Abstract
The ExPortal of Streptococcus pyogenes is a membrane microdomain dedicated to the secretion and folding of proteins. We investigated the lipid composition of the ExPortal by examining the distribution of anionic membrane phospholipids. Staining with 10-N-nonyl-acridine orange revealed a single microdomain enriched with an anionic phospholipid whose staining characteristics and behavior in a cardiolipin-deficient mutant were characteristic of phosphatidylglycerol. Furthermore, the location of the microdomain corresponded to the site of active protein secretion at the ExPortal. These results indicate that the ExPortal is an asymmetric lipid microdomain, whose enriched content of anionic phospholipids may play an important role in ExPortal organization and protein trafficking.
Secretion of proteins across the single cellular membrane plays a key role in the pathogenesis of infections caused by gram-positive pathogens. These virulence-associated proteins have a number of distinct postsecretion trafficking fates. Some must be delivered into the bacterial membrane, while others are routed to the bacterial cell wall for display on the cell surface. Yet others are secreted into the extracellular milieu, and a subset of these are routed to receptors on the host cell membrane. In certain cases, secreted bacterial effector proteins are translocated across a host cell membrane into the host cell's cytosolic compartment (26). How a gram-positive cell coordinates protein trafficking and the specific signals responsible for correct routing are only beginning to be understood.
A unique solution to the coordination problem has been observed with Streptococcus pyogenes, a pathogen responsible for a number of diseases ranging from superficial localized infection (e.g., impetigo) to life-threatening invasive diseases (e.g., necrotizing fasciitis). This bacterium possesses an ExPortal, a distinct membrane microdomain that contains a high concentration of the translocons of the general secretory (Sec) pathway, such that it is the primary cellular site for protein secretion (33). In addition, the microdomain also accumulates a high concentration of at least one protein (HtrA) that functions as an accessory factor in postsecretion folding (34), suggesting that the ExPortal functions to spatially couple secretion with protein maturation. Localized secretion has also been observed for two surface adhesins, M protein and protein F, though the mechanisms responsible for site-specific targeting remain unclear (7). Restricted distribution of the secretion machinery is not unique to S. pyogenes, since the translocons of Bacillus subtilis have been found to localize to specific clusters that follow a spiral-like pattern around the cell independently of any known helical structures (3, 6). Taken together, these data suggest that the ExPortal may play an important role in coordinating the trafficking of polypeptides to a number of different postsecretion fates.
An understanding of protein trafficking should also include an analysis of the signals responsible for retention of proteins at the ExPortal itself. The observation that the F1Fo ATPase of S. pyogenes is found in the peripheral membrane, rather than the ExPortal (34), shows that retention is not an intrinsic fate of all membrane proteins. The signals may be unique, since the localization patterns for M protein and protein F are distinct, indicating that different signals may be playing a role in the observed localization patterns (7). Interestingly, the ability of the translocons of B. subtilis to cluster to discrete sites was dependent on the presence of anionic phospholipids in the membrane (3). Thus, it was of interest to determine the distribution of anionic phospholipids in relation to the ExPortal in S. pyogenes.
MATERIALS AND METHODS
Strains, media, and culture conditions.
Strains used included Escherichia coli DH5α, B. subtilis JH462 (32), and S. pyogenes HSC5 (14). S. pyogenes strain ΩcovR is derived from HSC5 (8), and the construction of several additional mutant derivatives of HSC5 is described below. Routine culture of S. pyogenes was at 37°C and employed Todd-Hewitt medium (BBL) supplemented with 0.2% yeast extract (Difco). Culture of E. coli and B. subtilis utilized LB broth at 37°C with shaking. Proteolytic assays employed strains grown in C medium that were cultured under the conditions previously described (25). When required, antibiotics were used at the following concentrations: erythromycin, 750 μg/ml for E. coli and 1 μg/ml for S. pyogenes; spectinomycin, 100 μg/ml for both E. coli and S. pyogenes.
DNA and computational techniques.
Plasmid DNA was isolated via standard techniques and used to transform S. pyogenes as described previously (5). Restriction endonucleases, ligases, and polymerases were used according to the manufacturer's recommendations. The fidelity of all constructs derived by PCR was confirmed by DNA sequencing analyses. All references to genomic loci are based on the genome of S. pyogenes strain SF370 (12). Gene assignments were based on the information available in the Kyoto Encyclopedia of Genes and Genomes (www.genome.jp) and were supported by subsequent interrogation of the SF370 genome using BLAST (1) and query sequences derived from Bacillus subtilis gene products with experimentally confirmed activities, as noted.
Construction of mutants.
A mutant (JWR1) with an in-frame deletion of the gene encoding cardiolipin synthase (Spy1212) was constructed as follows: Primers clsFBamHI (AAG GAT CCG CTG ACT ACA AAC GTC TCT ATT CAG ATG AGG) and clsRXhoI (AAC TCG AG G CAA TGG TAA TAT ATC CAG CAT CCA TCA AAC G) were used to amplify a DNA fragment carrying Spy1212, which was subsequently inserted into pJRS233 using the restriction sites embedded in the primers (underlined). An in-frame deletion was constructed by an inverse PCR technique (23) to delete an internal fragment encompassing approximately 1 kb of the 1.6-kb sequence using the primers clsIFDF (AAC TGC AGG CTA AGG TAA GGT GCA GAC AGA TCT AC) and clsIFDR (AAC TGC AGG GAA CAA TTA ACT TAG ACT ATC GTA GCC TTT ATC). Similarly, an attempt to construct a mutant lacking phospytidyl glycerol synthase (Spy2196) was made using amplification primers pgsABam (AAG GAT CCG GGC ACG CCC TTC AAA AGG AGT CCC TGC TTG ATA AG) and pgsARXho (AAC TCG AGG GGA TTT CTT CCC ATT ACT TGC TGG CAA TGG AAT TGG) and inverse primers pgsAIFD-F (AAG AAT TCG GAA GTA ATA AAA AGA AAG AAA GGA ATC ATT GC) and pgsAIFD-R (AAG AAT TCC TGG ATA TGA TTA TTT TAA AGG AGC AAG CTT T). Allelic replacement proceeded as described previously (23), with confirmation of mutant genome structure by PCR using primers of the appropriate sequences. An insertion mutation in covR was introduced into JWR1 as described previously (8).
Measurement of cell-free protease activity.
The amount of SpeB cysteine protease activity in cell supernatants was determined using the substrate fluorescein isothiocyanate-casein (Sigma) as described elsewhere (15). Processing of the SpeB zymogen was monitored by Western blotting as described previously (24). Routine addition of the cysteine protease-specific inhibitor E64 (final concentration, 10 mM; Sigma) to selected samples confirmed that all protease activity monitored was due to SpeB.
Cellular fractionation and preparation of membranes.
Protoplasts were prepared as previously described (31) and then lysed by multiple freeze-thaw cycles (−80°C/37°C) followed by agitation with glass beads (106 μm; Sigma) using a reciprocating shaking device (FastPrep; Qbiogene) at a speed setting of 4.5 for 45 s, repeated four or five times. Membranes were collected by centrifugation (120,000 × g, 4°C, 60 min) and resuspended in 1 ml of distilled water. Extraction was performed as previously described (18). An internal standard was added (1,1,2,2-tetramyristoyl cardiolipin; Avanti Polar Lipids), followed by 1.25 ml of chloroform and 2.5 ml of methanol. Following 2 min of vortexing, an additional 1.25 ml of chloroform was added, followed by a 30-s vortex. Then, 1.25 ml of distilled water was added followed by a 30-s vortex. The solution was spun at 3,000 × g for 5 min and the lower phase collected. This solution was washed twice with a solution containing 1 part chloroform:1 part methanol:0.9 parts distilled water. Final extracts containing the lipids were then concentrated under nitrogen gas until samples were reduced to dryness.
Analysis of membrane composition.
Analysis of the anionic phospholipid content of membranes prepared as described above was done as described previously (17). Low-energy CAD tandem mass spectrometry experiments were conducted on a Finnigan (San Jose, CA) TSQ 7000 mass spectrometer equipped with an ICIS data system or on a LCQ DECA ion-trap mass spectrometer with the Xcalibur operation system.
Cellular staining and microscopy.
Nonyl acridine orange (NAO) (catalog no. A-1372; Molecular Probes) was added to growing cultures (C media; optical density at 600 nm = 0.4) at a final concentration of 1 μM, which did not inhibit growth of either S. pyogenes, B. subtilis, or E. coli upon overnight culture. Samples were stained for 15 min at 37°C, visualized with a model DM IRE 2 microscope, and examined for fluorescence in both the green (green fluorescent protein filter) and red (rhodamine filter) channels. Localization of the site of secretion of the SpeB cysteine protease was conducted using the red protease assay as described previously (33). Nile red (catalog no. N-1142; Molecular Probes) staining was performed under the same growth conditions at a final concentration of 10 μg/ml (9). Double staining to ensure membrane integrity was performed by staining with both 10-N-nonyl acridine orange and Nile red. Images were captured using a QImaging Retiga 1350 EX charged-coupled-device camera and Openlab software (Improvision) and processed for publication using Adobe Photoshop 7.0.
RESULTS AND DISCUSSION
Anionic lipid domains in S. pyogenes.
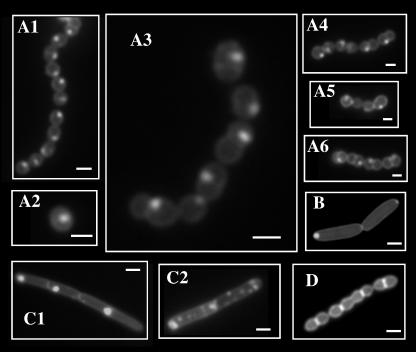
Anionic lipids have long been known to play an important role in protein translocation across the bacterial membrane (11) and in determination of the topology of membrane proteins (40). Visualization of anionic lipids using the fluorescent dye NAO revealed that Escherichia coli and B. subtilis membranes contain anionic lipid-rich domains at their septal regions and at their poles (19, 28). For examination of S. pyogenes, cells from exponentially growing liquid cultures were stained directly in the growth medium with NAO and examined essentially as described previously (28). This revealed a nonuniform punctate pattern of membrane staining (Fig. 1 A1 to A6). However, unlike the case with E. coli (Fig. 1 B) or B. subtilis (Fig. 1 C), where staining was observed primarily at the poles (Fig. 1 C1) or occasionally in a spiral-like pattern in B. subtilis (Fig. 1 C2), the single anionic lipid-rich domain of each S. pyogenes cell was located at a hemispherical position reminiscent of the location of the ExPortal (Fig. 1A). The NAO stain specifically recognized this hemispherical domain, since treatment with the nonpolar lipid stain Nile red did not stain a particular domain but rather the entire circumferential membrane (Fig. 1D).
FIG. 1.
S. pyogenes concentrates anionic phospholipids at discrete microdomains. Various bacterial species were stained with NAO and examined by fluorescence microscopy as described in Materials and Methods. Species included WT S. pyogenes (several representative images are shown in panels A1 to A6), E. coli (panel B), and B. subtilis (panels C1 and C2). Images presented were captured using the green fluorescent protein filter set and are shown in grayscale. WT S. pyogenes was also stained with Nile red (panel D). The bar in each panel is equivalent to 0.5 μm.
Analysis of genomic information (12) revealed that S. pyogenes has the capacity to synthesize at least two anionic phospholipids, phosphatidylglycerol (PG) and cardiolipin (CL). When bound to PG, NAO emits green fluorescence. However, due to its greater density of negative charge, CL arranges the NAO molecules in a manner that results in a shift to red fluorescence (28, 29). The NAO-stained polar domains of E. coli and B. subtilis exhibit predominantly red fluorescence (19, 28; also data not shown). In contrast, the NAO-stained domain of S. pyogenes consistently fluoresced green (Fig. 1A), suggesting that it is enriched for PG.
Analysis of S. pyogenes anionic phospholipids.
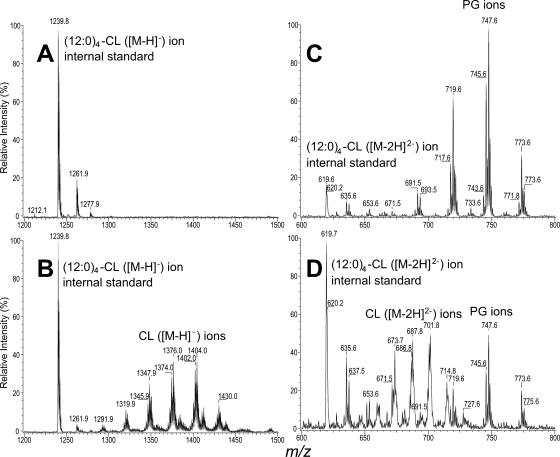
Lipid analysis of streptococci has indicated the presence of two acidic phospholipids, CL and PG, with a majority of the membrane being composed of glycolipids (37). The genome of S. pyogenes contains a single copy of pgsA (Spy2196), encoding the enzyme responsible for converting phosphatidic acid to PG, and a single copy of cls (Spy1212), which encodes the enzyme that converts PG to CL (38). Of the total phospholipid content of bacterial cells, cardiolipin typically comprises approximately 5% and PG about 20% (22). An in-frame deletion mutant lacking cls (herein referred to as the Cls− mutant) was constructed to lack both the region encoding the enzyme's predicted active site (38) and approximately 1 kb of additional sequence. Membranes were purified from both wild-type (WT) and Cls− strains and subjected to Bligh-Dryer extraction with addition of a (12:0)4-CL internal standard. The subsequent negative-ion electrospray ionization/mass spectrometry analysis of the Cls− mutant extracts showed only the [M-H]− ion of the internal standard (12:0)4-CL at m/z 1239.9 and no detectable CL ions in the mass range from m/z 1300 to 1500 (Fig. 2A). This contrasted with the abundant ions corresponding to CL that were detected in the same mass range for the WT control (Fig. 2B). These data confirm that S. pyogenes contains a single gene, cls, responsible for the biosynthesis of cardiolipin.
FIG. 2.
Lipid profile of streptococcal membranes. The electrospray ionization/mass spectrometry spectra of the lipid extracts arising from the [M-H]− ions of cardiolipin from Cls− cells (panel A) and from the WT (panel B). Panels C and D show the [M-H]− ions of phosphatidylglycerol species from the extracts shown in panels A and B, respectively. Ions from the (12:0)4-cardiolipin internal standard seen at m/z 1239.8 (panels A and B) are [M-H]− ions, while the ions seen at m/z 619.7 (panels C and D) are [M-2H]2− ions. Both the [M-H]− (panel A) and [M-2H]2− (panel C) ions of cardiolipin are absent in the lipid extract from Cls− cells but are abundant in the lipid extract from WT cells (panels B and D). In contrast, phosphatidyl glycerol is abundant from Cls− cells (panel C) and is of relatively low abundance in the WT (panel D).
The lipid extracts from both strains were then analyzed for the presence of PG species. In the mass range from m/z 600 to 800, where the [M-2H]2− ions of CL and the [M-H]− ions of PG lie, major ions at m/z 691, 717, 745, 747, and 773, arising from PG species, were observed for the Cls− cell extract with the CL species seen only as an [M-2H]2− ion at m/z 619.8 [(1240.9-2)/2], arising from the internal standard (Fig. 2C). In contrast, the WT sample had similar PG species in addition to the corresponding [M-H]2− ions of CL, as seen in Fig. 1B. The levels of PG appeared elevated in the Cls− mutant compared to the internal standard as a relative measure (Fig. 2D), although it is possible that this increase may result from an enhanced efficiency of PG extraction from membranes of the Cls− mutant. The absence of CL and the increase of PG in the Cls− mutant sample compared to data observed for the WT sample are consistent with the notion that in the prokaryotic biosynthesis pathway, two molecules of PG are involved in the synthesis of each molecule of CL (16). The blockage of the synthetic pathways of CL from PG in the Cls− mutant is consistent with the apparent increase in the amount of PG and the absence of CL (22).
Cls− mutant phenotypes.
Overall, the Cls− mutant showed a rather modest phenotype under all conditions examined. For example, the mutant grew at near-WT rates with all media examined and secreted normal amounts of several toxins, including streptolysin O and the SpeB cysteine protease, although the mutant did display a defect in processing the pro form of the latter to the active enzyme (approximately 25% of WT rate; data not shown). Transcription regulator CovR (CsrR) mutants overexpress the hyaluronic acid capsule and produce colonies with a characteristic mucoid appearance (8). Interestingly, a construction of a double CovR− Cls− strain showed no apparent defect in the ability to produce mucoid colonies compared to results for the CovR− mutant alone (data not shown), suggesting Cls is dispensable for production of capsule, despite the observation that the activity of the hyaluronate synthase enzyme is enhanced by CL in vitro (36). These data suggest that increased levels of PG may compensate for the absence of CL in membranes of the mutant. Consistent with the spectral characteristics of NAO staining in the WT, depletion of CL did not alter the staining pattern of the anionic lipid-rich microdomain (data not shown). These data are consistent with a lipid microdomain enriched in PG. However, analysis of a PG-deficient mutant was not possible, since deletion of pgsA was never recovered. The method for mutagenesis proceeds via the generation of a tandem duplication of mutant and wild-type alleles in the genome that can resolve to either allele by recombination. Typically, chromosomes with either allele are isolated at similar frequencies among the progeny (4). However, while it was possible to produce the merodiploid intermediate strain at the pgsA locus, all progeny recovered following resolution of the duplication contained a copy of the wild-type allele (30/30 tested from several independent pools). This bias towards recovery of the wild-type allele suggests that pgsA may be an essential gene in S. pyogenes, which would not be surprising, since depletion of PgsA in B. subtilis results in filamentous cells that eventually lyse (3, 22).
The ExPortal is a microdomain enriched in anionic phospholipids.
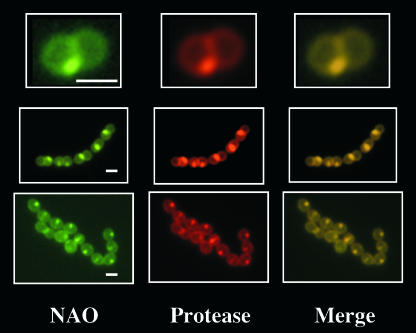
The ExPortal can be observed in live cells on the basis of visualizing the site of secretion of active SpeB protease in cells embedded in agarose along with a protease substrate that is intramolecularly quenched but becomes fluorescent when cleaved (33, 35). Examination of the Cls− mutant showed that CL is not required for ExPortal integrity, since the WT and the Cls− mutant showed identical patterns of SpeB secretion (data not shown). Examination of NAO-stained WT cells in the protease localization assay produced the expected single membrane domains revealed by both NAO staining (Fig. 3, NAO) and cleavage of SpeB protease substrate (Fig. 3, Protease). Furthermore, merging the NAO and protease localization images revealed a concordance between the positions of the anionic lipid-rich domain and the ExPortal (Fig. 3, Merge), indicating that the ExPortal is a microdomain enriched in anionic phospholipids.
FIG. 3.
The ExPortal is enriched in anionic lipids. WT S. pyogenes was stained with NAO and then examined in an assay which visualized the ExPortal as the site of secretion of active SpeB protease (33), as described in Materials and Methods. Cells were analyzed by fluorescence microscopy and examined for NAO staining and protease localization, as indicated. An overlay of NAO and protease images is shown on the right (Merge). Three representative groups of streptococcal cells, which are presented at various magnifications, are shown. Bars in leftmost panels are equivalent to 0.5 μm.
The presence of a high concentration of anionic lipids at the ExPortal is consistent with the importance of anionic lipids in promoting protein secretion. A negatively charged microdomain could provide an efficient scaffold for targeting proteins to the membrane for secretion via interaction with conserved regions of positive charge in signal sequences (10, 20). In addition, the SecA component of the translocon has a high affinity for anionic lipids (39), and its ATPase activity is enhanced by the presence of acidic phospholipids (21). A localized region of negative charge could also provide a mechanism of retention of membrane proteins at the ExPortal via interaction with the positively charged residues.
It is unclear whether the ExPortal is restricted to streptococci or is shared by gram-positive bacteria with different morphologies. The lipid biosynthetic machinery appears to be concentrated in the septal regions of B. subtilis cells (30), and the anionic lipids themselves are predominantly localized to the poles and nascent division sites (19). Depletion of these anionic lipids results in the rapid mislocalization of Sec components from sites of concentration along a helical axis, though localization may also rely upon the expression levels of Sec translocons (3). This may suggest that in bacilli, anionic lipids play a vital role in the organization of the helical subcellular apparatus around which the Sec translocons may be organized. Gram-positive cocci, which generally lack any known subcellular architecture proteins, may have developed a simpler system of organization of the Sec translocons at a single anionic lipid-rich microdomain. How such a domain is maintained remains an open question; however, it is interesting to note that acidic phospholipids may also play a role in the initiation of DNA replication via interaction with the initiator protein DnaA (27). Thus, one possibility for organization of the ExPortal is that a high concentration of a positively charged scaffolding protein may assist in the segregation of anionic lipids into distinct domains.
The fact that many naturally occurring antimicrobial agents are also highly positively charged raises some interesting possibilities as to their mechanisms of action. For example, defensins are cationic peptides produced by numerous host tissues to protect against bacterial infection (13). Polymyxin B also has a high affinity for PG (2) and can greatly decrease the efficiency of protein translocation via the Sec pathway in inverted membrane vesicles (41). This raises the possibility that these compounds may directly interfere with ExPortal-mediated protein secretion via their abilities to recognize anionic lipids, a property that could be exploited for further analysis of protein secretion in the gram-positive cocci.
Acknowledgments
This work was supported by Public Health Service grant 46433 from the National Institutes of Health.
We thank Petra Levin for the gift of B. subtilis strains.
Footnotes
Published ahead of print on 1 December 2006.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Cajal, Y., J. Ghanta, K. Easwaran, A. Surolia, and M. K. Jain. 1996. Specificity for the exchange of phospholipids through polymyxin B mediated intermembrane molecular contacts. Biochemistry 35:5684-5695. [DOI] [PubMed] [Google Scholar]
- 3.Campo, N., H. Tjalsma, G. Buist, D. Stepniak, M. Meijer, M. Veenhuis, M. Westermann, J. P. Muller, S. Bron, J. Kok, O. P. Kuipers, and J. D. H. Jongbloed. 2004. Subcellular sites for bacterial protein export. Mol. Microbiol. 53:1583-1599. [DOI] [PubMed] [Google Scholar]
- 4.Caparon, M. 2000. Gram-positive pathogens. ASM Press, Washington, DC.
- 5.Caparon, M. G., D. S. Stephens, A. Olsen, and J. R. Scott. 1991. Role of M protein in adherence of group A streptococci. Infect. Immun. 59:1811-1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carballido-Lopez, R., A. Formstone, Y. Li, S. D. Ehrlich, P. Noirot, and J. Errington. 2006. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev. Cell 11:399-409. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson, F., M. Stalhammar-Carlemalm, K. Flardh, C. Sandin, E. Carlemalm, and G. Lindahl. 2006. Signal sequence directs localized secretion of bacterial surface proteins. Nature 442:943-946. [DOI] [PubMed] [Google Scholar]
- 8.Cho, K. H., and M. G. Caparon. 2005. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 57:1545-1556. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, H., N. J. Garton, R. W. Horobin, D. E. Minnikin, and M. R. Barer. 1999. Lipid domains of mycobacteria studied with fluorescent molecular probes. Mol. Microbiol. 31:1561-1572. [DOI] [PubMed] [Google Scholar]
- 10.Demel, R. A., E. Goormaghtigh, and B. de Kruijff. 1990. Lipid and peptide specificities in signal peptide-lipid interactions in model membranes. Biochim. Biophys. Acta 1027:155-162. [DOI] [PubMed] [Google Scholar]
- 11.de Vrije, T., R. L. de Swart, W. Dowhan, J. Tommassen, and B. de Kruijff. 1988. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature 334:173-175. [DOI] [PubMed] [Google Scholar]
- 12.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 14.Hanski, E., P. A. Horwitz, and M. G. Caparon. 1992. Expression of protein F, the fibronectin-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect. Immun. 60:5119-5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser, A. R., and P. M. Schlievert. 1990. Nucleotide sequence of the streptococcal pyogenic exotoxin B and relationship between the toxin and the streptococcal proteinase precursor. J. Bacteriol. 172:4536-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschberg, C. B., and E. P. Kennedy. 1972. Mechanism of the enzymatic synthesis of cardiolipin in Escherichia coli. Proc. Natl. Acad. Sci. USA 69:648-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu, F., J. Turk, E. R. Rhoades, D. G. Russell, S. Yixin, and E. A. Groisman. 2005. Structural characterization by tandem and multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 16:491-504. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, F., J. Turk, Y. Shi, and E. A. Groisman. 2004. Characterization of acylphosphatidylglycerols from Salmonella typhimurium by tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 15:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Kawai, F., M. Shoda, R. Harashima, Y. Sadaie, H. Hara, and K. Matsumoto. 2004. Cardiolipin domains in Bacillus subtilus Marburg membranes. J. Bacteriol. 186:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller, R. C., J. A. Killian, and B. de Kruijff. 1992. Anionic phospholipids are essential for alpha-helix formation of the signal peptide of prePhoE upon interaction with phospholipid vesicles. Biochemistry 31:1672-1677. [DOI] [PubMed] [Google Scholar]
- 21.Lill, R., W. Dowhan, and W. Wickner. 1990. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 60:271-280. [DOI] [PubMed] [Google Scholar]
- 22.Lopez, C. S., A. F. Alice, H. Heras, E. A. Rivas, and C. Sanchez-Rivas. 2006. Role of anionic phospholipids in the adaptation of Bacillus subtilis to high salinity. Microbiology 152:605-616. [DOI] [PubMed] [Google Scholar]
- 23.Lyon, W. R., and M. G. Caparon. 2004. Role for the serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect. Immun. 72:1618-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyon, W. R., and M. G. Caparon. 2003. Trigger factor-mediated prolyl isomerization influences maturation of Streptococcus pyogenes cysteine protease. J. Bacteriol. 185:3661-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyon, W. R., C. M. Gibson, and M. G. Caparon. 1998. A role for Trigger Factor and an Rgg-like regulator in the transcription, secretion, and processing of the cysteine proteinase of Streptococcus pyogenes. EMBO J. 17:6263-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madden, J. C., N. Ruiz, and M. Caparon. 2001. Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104:143-152. [DOI] [PubMed] [Google Scholar]
- 27.Makise, M., S. Mima, T. Katsu, T. Tsuchiya, and T. Mizushima. 2002. Acidic phospholipids inhibit the DNA-binding activity of DnaA protein, the initiator of chromosomal DNA replication in Escherichia coli. Mol. Microbiol. 46:245-256. [DOI] [PubMed] [Google Scholar]
- 28.Mileykovskaya, E., and W. Dowhan. 2000. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 182:1172-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mileykovskaya, E., W. Dowhan, R. L. Birke, D. Zheng, L. Lutterodt, and T. H. Haines. 2001. Cardiolipin binds nonyl acridine orange by aggregating the dye at exposed hydrophobic domains on bilayer surfaces. FEBS Lett. 507:187-190. [DOI] [PubMed] [Google Scholar]
- 30.Nishibori, A., J. Kusaka, H. Hara, M. Umeda, and K. Matsumoto. 2005. Phosphatidylethanolamine domains and localization of phospholipid synthases in Bacillus subtilis membranes. J. Bacteriol. 187:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pancholi, V., and V. A. Fischetti. 1989. Identification of an endogenous membrane anchor-cleaving enzyme for group A streptococcal M protein. J. Exp. Med. 170:2119-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perego, M., G. B. Spiegelman, and J. A. Hoch. 1988. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol. Microbiol. 2:689-699. [DOI] [PubMed] [Google Scholar]
- 33.Rosch, J., and M. Caparon. 2004. A microdomain for protein secretion in gram-positive bacteria. Science 304:1513-1515. [DOI] [PubMed] [Google Scholar]
- 34.Rosch, J. W., and M. G. Caparon. 2005. The ExPortal: an organelle dedicated to protein secretion and folding in Streptococcus pyogenes. Mol. Microbiol. 58:959-968. [DOI] [PubMed] [Google Scholar]
- 35.Scott, M. E., Z. Y. Dossani, and M. Sandkvist. 2001. Directed polar secretion of protease from single cells of Vibrio cholerae via the type II secretion pathway. Proc. Natl. Acad. Sci. USA 98:13978-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tlapak-Simmons, V. L., B. A. Baggenstoss, T. Clyne, and P. H. Weigel. 1999. Purification and lipid dependence of the recombinant hyaluronan synthases from Streptococcus pyogenes and Streptococcus equisimilis. J. Biol. Chem. 274:4239-4245. [DOI] [PubMed] [Google Scholar]
- 37.Trombe, M. C., M. A. Laneelle, and G. Laneelle. 1979. Lipid composition of aminopterin-resistant and sensitive strains of Streptococcus pneumoniae. Effect of aminopterin inhibition. Biochim. Biophys. Acta 574:290-300. [DOI] [PubMed] [Google Scholar]
- 38.Tropp, B. E. 1997. Cardiolipin synthase from Escherichia coli. Biochim. Biophys. Acta 1348:192-200. [DOI] [PubMed] [Google Scholar]
- 39.Ulbrandt, N. D., V. Ramamurthy, and D. Oliver. 1992. Deep penetration of a portion of Escherichia coli SecA protein into model membranes is promoted by anionic phospholipids and by partial unfolding. J. Biol. Chem. 267:184-192. [PubMed] [Google Scholar]
- 40.van Klompenburg, W., I. Nilsson, G. von Heijne, and B. de Kruijff. 1997. Anionic phospholipids are determinants of membrane protein topology. EMBO J. 16:4261-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Klompenburg, W., A. N. J. A. Ridder, A. L. J. van Raalre, A. J. Killian, G. von Heijne, and B. de Kruijff. 1997. In vitro membrane integration of leader peptidase depends on the Sec machinery and anionic phospholipids and can occur translationally. FEBS Lett. 413:109-114. [DOI] [PubMed] [Google Scholar]