl2), as well as the cytokines interleukin-6 and interleukin-1α (Table 1). The K15 F481 mutant only marginally affected expression of these five inflammatory genes (Table 1).
Among the K15-induced genes were also several whose expression was upregulated to the same extent by K15 WT and the K15 F481 mutant (depicted in bold in Table 1), e.g., death-associated protein kinase 2 (dapk2), neuronal pentraxin receptor (nptxr), syntrophin (sntg2), prostate differentiation factor (plab), hmp19 protein, and fibroblast growth factor 12 (fgf12).
We extended the analysis of K15-regulated inflammatory genes by an additional series of experiments using a DNA oligonucleotide microarray containing 155 validated oligonucleotide probes for 136 inflammatory genes. Among the 136 inflammatory genes are chemokines, cytokines, growth factors and the respective receptors, adhesion molecules, enzymes, matrix proteins, signal transduction transcription factors, and matrix metalloproteases (52, 53). Using this type of microarray, 27 genes were found to be upregulated by K15 more than 1.5-fold in two independent experiments (Table 1).
As on the high-density array, IL-8 was the cellular gene most strongly upregulated (36-fold) by K15 WT (Table 1), whereas the K15 F481 mutant was strongly impaired in its ability to upregulate IL-8 (3.5-fold) (Table 1). Besides IL-8, the genes of the chemokines CCL20, CXCL3, and CCL2 and of the interleukins IL-1α, IL-1β, and IL-6 were significantly upregulated by wild-type K15 but not by mutant K15 F481 (Table 1).
Notably, K15 induced the expression of three genes that are downstream targets of vascular endothelial growth factor (VEGF) (Table 1): Down syndrome critical region 1 (dscr1; 5.6-fold), cyclooxygenase 2 (ptgs2/cox-2; 5.8-fold), and tissue factor (f3; 1.6-fold). The K15 F481 mutant slightly activated dscr1 (1.8-fold) and cox-2 (2.1-fold) gene expression and did not activate tissue factor/f3 gene expression (0.9-fold) (Table 1).
In summary, we have shown that K15 has the capacity to modulate the host cell response toward an inflammatory phenotype: K15 induces gene expression of chemokines (C-X-C chemokines CXCL8/IL-8, CXCL3/Groγ and CCL2; C-C chemokines CCL5 and CCL20/MIP-3α), cytokines (IL-1α, IL-1β, and IL-6), antiapoptotic genes (tnfaip3/A20, bf, birc3, birc2, and bcl2a1), genes involved in signaling (NF-κbia and rgs2), angiogenesis (IL-8, Dscr1, Mmp1, Mmp3, and tissue factor), and various other classical inflammatory genes such as Cox-2, heme oxygenase 1, Ptx3/pentraxin-3, and Sod2.
K15 induces the secretion of inflammatory cytokines.
In order to confirm the data obtained with the DNA microarrays, we analyzed cytokine secretion of K15-transfected HeLa cells. We used a cytokine antibody array spotted with 42 different human cytokine antibodies. The supernatants of HeLa cells transiently transfected with empty vector pFJ-EA, K15 WT, or K15 F481 were incubated with the antibody array membrane, and binding of secreted cytokines was measured as described in Materials and Methods. This assay showed that K15 expression induced the secretion of IL-6, IL-8, and GROγ/CXCL3 proteins in HeLa cells (Fig. 3A). The control spots (Fig. 3A) are marked as well as the spots for IL-8, IL-6, and GROγ. Quantification of the spots in Fig. 3A shows that K15 upregulated IL-8 secretion 16-fold, IL-6 expression 4-fold, and GROγ expression 2-fold (Fig. 3B), which confirms the changes seen at the mRNA level (Table 1). The mutant K15 F481 was significantly impaired in the induction of cytokine secretion (IL-6, 1.5-fold; IL-8, 1; GROγ, 0.3-fold) (Fig. 3A and B).
FIG. 3.
Induction of cytokine and chemokine secretion by K15. (A) HeLa cells were transiently transfected with K15 expression constructs (K15 WT and K15 F481) or the empty vector pFJ-EA (mock). At 32 h posttransfection, the conditioned medium was collected and analyzed with a cytokine antibody array encompassing 42 different human cytokines spotted in duplicate onto a nitrocellulose membrane. The cytokine antibody array contains two different controls (c), one spotted in quadruplicate (upper left corner) and one in duplicate (lower right corner) and was performed as described in Materials and Methods. Arrays are labeled at left. The spots that were upregulated by K15 are marked as indicated on the figure. (B) Quantification of the cytokine antibody array shown in panel A. The intensity of the spots was measured by densitometry and normalized to the control spots (c). The signal reached with medium of cells that were mock-transfected was set at 1. Standard deviation is shown. (C) The ELISA was carried out according to the manufacturer's instructions with supernatant from transiently transfected HeLa cells as described in panel A. Shown is the mean value of two independent experiments. (D) After the conditioned medium was collected from transiently transfected HeLa cells for the cytokine antibody array (A) and ELISA (C), cells were lysed and analyzed by Western blotting for K15 protein expression with the K15 polyclonal antibody. Actin served as a loading control. (E) Real-time PCR analysis for IL-8 and VEGF was performed with 7 ng of cDNA from HeLa cells transiently transfected with K15 expression constructs (K15 WT and K15 F481) or pFJ-EA (mock). The graph shows the relative changes of mRNA expression after normalization against β-actin (ΔΔCT method; see Material and Methods).WB, Western blotting.
To quantitatively determine the specific amounts of IL-6 and IL-8 secreted from HeLa cells transiently transfected with K15, an ELISA was performed. Consistent with the DNA microarray and cytokine antibody array, K15 WT significantly induced IL-6 and IL-8 production (Fig. 3C) (IL-6, 762 pg/ml; IL-8, 1318 pg/ml), while K15 F481 did not induce IL-6 and IL-8 production compared to empty vector-transfected HeLa cells (Fig. 3C) (mock IL-6, 167 pg/ml; K15 F481 IL-6, 160 pg/ml; mock IL-8, 51 pg/ml; K15 F481 IL-8, 51 pg/ml). Equal protein expression levels for K15 WT and K15 F481 in HeLa cells used for the cytokine antibody array and ELISA are shown in Fig. 3D.
To test whether K15 can induce gene expression of VEGF, we performed a real-time PCR with probes for VEGF and, as a positive control, IL-8. IL-8 was significantly upregulated by K15 (101-fold) but only marginally by K15 F481 (3.4-fold) as expected, whereas VEGF levels were only slightly upregulated (1.9-fold) in K15-expressing HeLa cells (Fig. 3E).
Activation of the dscr1 and cox-2 promoters by K15 in 293-T cells.
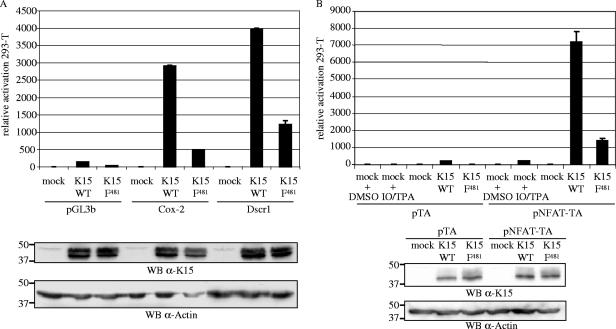
The DNA microarrays showed that expression of dscr1 and cox-2 was significantly upregulated by K15. dscr1 was only recently described as a target for VEGF and seems to have a role in angiogenesis (50, 54, 132). To confirm the DNA microarray data, we performed luciferase-based reporter assays in 293-T and HeLa cells with reporter plasmids containing the promoter region of either dscr1 or cox-2 cloned upstream of the luciferase gene in the promoterless reporter vector pGL3b (132). Figure 4A shows the luciferase activity of 293-T cells cotransfected with K15 WT or K15 F481 with the reporter plasmids pGL3b, Cox-2, or Dscr1 in relation to cells cotransfected with pFJ-EA (mock) and the respective reporter plasmid (luciferase activity of mock-transfected cells was set at 1 for each reporter plasmid). K15 WT was found to upregulate the cox-2 and dscr1 promoters in 293-T cells, whereas the mutant K15 F481 was significantly impaired in the activation of the two promoters (Fig. 4A). Similar results were obtained in HeLa cells (data not shown). Expression of the K15 WT and K15 F481 proteins was comparable (Fig. 4A).
FIG. 4.
K15 activates the cox-2 and dscr1 promoter and the NFAT transcription factor in luciferase-based reporter assays in 293-T cells. 293-T cells were transiently transfected with 1 μg of K15 WT or K15 F481 and 100 ng of reporter plasmid per well of a six-well plate. The Dscr1 and Cox-2 reporter plasmids contain the promoter regions of the dscr1 and cox-2 genes, respectively, cloned in the promoterless pGL3b vector (A). The pTA-Luc reporter plasmid contains the minimal TA promoter upstream of the luciferase reporter gene. The pNFAT-TA-Luc reporter plasmid additionally contains three tandem copies of the NFAT transcription factor consensus sequence (B). At 20 h posttransfection, cells were serum starved by reducing the serum concentration from 10% to 1% (for dscr1 and cox-2) or 0% (for NFAT). At 29 h posttransfection, cells were lysed, and luciferase activities were determined. As a positive control for NFAT activation, mock-transfected 293-T cells were incubated with TPA and calcium ionophore (IO; both dissolved in DMSO) 4 h before cells were lysed, and treatment of mock-transfected cells with DMSO served as negative control (B). The luciferase activities are depicted as relative activity compared to mock transfections (luciferase activity of mock and pGL3b, Dscr1, and Cox-2 transfections in panel A or mock- and pTA- and pNFAT-TA-cotransfected cells treated with DMSO in panel B was set at 1). The data are representative of three independent experiments, each performed in duplicate. Standard deviations are shown. For both panels, lysates were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting (WB) with the K15 polyclonal antibody. Membranes were stripped and probed with an α-actin antibody as loading control.
K15 activates the NFAT transcription factor in 293-T cells.
Recently, VEGF was reported to induce dscr1 expression via activation of the NFAT transcription factor (50). Given the activation of dscr1 by K15, we investigated whether K15 was able to induce NFAT activity. 293-T cells were transiently cotransfected with K15 expression constructs or the empty vector pFJ-EA together with the control reporter plasmid pTA, containing the TA promoter, or the reporter plasmid pNFAT-TA, which additionally contains three tandem copies of the NFAT transcription factor consensus sequence upstream of the TA promoter. As a positive control for NFAT activation, empty vector-transfected 293-T cells were stimulated with TPA and calcium ionophore 4 h before cells were lysed. In order to reduce serum-induced signaling pathways, cells were serum starved 9 h prior to cell lysis. The luciferase activity of mock-transfected 293-T cells was set at 1 for each reporter plasmid. K15 significantly induced the activity of the NFAT transcription factor in 293-T cells (Fig. 4B), and similar results were obtained in HeLa cells (data not shown). The mutant K15 F481 was impaired in the activation of NFAT in 293-T (Fig. 4B) and HeLa cells (data not shown). K15 WT and mutant K15 F481 were expressed at comparable levels (Fig. 4B).
K15 activates the dscr1 and cox-2 promoters and the NFAT transcription factor in SLK endothelial cells.
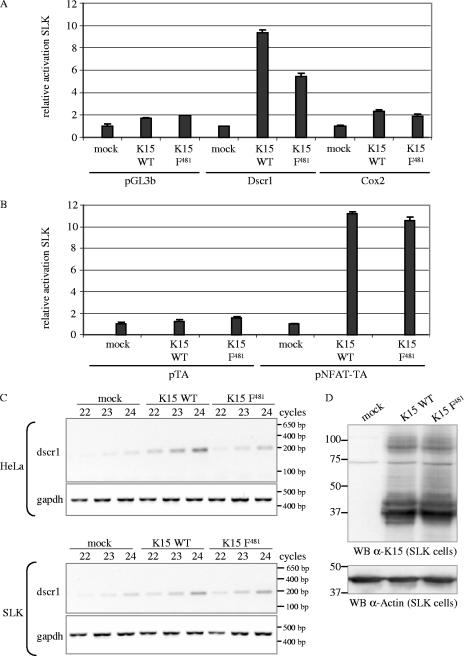
Since KS is a tumor of endothelial origin, we investigated the capability of K15 to induce dscr1 and cox-2 gene expression and NFAT activity in the KSHV-negative SLK endothelial cell line that was established from a KS tumor (49). K15 expression constructs or pFJ-EA were cotransfected together with the Dscr1, Cox-2 (Fig. 5A), or NFAT (Fig. 5B) luciferase reporter plasmids. The luciferase activity is shown as relative activity compared to empty-vector-transfected cells for each reporter. As observed for epithelial cells, K15 WT was able to activate the dscr1 and cox-2 promoters (Fig. 5A) and the NFAT transcription factor (Fig. 5B) in endothelial cells. The K15 F481 mutant was only slightly impaired in the activation of the dscr1 and cox-2 promoters (Fig. 5A) and as potent as K15 WT to induce NFAT activity (Fig. 5B), unlike its effect in epithelial cells (Fig. 4A and B).
FIG. 5.
K15 activates the cox-2 and dscr1 promoter and the NFAT transcription factor in SLK endothelial cells. SLK cells were transiently cotransfected with 3 μg of K15 WT, K15 F481, or pFJ-EA (empty vector, mock) and 1 μg of reporter plasmids pGL3b (negative control), Dscr1, or Cox-2 (A) or 1 μg of reporter plasmids pTA (negative control) or pNFAT-TA (B). At 21 h posttransfection, cells were serum starved by reducing the serum concentration from 10% to 0%. At 29 h posttransfection, cells were lysed, and luciferase activities were determined. The luciferase activities are depicted as relative activity compared to mock transfections. The data are from one experiment representative of three independent experiments, each performed in duplicate. Standard deviations are shown. Equal expression levels of K15 proteins were analyzed by Western blotting (data not shown). (C) Dscr1 and GAPDH semiquantitative RT-PCR. cDNA of SLK or HeLa cells transiently transfected with empty vector (mock) or K15 expression constructs K15 WT or K15 F481 was generated as described in Material and Methods. A semiquantitative PCR with 22, 23, or 24 PCR cycles was performed with dscr1-for and dscr1-rev primers amplifying a spliced transcript of 196 bp. The GAPDH PCR shows that equal amounts of cDNA were utilized for the PCR. (D) To control equal protein expression of K15 WT and K15 F481 in transiently transfected SLK cells, cells were lysed for Western blotting (WB) analysis in parallel to the cells being lysed for RNA preparation and subsequent RT-PCR analysis. K15 proteins were detected with the K15 polyclonal antibody. Blots were stripped and probed with an α-actin antibody as loading control. The transfection efficiency of SLK cells with K15 expression constructs was about 40% as judged by immunofluorescence (data not shown).
In order to confirm the SLK luciferase reporter assay data, we performed a semiquantitative RT-PCR analysis for the dscr1 transcript. RNA was extracted 32 h posttransfection from transfected HeLa and SLK cells, and cDNA was prepared as described for the DNA microarray. The dscr1 primers detect, as do the probes for the inflammatory gene array, three different splice variants of the dscr1 gene. As depicted in Fig. 5C, the dscr1 transcript appeared after fewer PCR cycles in HeLa and SLK cells that were transfected with K15 WT compared to cells transfected with empty vector. The K15 F481 mutant was impaired in the upregulation of dscr1 expression in HeLa but not in SLK cells (Fig. 5C), which confirms the results obtained with the luciferase reporter assays (Fig. 5A). The PCR with primers for the housekeeping gene GAPDH shows equal amounts of cDNA used for the PCRs (Fig. 5C), and equal protein expression of K15 WT and K15 F481 in the SLK cells is shown in Fig. 5D.
DISCUSSION
Previous studies with transiently transfected K15 (exons 1 to 8; aa 1 to 489) had shown that it has an apparent molecular mass of approximately 45 kDa (13, 23, 116). However, it had not been shown up to now whether this protein is expressed in the context of a complete viral genome. Here we show the existence of a 45-kDa K15-derived protein in cells stably transfected with KSHV BAC36 undergoing productive replication. Based on its molecular size and intracellular distribution, this K15 protein appears to be similar to the transiently expressed full-length (exons 1 to 8) K15 protein. The increased expression of the 45-kDa K15 protein in the 293 KSHV BAC36 cell line during the lytic replication cycle correlates well with published RT-PCR analyses (23, 44, 100) and KSHV gene arrays (57, 98) that describe upregulation of K15 mRNA expression upon lytic cycle induction in PEL cell lines. However, low expression levels of K15 transcripts (23, 44, 100) and K15 protein (this study) have been detected in uninduced KSHV-infected cells. Since a small percentage of latently infected cells are known to undergo spontaneous lytic replication (46, 134, 135), we favor the interpretation that K15 expression observed in uninduced KSHV-infected cell cultures may be attributable to the small percentage of cells undergoing spontaneous lytic replication.
In PEL cell lines, a 23-kDa protein recognized by a monoclonal antibody to the cytoplasmic domain of K15 has previously been reported (116). The polyclonal antibody to the cytoplasmic domain of K15 used in our studies (13, 44) detected a similar protein (data not shown). However, we found this protein not to be associated with cellular membranes (data not shown), although a K15-derived protein of similar size and containing the cytoplasmic domain (K15 exons 6 to 8; aa 296 to 489) was found in the membrane fraction (data not shown). An artificially initiated protein containing only the (hydrophilic) cytoplasmic domain (aa 355 to 489) had a lower molecular mass (15 kDa; data not shown), suggesting that the 23-kDa protein in PEL cells is unlikely to represent a proteolytic fragment of a precursor K15 protein that only contains the cytoplasmic domain. We cannot exclude that the 23-kDa protein in PEL cells is derived from K15, but its nature and derivation are not yet resolved.
Since the 293 BAC36 cell line is so far the only cell system in which expression of the endogenous 45-kDa K15 protein could be observed in the context of the entire viral genome, we decided to study its function in epithelial cells. Epithelial cells play a significant role in the establishment of KSHV infection and viral transmission in vivo and in vitro. KSHV DNA has been detected in scattered keratinocytes of the epidermis overlying cutaneous lesions and in eccrine ductular epithelial cells and in spindle and endothelial cells lining the well-formed blood vessels within and surrounding KS lesions (102). Further, KSHV DNA was detected in epithelial cells from several organs (111). Moreover, detection of infectious viral particles in the saliva of KSHV-infected patients suggests that the epithelial cells in the oral mucosa or in salivary glands are a source for the virus (58, 97, 127). KSHV shows a broad host range in culture in that it can efficiently establish latency after de novo infection in many human cells such as HeLa (epithelial origin), human foreskin fibroblasts, and SLK cells (7). Other studies have demonstrated that the human embryonal kidney epithelial cell line 293 (7, 39, 106) as well as cultured primary oral epithelial cells (33) can support KSHV infection in vitro. Furthermore, KSHV can infect and replicate in primary human keratinocytes in vitro (18).
We have now analyzed the downstream targets of K15 signaling using DNA gene array analyses. Our study establishes K15 as one of the KSHV genes that is capable of inducing the expression of multiple cytokines, including in particular IL-8 and IL-6 (Table 1 and Fig. 3), which have been shown to play a role in KSHV-associated pathogenesis. The IL-8 promoter contains an NF-κB element that is required for activation in all cell types studied, as well as a single consensus AP-1 site (22, 51). Numerous lines of evidence exist suggesting that coordinated activation of the MAP kinases ERK, JNK, and p38 is important for IL-8 regulation in response to proinflammatory cytokines such as IL-1 (63), viruses (15), bacteria (8, 45, 80), and various stress inducers (51). The 5′ flanking region of the IL-6 gene contains a number of cis-acting elements, among them an AP-1 (126) and NF-κB (69) consensus site. The existence of these cis elements in the IL-8 and IL-6 gene promoter suggests that K15-mediated activation of cellular transcription factor activity results in the upregulation of cytokine expression. This notion is further supported by the crucial role of the Y481 residue of the K15 SH2-B motif Y481EEV, both in activation of MAP kinase pathways and transcription factors NF-κB and AP-1 (13), as well as in the induction of cytokine expression (Table 1 and Fig. 3). However, K15-induced upregulation of IL-8 and IL-6 may also be mediated indirectly. Expression of IL-1α and IL-1β was induced by wild-type K15 but not by the K15 mutant K15 F481 (Table 1), and IL-1 is known to induce IL-6 and IL-8 production via the JNK MAP kinase pathway (63). Induction of the chemokine CCL20/MIP-3α, which was significantly upregulated by K15 (7.4-fold) but not by K15 F481, has been attributed to tumor necrosis factor alpha and IL-1β, probably via MAP kinases Erk1/2 and p38, in epithelial cells (105). Whether K15 signaling directly upregulates IL-6, IL-8, and/or CCL20/MIP-3α expression cannot be answered at this stage.
Notably, several other KSHV-encoded genes upregulate expression of IL-8 and IL-6, suggesting that dysregulated expression of these cytokines is important for KSHV-associated pathogenesis. The lytic vGPCR constitutively activates NF-κB and secretion of IL-8 in HeLa cells (117) and IL-6 and IL-8 in endothelial cells (95). Kaposin B increases the expression of cytokines by blocking the degradation of their mRNAs (e.g., granulocyte-macrophage colony-stimulating factor and IL-6) via activation of the p38/MK2 pathway in HeLa cells (75). Inflammatory cytokines have been proposed to cooperate with KSHV in promoting the pathogenesis of the virus (36). KS lesions contain elevated levels of mRNA of inflammatory cytokines such as IL-1, IL-6, gamma interferon, tumor necrosis factor alpha and VEGF, and cultured KS spindle cells express IL-1, IL-6, IL-8, and VEGF (34, 35). IL-6 is expressed in KSHV-associated tumors (5, 30, 31, 68, 77, 89, 131, 133) and is highly upregulated and escapes the viral host gene expression shutoff mediated by the KSHV-encoded shutoff exonuclease (ORF37) during lytic KSHV growth (42, 43).
Viral infections are frequently associated with induction of IL-8 production: IL-8 and GROα are induced by KSHV infection of endothelial cells and are crucial to the angiogenic phenotype developed by KSHV-infected endothelial cells in cell culture and upon implantation into SCID mice (66). In addition, human herpesviruses cytomegalovirus (47, 83), EBV (61, 74), and HHV-6 (55) induce IL-8 expression. Dengue virus infection and dengue virus nonstructural protein NS5 and hepatitis C virus nonstructural proteins 4A and 4B were shown to induce IL-8 transcription and secretion in 293 and HeLa cells, respectively (59, 76). Adenovirus-induced activation of the Ras/Erk MAP kinase pathway contributes to IL-8 production (15). A member of the α-Herpesvirinae, Marek's disease virus, causes T-cell lymphomas in chicken and codes for a viral homologue of human IL-8 (vIL-8) (71, 92, 93). Studies with vIL-8 deletion in Marek's disease virus mutants suggest that vIL-8 may serve as a chemoattractant for target cells to the site of infection (27, 28, 93). KSHV does not encode its own vIL-8, but the fact that multiple KSHV-encoded genes upregulate IL-8 expression, such as vGPCR (117), K1 (67), vFLIP (124), and, as shown in this study, K15, underlines the importance of IL-8 expression for KSHV.
We further showed that K15 induced expression of chemokines CXCL3/GROγ, CCL2, CCL5, and CCL20/MIP-3α (Table 1 and Fig. 3). Interestingly, IL-8 and GROγ can bind to the KSHV vGPCR with high affinity and stimulate its signaling activity (41, 108). Since both K15 and vGPCR are expressed during the lytic viral life cycle, K15 might contribute to activation of vGPCR signaling by exerting paracrine effects. vGPCR signaling potently induces cytokine, growth factor, and VEGF secretion, and its angiogenic and tumorigenic activity has been clearly demonstrated in several models (88).
Cytokines IL-1α, IL-1β, and IL-8 are also upregulated by the lytic K1 protein, depending on the intact ITAM motif (67). K15 has no ITAM motif, but the importance of the Y481 residue of the SH2-binding motif Y481EEV for K15 signaling activity in epithelial cells was already demonstrated by our group (13). In this study, we confirm the important role of the Y481 residue in epithelial cells, since the K15 Y481→F481 mutant was impaired in the induction of cytokine, Dscr1, and Cox-2 expression and activation of NFAT. However, in SLK endothelial cells, the K15 F481 mutant was only slightly impaired concerning upregulation of dscr1 and cox-2 gene expression and NFAT activity. This would suggest that different intracellular mediators may be required for K15-dependent signaling in endothelial versus epithelial cells. K15 has multiple binding motifs suitable for interaction with cellular proteins and was already shown to bind TRAFs (13). In addition, the proline-rich motif (PP387PLPP) in the K15 cytoplasmic domain functions as an SH3-B motif for the protein tyrosine kinases Lyn and Hck (M. M. Brinkmann, unpublished data). Studies to identify the role of K15 interaction partners in endothelial cells are currently in progress.
The majority of genes upregulated by K15 were not induced by the K15 F481 mutant (Table 1). However, a subset of genes was upregulated by this mutant (Table 1, highlighted in bold). Among them are (i) death-associated protein kinase 2, which is a Ca2+/calmodulin-dependent cytoskeletal-associated protein kinase involved in apoptosis (25, 29); (ii) syntrophin γ2, which belongs to a family of genes that are suggested to provide a link between the actin cytoskeleton and membrane-associated proteins including ion channels, enzymes, and receptors (12, 40, 91); (iii) prostate differentiation factor/growth differentiation factor 15/macrophage inhibitory cytokine 1, which is a member of the transforming growth factor β superfamily (37); and (iv) fibroblast growth factor 12, which is described to associate with the MAP kinase scaffold protein islet-brain-2, which facilitates recruitment of the MAP kinase p38δ (112, 113). This suggests that other K15 functions, independent of SH2-B interactions and triggering of MAP kinase and transcription factor activity, may be important for K15 activity.
In addition to IL-8, the K15-regulated genes dscr1, cox-2, matrix metalloprotease 1, and tissue factor are also involved in angiogenesis. Upregulation of dscr1 and cox-2 gene expression by K15 was confirmed in luciferase reporter assays and by semiquantitative RT-PCR in epithelial (Fig. 4 and 5) and endothelial cells (Fig. 5). dscr1 has several NFAT binding sites in its promoter region (79) and is the most prominent VEGF-induced gene in endothelial cells (1, 50, 54, 79, 132). The cox-2 promoter encompasses NF-κB, AP-1, and NFAT binding sites (118), and cox-2 is induced by VEGF in an NFAT-dependent manner (1, 48). As we have shown here, K15 activates, similarly to the KSHV lytic genes K1 (65, 67) and vGPCR (17, 96), the NFAT transcription factor in epithelial (Fig. 4 and 5) and endothelial cells (Fig. 5).
VEGF plays a key role in KSHV-associated pathogenesis (3, 26, 72, 109, 110) and is induced by a variety of KSHV genes: K1 (129), vGPCR (6, 119), vIL-6 (2), and vMIP-IA (70). K15 induced vegf gene expression only slightly (Fig. 3E). This could imply that K15 acts by itself as a constitutive activator of VEGF-dependent pathways, able to induce the activity/expression of VEGF-downstream targets NFAT, dscr1, and cox-2. Notably, VEGF-triggered signaling activates the NFAT and AP-1 transcription factors in endothelial cells (4). Interestingly, the MAP kinase JNK, which is induced by K15, was shown to mediate NFATc2 activation (90). Our observation that activation of NFAT and dscr1 and cox-2 gene expression by K15 requires an intact Y481 in epithelial but not in endothelial cells provides indirect evidence that K15-induced gene expression of dscr1 and cox-2 and NFAT activity may indeed be functionally linked.
The dscr1 gene, designated as such because it resides within the Down syndrome critical region of human chromosome 21, belongs to a family of proteins also termed calcipressins or modulatory calcineurin-interacting proteins. Several potential target genes of the serine/threonine phosphatase calcineurin were described in activated endothelial cells, including Cox-2 (48), IL-8 (11), and tissue factor (4), which were all shown to be induced by K15 (Table 1 and Fig. 3, 4, and 5). Induction of dscr1 expression has so far not been described for other KSHV-encoded genes, although several are able to induce VEGF and/or NFAT (see above). However, further experiments are needed to address the role of K15-mediated dcsr1 and cox-2 upregulation. Notably, NFAT was shown to be an important factor for KSHV lytic gene expression (137). These authors have shown that calcineurin-NFAT-dependent signal transduction induces Ca2+-dependent KSHV replication and virus production in PEL cells and endothelial cells. Whether K15-triggered NFAT activity contributes to KSHV replication and/or virus production is currently being addressed in our laboratory.
Cox-2 is rapidly induced by both inflammatory and mitogenic stimuli resulting in increased prostaglandin E2 synthesis (118). Prostaglandins are lipid mediators that have been shown to participate in the regulation of virus replication and modulation of inflammatory responses following infection (123). Cox-2 is induced in murine herpesvirus 68-infected cells, and prostaglandin E2 was shown to increase the production of multiple gene products of murine herpesvirus 68 (125). EBV LMP1 induces Cox-2 protein expression via NF-κB in epithelial cell lines (84). Cox-2 expression was also reported to be increased after KSHV infection (86) and HHV-6 infection (56), and cytomegalovirus replication was shown to be blocked by Cox-2 inhibitors (121, 136).
K15 upregulated the acute phase protein pentraxin-3 (Table 1), which has also been described to be upregulated by KSHV vIL-6 (62). Expression of heme-oxygenase 1, which was slightly induced by K15, was found to be upregulated in KSHV-infected endothelial cells, and increased heme-oxygenase 1 activity in vitro enhanced proliferation of KSHV-infected cells in the presence of free heme (73). Thus, K15 may contribute to the expression of several cellular genes previously noted to be upregulated in virus-infected cells.
Moreover, we have found that K15 upregulated the expression of antiapoptotic genes such as tnfaip3/A20, bf, birc3, birc2, and bcl2a1 (Table 1). K15 has been described to interact with the protein Hax-1 (116). Hax-1 was shown to inhibit Bax-induced apoptosis, but the relevance of its interaction with K15 is not yet clear (116). Taken together, these findings suggest that K15 may also contribute to the inhibition of apoptosis in lytically replicating, virus-producing cells.
Acknowledgments
The luciferase reporter plasmids Dscr1 and Cox-2 were generous gifts of Elia Duh, and the KSHV BAC36 construct was kindly provided by S. J. Gao. We thank V. Kaever for performing the IL-6 and IL-8 ELISA assay. The excellent technical assistance of Heike Schneider and Christa Urban is gratefully acknowledged. We thank Matthias Ottinger for critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 566 B11).
Footnotes
Published ahead of print on 18 October 2006.
REFERENCES
- 1.Abe, M., and Y. Sato. 2001. cDNA microarray analysis of the gene expression profile of VEGF-activated human umbilical vein endothelial cells. Angiogenesis 4:289-298. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 3.Aoki, Y., and G. Tosato. 1999. Role of vascular endothelial growth factor/vascular permeability factor in the pathogenesis of Kaposi's sarcoma-associated herpesvirus-infected primary effusion lymphomas. Blood 94:4247-4254. [PubMed] [Google Scholar]
- 4.Armesilla, A. L., E. Lorenzo, d. A. Gomez, S. Martinez-Martinez, A. Alfranca, and J. M. Redondo. 1999. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol. Cell. Biol. 19:2032-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asou, H., J. W. Said, R. Yang, R. Munker, D. J. Park, N. Kamada, and H. P. Koeffler. 1998. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 91:2475-2481. [PubMed] [Google Scholar]
- 6.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, E. A. Mesri, and M. C. Gerhengorn. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 7.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharyya, A., S. Pathak, S. Datta, S. Chattopadhyay, J. Basu, and M. Kundu. 2002. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem. J. 368:121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boshoff, C., S. J. Gao, L. E. Healy, S. Matthews, A. J. Thomas, L. Coignet, R. A. Warnke, J. A. Strauchen, E. Matutes, O. W. Kamel, P. S. Moore, R. A. Weiss, and Y. Chang. 1998. Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood 91:1671-1679. [PubMed] [Google Scholar]
- 10.Boshoff, C., T. F. Schulz, M. M. Kennedy, A. K. Graham, C. Fisher, A. Thomas, J. O. McGee, R. A. Weiss, and J. J. O'Leary. 1995. Kaposi's sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat. Med. 1:1274-1278. [DOI] [PubMed] [Google Scholar]
- 11.Boss, V., X. Wang, L. F. Koppelman, K. Xu, and T. J. Murphy. 1998. Histamine induces nuclear factor of activated T cell-mediated transcription and cyclosporin A-sensitive interleukin-8 mRNA expression in human umbilical vein endothelial cells. Mol. Pharmacol. 54:264-272. [DOI] [PubMed] [Google Scholar]
- 12.Brenman, J. E., D. S. Chao, S. H. Gee, A. W. McGee, S. E. Craven, D. R. Santillano, Z. Wu, F. Huang, H. Xia, M. F. Peters, S. C. Froehner, and D. S. Bredt. 1996. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell 84:757-767. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann, M. M., M. Glenn, L. Rainbow, A. Kieser, C. Henke-Gendo, and T. F. Schulz. 2003. Activation of mitogen-activated protein kinase and NF-κB pathways by a Kaposi's sarcoma-associated herpesvirus K15 membrane protein. J. Virol. 77:9346-9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann, M. M., and T. F. Schulz. 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J. Gen. Virol. 87:1047-1074. [DOI] [PubMed] [Google Scholar]
- 15.Bruder, J. T., and I. Kovesdi. 1997. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J. Virol. 71:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon, J. S., D. Ciufo, A. L. Hawkins, C. A. Griffin, M. J. Borowitz, G. S. Hayward, and R. F. Ambinder. 2000. A new primary effusion lymphoma-derived cell line yields a highly infectious Kaposi's sarcoma herpesvirus-containing supernatant. J. Virol. 74:10187-10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cannon, M., N. J. Philpott, and E. Cesarman. 2003. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 77:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerimele, F., F. Curreli, S. Ely, A. E. Friedman-Kien, E. Cesarman, and O. Flore. 2001. Kaposi's sarcoma-associated herpesvirus can productively infect primary human keratinocytes and alter their growth properties. J. Virol. 75:2435-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 20.Cesarman, E., and D. M. Knowles. 1999. The role of Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in lymphoproliferative diseases. Semin. Cancer Biol. 9:165-174. [DOI] [PubMed] [Google Scholar]
- 21.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 22.Chinenov, Y., and T. K. Kerppola. 2001. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438-2452. [DOI] [PubMed] [Google Scholar]
- 23.Choi, J. K., B. S. Lee, S. N. Shim, M. Li, and J. U. Jung. 2000. Identification of the novel K15 gene at the rightmost end of the Kaposi's sarcoma-associated herpesvirus genome. J. Virol. 74:436-446. [PMC free article] [PubMed] [Google Scholar]
- 24.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen, O., E. Feinstein, and A. Kimchi. 1997. DAP-kinase is a Ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. EMBO J. 16:998-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornali, E., C. Zietz, R. Benelli, W. Weninger, L. Masiello, G. Breier, E. Tschachler, A. Albini, and M. Sturzl. 1996. Vascular endothelial growth factor regulates angiogenesis and vascular permeability in Kaposi's sarcoma. Am. J. Pathol. 149:1851-1869. [PMC free article] [PubMed] [Google Scholar]
- 27.Cui, X., L. F. Lee, H. D. Hunt, W. M. Reed, B. Lupiani, and S. M. Reddy. 2005. A Marek's disease virus vIL-8 deletion mutant has attenuated virulence and confers protection against challenge with a very virulent plus strain. Avian Dis. 49:199-206. [DOI] [PubMed] [Google Scholar]
- 28.Cui, X., L. F. Lee, W. M. Reed, H. J. Kung, and S. M. Reddy. 2004. Marek's disease virus-encoded vIL-8 gene is involved in early cytolytic infection but dispensable for establishment of latency. J. Virol. 78:4753-4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deiss, L. P., E. Feinstein, H. Berissi, O. Cohen, and A. Kimchi. 1995. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 9:15-30. [DOI] [PubMed] [Google Scholar]
- 30.de Wit, R., M. H. Raasveld, R. J. ten Berge, P. A. van der Wouw, P. J. Bakker, and C. H. Veenhof. 1991. Interleukin-6 concentrations in the serum of patients with AIDS-associated Kaposi's sarcoma during treatment with interferon-alpha. J. Intern. Med. 229:539-542. [DOI] [PubMed] [Google Scholar]
- 31.Dourado, I., O. Martinez-Maza, T. Kishimoto, H. Suzuki, and R. Detels. 1997. Interleukin 6 and AIDS-associated Kaposi's sarcoma: a nested case control study within the Multicenter AIDS Cohort Study. AIDS Res. Hum. Retrovir. 13:781-788. [DOI] [PubMed] [Google Scholar]
- 32.Dupin, N., C. Fisher, P. Kellam, S. Ariad, M. Tulliez, N. Franck, E. van Marck, D. Salmon, I. Gorin, J. P. Escande, R. A. Weiss, K. Alitalo, and C. Boshoff. 1999. Distribution of human herpesvirus-8 latently infected cells in Kaposi's sarcoma, multicentric Castleman's disease, and primary effusion lymphoma. Proc. Natl. Acad. Sci. USA 96:4546-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duus, K. M., V. Lentchitsky, T. Wagenaar, C. Grose, and J. Webster-Cyriaque. 2004. Wild-type Kaposi's sarcoma-associated herpesvirus isolated from the oropharynx of immune-competent individuals has tropism for cultured oral epithelial cells. J. Virol. 78:4074-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ensoli, B., and M. Sturzl. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63-83. [DOI] [PubMed] [Google Scholar]
- 35.Ensoli, B., M. Sturzl, and P. Monini. 2000. Cytokine-mediated growth promotion of Kaposi's sarcoma and primary effusion lymphoma. Semin. Cancer Biol. 10:367-381. [DOI] [PubMed] [Google Scholar]
- 36.Ensoli, B., M. Sturzl, and P. Monini. 2001. Reactivation and role of HHV-8 in Kaposi's sarcoma initiation. Adv. Cancer Res. 81:161-200. [DOI] [PubMed] [Google Scholar]
- 37.Fairlie, W. D., A. G. Moore, A. R. Bauskin, P. K. Russell, H. P. Zhang, and S. N. Breit. 1999. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 65:2-5. [DOI] [PubMed] [Google Scholar]
- 38.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 39.Foreman, K. E., J. Friborg, Jr., W. P. Kong, C. Woffendin, P. J. Polverini, B. J. Nickoloff, and G. J. Nabel. 1997. Propagation of a human herpesvirus from AIDS-associated Kaposi's sarcoma. N. Engl. J. Med. 336:163-171. [DOI] [PubMed] [Google Scholar]
- 40.Gee, S. H., R. Madhavan, S. R. Levinson, J. H. Caldwell, R. Sealock, and S. C. Froehner. 1998. Interaction of muscle and brain sodium channels with multiple members of the syntrophin family of dystrophin-associated proteins. J. Neurosci. 18:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gershengorn, M. C., E. Geras-Raaka, A. Varma, and I. Clark-Lewis. 1998. Chemokines activate Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor in mammalian cells in culture. J. Clin. Investig. 102:1469-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glaunsinger, B., and D. Ganem. 2004. Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J. Exp. Med. 200:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glaunsinger, B., and D. Ganem. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13:713-723. [DOI] [PubMed] [Google Scholar]
- 44.Glenn, M., L. Rainbow, F. Aurade, A. Davison, and T. F. Schulz. 1999. Identification of a spliced gene from Kaposi's sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J. Virol. 73:6953-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grassl, G. A., M. Kracht, A. Wiedemann, E. Hoffmann, M. Aepfelbacher, C. Eichel-Streiber, E. Bohn, and I. B. Autenrieth. 2003. Activation of NF-kappaB and IL-8 by Yersinia enterocolitica invasin protein is conferred by engagement of Rac1 and MAP kinase cascades. Cell Microbiol. 5:957-971. [DOI] [PubMed] [Google Scholar]
- 46.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113:124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grundy, J. E., K. M. Lawson, L. P. MacCormac, J. M. Fletcher, and K. L. Yong. 1998. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 177:1465-1474. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez, G. L., O. V. Volpert, M. A. Iniguez, E. Lorenzo, S. Martinez-Martinez, R. Grau, M. Fresno, and J. M. Redondo. 2001. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J. Exp. Med. 193:607-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herndier, B. G., A. Werner, P. Arnstein, N. W. Abbey, F. Demartis, R. L. Cohen, M. A. Shuman, and J. A. Levy. 1994. Characterization of a human Kaposi's sarcoma cell line that induces angiogenic tumors in animals. AIDS 8:575-581. [DOI] [PubMed] [Google Scholar]
- 50.Hesser, B. A., X. H. Liang, G. Camenisch, S. Yang, D. A. Lewin, R. Scheller, N. Ferrara, and H. P. Gerber. 2004. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood 104:149-158. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann, E., O. Dittrich-Breiholz, H. Holtmann, and M. Kracht. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847-855. [PubMed] [Google Scholar]
- 52.Hoffmann, E., A. Thiefes, D. Buhrow, O. Dittrich-Breiholz, H. Schneider, K. Resch, and M. Kracht. 2005. MEK1-dependent delayed expression of Fos-related antigen-1 counteracts c-Fos and p65 NF-κB-mediated interleukin-8 transcription in response to cytokines or growth factors. J. Biol. Chem. 280:9706-9718. [DOI] [PubMed] [Google Scholar]
- 53.Holzberg, D., C. G. Knight, O. Dittrich-Breiholz, H. Schneider, A. Dorrie, E. Hoffmann, K. Resch, and M. Kracht. 2003. Disruption of the c-JUN-JNK complex by a cell-permeable peptide containing the c-JUN delta domain induces apoptosis and affects a distinct set of interleukin-1-induced inflammatory genes. J. Biol. Chem. 278:40213-40223. [DOI] [PubMed] [Google Scholar]
- 54.Iizuka, M., M. Abe, K. Shiiba, I. Sasaki, and Y. Sato. 2004. Down syndrome candidate region 1,a downstream target of VEGF, participates in endothelial cell migration and angiogenesis. J. Vasc. Res. 41:334-344. [DOI] [PubMed] [Google Scholar]
- 55.Inagi, R., R. Guntapong, M. Nakao, Y. Ishino, K. Kawanishi, Y. Isegawa, and K. Yamanishi. 1996. Human herpesvirus 6 induces IL-8 gene expression in human hepatoma cell line, Hep G2. J. Med. Virol. 49:34-40. [DOI] [PubMed] [Google Scholar]
- 56.Janelle, M. E., A. Gravel, J. Gosselin, M. J. Tremblay, and L. Flamand. 2002. Activation of monocyte cyclooxygenase-2 gene expression by human herpesvirus 6. Role for cyclic AMP-responsive element-binding protein and activator protein-1. J. Biol. Chem. 277:30665-30674. [DOI] [PubMed] [Google Scholar]
- 57.Jenner, R. G., M. M. Alba, C. Boshoff, and P. Kellam. 2001. Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J. Virol. 75:891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson, A. S., N. Maronian, and J. Vieira. 2005. Activation of Kaposi's sarcoma-associated herpesvirus lytic gene expression during epithelial differentiation. J. Virol. 79:13769-13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kadoya, H., M. Nagano-Fujii, L. Deng, N. Nakazono, and H. Hotta. 2005. Nonstructural proteins 4A and 4B of hepatitis C virus transactivate the interleukin 8 promoter. Microbiol. Immunol. 49:265-273. [DOI] [PubMed] [Google Scholar]
- 60.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 2000. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi's sarcoma, and multicentric Castleman's disease. Virology 269:335-344. [DOI] [PubMed] [Google Scholar]
- 61.Klein, S. C., D. Kube, H. Abts, V. Diehl, and H. Tesch. 1996. Promotion of IL8, IL10, TNF alpha and TNF beta production by EBV infection. Leuk. Res. 20:633-636. [DOI] [PubMed] [Google Scholar]
- 62.Klouche, M., N. Brockmeyer, C. Knabbe, and S. Rose-John. 2002. Human herpesvirus 8-derived viral IL-6 induces PTX3 expression in Kaposi's sarcoma cells. AIDS 16:F9-F18. [DOI] [PubMed] [Google Scholar]
- 63.Krause, A., H. Holtmann, S. Eickemeier, R. Winzen, M. Szamel, K. Resch, J. Saklatvala, and M. Kracht. 1998. Stress-activated protein kinase/Jun N-terminal kinase is required for interleukin (IL)-1-induced IL-6 and IL-8 gene expression in the human epidermal carcinoma cell line KB. J. Biol. Chem. 273:23681-23689. [DOI] [PubMed] [Google Scholar]
- 64.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lagunoff, M., R. Majeti, A. Weiss, and D. Ganem. 1999. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 96:5704-5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane, B. R., J. Liu, P. J. Bock, D. Schols, M. J. Coffey, R. M. Strieter, P. J. Polverini, and D. M. Markovitz. 2002. Interleukin-8 and growth-regulated oncogene alpha mediate angiogenesis in Kaposi's sarcoma. J. Virol. 76:11570-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee, B. S., S. H. Lee, P. Feng, H. Chang, N. H. Cho, and J. U. Jung. 2005. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. J. Virol. 79:12173-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leger-Ravet, M. B., M. Peuchmaur, O. Devergne, J. Audouin, M. Raphael, J. Van Damme, P. Galanaud, J. Diebold, and D. Emilie. 1991. Interleukin-6 gene expression in Castleman's disease. Blood 78:2923-2930. [PubMed] [Google Scholar]
- 69.Libermann, T. A., and D. Baltimore. 1990. Activation of interleukin-6 gene expression through the NF-κ B transcription factor. Mol. Cell. Biol. 10:2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu, C., Y. Okruzhnov, H. Li, and J. Nicholas. 2001. Human herpesvirus 8 (HHV-8)-encoded cytokines induce expression of and autocrine signaling by vascular endothelial growth factor (VEGF) in HHV-8-infected primary-effusion lymphoma cell lines and mediate VEGF-independent antiapoptotic effects. J. Virol. 75:10933-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu, J. L., S. F. Lin, L. Xia, P. Brunovskis, D. Li, I. Davidson, L. F. Lee, and H. J. Kung. 1999. MEQ and V-IL8: cellular genes in disguise? Acta Virol. 43:94-101. [PubMed] [Google Scholar]
- 72.Masood, R., E. Cesarman, D. L. Smith, P. S. Gill, and O. Flore. 2002. Human herpesvirus-8-transformed endothelial cells have functionally activated vascular endothelial growth factor/vascular endothelial growth factor receptor. Am. J. Pathol. 160:23-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McAllister, S. C., S. G. Hansen, R. A. Ruhl, C. M. Raggo, V. R. DeFilippis, D. Greenspan, K. Fruh, and A. V. Moses. 2004. Kaposi sarcoma-associated herpesvirus (KSHV) induces heme oxygenase-1 expression and activity in KSHV-infected endothelial cells. Blood 103:3465-3473. [DOI] [PubMed] [Google Scholar]
- 74.McColl, S. R., C. J. Roberge, B. Larochelle, and J. Gosselin. 1997. EBV induces the production and release of IL-8 and macrophage inflammatory protein-1 alpha in human neutrophils. J. Immunol. 159:6164-6168. [PubMed] [Google Scholar]
- 75.McCormick, C., and D. Ganem. 2005. The kaposin B protein of KSHV activates the p38/MK2 pathway and stabilizes cytokine mRNAs. Science 307:739-741. [DOI] [PubMed] [Google Scholar]
- 76.Medin, C. L., K. A. Fitzgerald, and A. L. Rothman. 2005. Dengue virus nonstructural protein NS5 induces interleukin-8 transcription and secretion. J. Virol. 79:11053-11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miles, S. A., A. R. Rezai, J. F. Salazar-Gonzalez, M. M. Vander, R. H. Stevens, D. M. Logan, R. T. Mitsuyasu, T. Taga, T. Hirano, and T. Kishimoto. 1990. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc. Natl. Acad. Sci. USA 87:4068-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minami, T., K. Horiuchi, M. Miura, M. R. Abid, W. Takabe, N. Noguchi, T. Kohro, X. Ge, H. Aburatani, T. Hamakubo, T. Kodama, and W. C. Aird. 2004. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J. Biol. Chem. 279:50537-50554. [DOI] [PubMed] [Google Scholar]
- 80.Mitsuno, Y., H. Yoshida, S. Maeda, K. Ogura, Y. Hirata, T. Kawabe, Y. Shiratori, and M. Omata. 2001. Helicobacter pylori induced transactivation of SRE and AP-1 through the ERK signalling pathway in gastric cancer cells. Gut 49:18-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 82.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murayama, T., N. Mukaida, H. Sadanari, N. Yamaguchi, K. S. Khabar, J. Tanaka, K. Matsushima, S. Mori, and Y. Eizuru. 2000. The immediate early gene 1 product of human cytomegalovirus is sufficient for up-regulation of interleukin-8 gene expression. Biochem. Biophys. Res. Commun. 279:298-304. [DOI] [PubMed] [Google Scholar]
- 84.Murono, S., H. Inoue, T. Tanabe, I. Joab, T. Yoshizaki, M. Furukawa, and J. S. Pagano. 2001. Induction of cyclooxygenase-2 by Epstein-Barr virus latent membrane protein 1 is involved in vascular endothelial growth factor production in nasopharyngeal carcinoma cells. Proc. Natl. Acad. Sci. USA 98:6905-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakamura, H., M. Lu, Y. Gwack, J. Souvlis, S. L. Zeichner, and J. U. Jung. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J. Virol. 77:4205-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 87.Neipel, F., J. C. Albrecht, and B. Fleckenstein. 1997. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J. Virol. 71:4187-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nicholas, J. 2003. Human herpesvirus-8-encoded signalling ligands and receptors. J. Biomed. Sci. 10:475-489. [DOI] [PubMed] [Google Scholar]
- 89.Oksenhendler, E., G. Carcelain, Y. Aoki, E. Boulanger, A. Maillard, J. P. Clauvel, and F. Agbalika. 2000. High levels of human herpesvirus 8 viral load, human interleukin-6, interleukin-10, and C reactive protein correlate with exacerbation of multicentric castleman disease in HIV-infected patients. Blood 96:2069-2073. [PubMed] [Google Scholar]
- 90.Ortega-Perez, I., E. Cano, F. Were, M. Villar, J. Vazquez, and J. M. Redondo. 2005. c-Jun N-terminal kinase (JNK) positively regulates NFATc2 transactivation through phosphorylation within the N-terminal regulatory domain. J. Biol. Chem. 280:20867-20878. [DOI] [PubMed] [Google Scholar]
- 91.Ou, Y., P. Strege, S. M. Miller, J. Makielski, M. Ackerman, S. J. Gibbons, and G. Farrugia. 2003. Syntrophin gamma 2 regulates SCN5A gating by a PDZ domain-mediated interaction. J. Biol. Chem. 278:1915-1923. [DOI] [PubMed] [Google Scholar]
- 92.Parcells, M. S., V. Arumugaswami, J. T. Prigge, K. Pandya, and R. L. Dienglewicz. 2003. Marek's disease virus reactivation from latency: changes in gene expression at the origin of replication. Poult. Sci. 82:893-898. [DOI] [PubMed] [Google Scholar]
- 93.Parcells, M. S., S. F. Lin, R. L. Dienglewicz, V. Majerciak, D. R. Robinson, H. C. Chen, Z. Wu, G. R. Dubyak, P. Brunovskis, H. D. Hunt, L. F. Lee, and H. J. Kung. 2001. Marek's disease virus (MDV) encodes an interleukin-8 homolog (vIL-8): characterization of the vIL-8 protein and a vIL-8 deletion mutant MDV. J. Virol. 75:5159-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pati, S., J. S. Foulke, Jr., O. Barabitskaya, J. Kim, B. C. Nair, D. Hone, J. Smart, R. A. Feldman, and M. Reitz. 2003. Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat. J. Virol. 77:5759-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pauk, J., M. L. Huang, S. J. Brodie, A. Wald, D. M. Koelle, T. Schacker, C. Celum, S. Selke, and L. Corey. 2000. Mucosal shedding of human herpesvirus 8 in men. N. Engl. J. Med. 343:1369-1377. [DOI] [PubMed] [Google Scholar]
- 98.Paulose-Murphy, M., N. K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Polson, A. G., D. Wang, J. DeRisi, and D. Ganem. 2002. Modulation of host gene expression by the constitutively active G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus. Cancer Res. 62:4525-4530. [PubMed] [Google Scholar]
- 100.Poole, L. J., J. C. Zong, D. M. Ciufo, D. J. Alcendor, J. S. Cannon, R. Ambinder, J. M. Orenstein, M. S. Reitz, and G. S. Hayward. 1999. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J. Virol. 73:6646-6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by ORF73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reed, J. A., R. G. Nador, D. Spaulding, Y. Tani, E. Cesarman, and D. M. Knowles. 1998. Demonstration of Kaposi's sarcoma-associated herpes virus cyclin D homolog in cutaneous Kaposi's sarcoma by colorimetric in situ hybridization using a catalyzed signal amplification system. Blood 91:3825-3832. [PubMed] [Google Scholar]
- 103.Regezi, J. A., L. A. Macphail, T. E. Daniels, Y. G. DeSouza, J. S. Greenspan, and D. Greenspan. 1993. Human immunodeficiency virus-associated oral Kaposi's sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am. J. Pathol. 143:240-249. [PMC free article] [PubMed] [Google Scholar]
- 104.Regezi, J. A., L. A. Macphail, T. E. Daniels, J. S. Greenspan, D. Greenspan, C. L. Dodd, F. Lozada-Nur, G. S. Heinic, H. Chinn, and S. Silverman, Jr. 1993. Oral Kaposi's sarcoma: a 10-year retrospective histopathologic study. J. Oral Pathol. Med. 22:292-297. [DOI] [PubMed] [Google Scholar]
- 105.Reibman, J., Y. Hsu, L. C. Chen, B. Bleck, and T. Gordon. 2003. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am. J. Respir. Cell Mol. Biol. 28:648-654. [DOI] [PubMed] [Google Scholar]
- 106.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 108.Rosenkilde, M. M., T. N. Kledal, H. Brauner-Osborne, and T. W. Schwartz. 1999. Agonists and inverse agonists for the herpesvirus 8-encoded constitutively active seven-transmembrane oncogene product, ORF-74. J. Biol. Chem. 274:956-961. [DOI] [PubMed] [Google Scholar]
- 109.Samaniego, F., P. D. Markham, R. C. Gallo, and B. Ensoli. 1995. Inflammatory cytokines induce AIDS-Kaposi's sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi's sarcoma-like lesion formation in nude mice. J. Immunol. 154:3582-3592. [PubMed] [Google Scholar]
- 110.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, J. A. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 152:1433-1443. [PMC free article] [PubMed] [Google Scholar]
- 111.Sanchez-Velasco, P., J. G. Ocejo-Vinyals, R. Flores, J. J. Gomez-Roman, M. J. Lozano, and F. Leyva-Cobian. 2001. Simultaneous multiorgan presence of human herpesvirus 8 and restricted lymphotropism of Epstein-Barr virus DNA sequences in a human immunodeficiency virus-negative immunodeficient infant. J. Infect. Dis. 183:338-342. [DOI] [PubMed] [Google Scholar]
- 112.Schoorlemmer, J., and M. Goldfarb. 2001. Fibroblast growth factor homologous factors are intracellular signaling proteins. Curr. Biol. 11:793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schoorlemmer, J., and M. Goldfarb. 2002. Fibroblast growth factor homologous factors and the islet brain-2 scaffold protein regulate activation of a stress-activated protein kinase. J. Biol. Chem. 277:49111-49119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schulz, T. F. 2000. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8): epidemiology and pathogenesis. J. Antimicrob. Chemother. 45(Suppl. T3):15-27. [DOI] [PubMed] [Google Scholar]
- 115.Schulz, T. F. 2001. KSHV/HHV8-associated lymphoproliferations in the AIDS setting. Eur. J. Cancer. 37:1217-1226. [DOI] [PubMed] [Google Scholar]
- 116.Sharp, T. V., H. W. Wang, A. Koumi, D. Hollyman, Y. Endo, H. Ye, M. Q. Du, and C. Boshoff. 2002. K15 protein of Kaposi's sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J. Virol. 76:802-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shepard, L. W., M. Yang, P. Xie, D. D. Browning, T. Voyno-Yasenetskaya, T. Kozasa, and R. D. Ye. 2001. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve G α(13) and RhoA. J. Biol. Chem. 276:45979-45987. [DOI] [PubMed] [Google Scholar]
- 118.Smith, W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145-182. [DOI] [PubMed] [Google Scholar]
- 119.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 120.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, and L. Degos. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 121.Speir, E., Z. X. Yu, V. J. Ferrans, E. S. Huang, and S. E. Epstein. 1998. Aspirin attenuates cytomegalovirus infectivity and gene expression mediated by cyclooxygenase-2 in coronary artery smooth muscle cells. Circ. Res. 83:210-216. [DOI] [PubMed] [Google Scholar]
- 122.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steer, S. A., and J. A. Corbett. 2003. The role and regulation of COX-2 during viral infection. Viral Immunol. 16:447-460. [DOI] [PubMed] [Google Scholar]
- 124.Sun, Q., H. Matta, G. Lu, and P. M. Chaudhary. 2006. Induction of IL-8 expression by human herpesvirus 8 encoded vFLIP K13 via NF-κB activation. Oncogene 25:2717-2726. [DOI] [PubMed] [Google Scholar]
- 125.Symensma, T. L., D. Martinez-Guzman, Q. Jia, E. Bortz, T. T. Wu, N. Rudra-Ganguly, S. Cole, H. Herschman, and R. Sun. 2003. COX-2 induction during murine gammaherpesvirus 68 infection leads to enhancement of viral gene expression. J. Virol. 77:12753-12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tanabe, O., S. Akira, T. Kamiya, G. G. Wong, T. Hirano, and T. Kishimoto. 1988. Genomic structure of the murine IL-6 gene. High degree conservation of potential regulatory sequences between mouse and human. J. Immunol. 141:3875-3881. [PubMed] [Google Scholar]
- 127.Vieira, J., M. L. Huang, D. M. Koelle, and L. Corey. 1997. Transmissible Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in saliva of men with a history of Kaposi's sarcoma. J. Virol. 71:7083-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vieira, J., and P. M. O'Hearn. 2004. Use of the red fluorescent protein as a marker of Kaposi's sarcoma-associated herpesvirus lytic gene expression. Virology 325:225-240. [DOI] [PubMed] [Google Scholar]
- 129.Wang, L., N. Wakisaka, C. C. Tomlinson, S. M. DeWire, S. Krall, J. S. Pagano, and B. Damania. 2004. The Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) K1 protein induces expression of angiogenic and invasion factors. Cancer Res. 64:2774-2781. [DOI] [PubMed] [Google Scholar]
- 130.Wong, E. L., and B. Damania. 2006. Transcriptional regulation of the Kaposi's sarcoma-associated herpesvirus K15 gene. J. Virol. 80:1385-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang, J., M. K. Hagan, and M. K. Offermann. 1994. Induction of IL-6 gene expression in Kaposi's sarcoma cells. J. Immunol. 152:943-955. [PubMed] [Google Scholar]
- 132.Yao, Y. G., and E. J. Duh. 2004. VEGF selectively induces Down syndrome critical region 1 gene expression in endothelial cells: a mechanism for feedback regulation of angiogenesis? Biochem. Biophys. Res. Commun. 321:648-656. [DOI] [PubMed] [Google Scholar]
- 133.Yoshizaki, K., T. Matsuda, N. Nishimoto, T. Kuritani, L. Taeho, K. Aozasa, T. Nakahata, H. Kawai, H. Tagoh, and T. Komori. 1989. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood 74:1360-1367. [PubMed] [Google Scholar]
- 134.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou, F. C., Y. J. Zhang, J. H. Deng, X. P. Wang, H. Y. Pan, E. Hettler, and S. J. Gao. 2002. Efficient infection by a recombinant Kaposi's sarcoma-associated herpesvirus cloned in a bacterial artificial chromosome: application for genetic analysis. J. Virol. 76:6185-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu, H., J. P. Cong, D. Yu, W. A. Bresnahan, and T. E. Shenk. 2002. Inhibition of cyclooxygenase 2 blocks human cytomegalovirus replication. Proc. Natl. Acad. Sci. USA 99:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zoeteweij, J. P., A. V. Moses, A. S. Rinderknecht, D. A. Davis, W. W. Overwijk, R. Yarchoan, J. M. Orenstein, and A. Blauvelt. 2001. Targeted inhibition of calcineurin signaling blocks calcium-dependent reactivation of Kaposi sarcoma-associated herpesvirus. Blood 97:2374-2380. [DOI] [PubMed] [Google Scholar]