Abstract
Malignant gliomas are the most common neoplasm in the central nervous system. When treated with conventional treatments including surgery, irradiation, and chemotherapy, the average life expectancy of the most malignant type, glioblastoma multiforme is usually less than 1 year. Therefore, gene therapy is expected to be an effective and possibly curative treatment. Many gene therapeutic approaches have demonstrated efficacy in experimental animal models. However, the current clinical trials are disappointing. This review focuses on current therapeutic genes/vectors/delivery systems/targeting strategies in order to introduce updated trends and hopefully indicate prospective gene therapy for malignant gliomas.
INTRODUCTION
Malignant gliomas are the most common primary tumors arising in the human brain [1]. The most malignant type of them, the glioblastoma multiforme, represents 29% of all primary brain tumors or 5,000 new cases per year in the United States [2]. Despite surgery, chemotherapy, and radiotherapy, glioblastomas are almost always fatal, with a median survival rate of less than a year and a 5-year survival rate of 5% or less [1, 2, 3]. No therapeutic modality has substantially changed the outcome of patients with glioblastoma [2, 3]. Therefore, it is no wonder that one of the earliest targets of cancer gene therapy was malignant glioma.
The epoch-making human trial of herpes simplex virus thymidine kinase gene/ganciclovir (HSV-tk/GCV) using retroviral vector started in early 1990s [4]. Although the antitumor effect of HSV-tk/GCV therapy had looked very promising in the animal model, the effect on human patients was disappointing. To augment the effect of gene therapy, adenoviral vectors were developed and advanced to human trials [5]. Adenoviral vectors significantly improved transduction efficacy but raised other problems, as discussed later in this review. Additionally, there was a death of a patient who received gene therapy using an adenoviral vector [6]. This incident raised a nation-wide debate especially on the safety of gene therapy using viral vectors. Most of clinical trials were put on hold for several months to make sure that safety guidelines are strictly followed. However, hopes for gene therapy have not been quenched out. Researchers have been exploring many candidate genes, developing improved viral and nonviral vectors, trying different methods to deliver genes, and combining gene therapies with other modalities such as immunotherapy. In this review, we focus on animal and human studies of gene therapy for malignant gliomas. We have collected relatively recent references to introduce updated trends and hopefully to indicate future directions in this field.
THERAPEUTIC GENES
Many therapeutic genes have shown efficacy in experimental models and been divided into three categories. First, therapeutic genes are used to induce direct killing effect (Table 1). In this category, HSV-tk/GCV [5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16], toxin [17], tumor suppressor genes [18, 19, 20, 21, 22, 23, 24, 25, 26], apoptosis-inducers [27, 28, 29, 30, 31, 32], antisense against telomerase [25, 33, 34, 35], and oncolytic viruses [36, 37, 38, 39, 40] are included. Second, immunomodulation has been performed to elicit immune response against malignant gliomas [41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54]. Third, angiogenesis inhibitors [55, 56, 57, 58] or neural stem cells [59, 60, 61] are used to induce antitumor effect, although direct killing effect or direct immunoreaction may be unrelated.
Table 1.
Therapeutic genes.
| Direct killing effect | References |
| HSV-tk/GCV | [5, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16] |
| Toxin | [17] |
| Tumor suppresor gene | [18, 19, 20, 21, 22, 23, 24, 25, 26] |
| Apoptosis inducer | [27, 28, 29, 30, 31, 32] |
| Antisense therapy for telomerase | [25, 33, 34, 35] |
| Oncolytic virus | [36, 37, 38, 39, 40] |
| Immunomodulation | References |
| IL-2 | [41, 42, 43] |
| IL-4 | [44, 45, 46] |
| IL-12 | [47, 48] |
| IFN | [49, 50] |
| TNF-α | [51, 52, 53, 54] |
| Others | References |
| Angiogenesis inhibitor | [55, 56, 57, 58] |
| Neural stem cell | [59, 60, 61] |
Direct killing effect
HSV-tk/GCV
Herpes simplex virus thymidine kinase gene/ganciclovir (HSV-tk/GCV) therapy is a two-step strategy [4]. First, HSV-tk gene is transduced into tumor cells. Second, GCV is administered systematically. GCV is harmless to normal cells without HSV-tk. When tumor cells express HSV-tk, this enzyme converts GCV into a cytotoxic molecule, resulting in cell death. This HSV-tk/GCV therapy is also called suicide gene therapy. When HSV-tk gene is transduced with retroviral vectors, these vectors are selectively incorporated into dividing cells, predominantly into brain tumor cells. However, the original scheme using retroviral vectors turned out to be not potent enough. In vivo studies showed that effective tumor-cell killing depends on bystander effect and transduction efficacy [7, 13]. Bystander effect refers to killing cells that do not express HSV-tk, but closely located with the cells expressing the enzyme. On the other hand, to improve transduction efficacy, retroviral-vector producing cells or adenoviral vectors were developed. Many human trials of HSV-tk/GCV therapy were carried out using either retroviral-vector producing cells or adenoviral vectors. Some phase I/II studies reported significant therapeutic responses in small groups of patients [10, 14], but others claimed only marginal benefit [5, 11, 15]; overall, results were disappointing. The main reasons of failure are assumed to be low transduction efficacy and lack of bystander effect. Additionally, there was a report warning that adenoviral vector induced long-term active inflammation in the animal model [12]. More recently, HSV-tk/GCV therapy is used in in vivo experiments with adeno-associated virus [9] or combined with other therapy [16].
Toxin
Martin et al constructed retroviral vectors with a toxin gene (the Pseudomonas exotoxin or the Ricinus toxin, ricin) placed under the control of the thyroid hormone (T3) regulatable promoter of the myelin basic protein (MBP) [17]. They showed that malignant glioma cells, stably transducted with the vector, failed to establish a tumor or regressed the tumor mass in the rat brain.
Tumor suppressor genes
Tumor suppressor genes are often mutated or deleted in malignant gliomas and the lack of function of these genes is supposed to cause tumorigenicity. Therefore, it is quite reasonable to replace them. The most extensively studied is the p53 tumor suppressor gene. Since alterations in the p53 gene occur in 35–60% of human malignant glioma [62, 63], p53 gene therapy is logically appropriate for these tumors. Accumulating evidences show that replacement of p53 significantly inhibits tumor growth in the subcutaneous [19] or intracranial [18, 22, 24] tumor model. However, many gliomas are mixture of cells with mutated p53 and wild-type p53 (wt-p53), and p53 gene transfer is known to be ineffective for the cells with wt-p53 [23, 25]. That is, p53 gene therapy is supposed to be effective only for part of malignant gliomas. There is a conflicting report claiming that p53 gene therapy induces apoptosis in glioma cells with wt-p53 [24]. New studies show that the combination of p53 gene therapy and irradiation is effective for malignant gliomas with heterogenous p53 status [20, 21]. Other tumor suppressor genes (p21, p16, and p27) belong to cyclin-dependent kinase inhibitors (CDKIs) and they are involved in the regulation of cell cycle. Wang et al showed that retroviral transfer of p16 and p21 inhibited tumor growth [26].
Apoptosis-inducers
Apoptosis, also called programmed cell death, is a genetically-encoded program to get rid of unwanted cells. It is well known that apoptotic pathways are suppressed in malignancies including malignant gliomas [64]. The rationale to use apoptosis-inducers is to activate apoptotic pathways and induce cell death effectively in malignant glioma cells. The genes used in the studies so far are caspase-1/interleukin-1β-converting enzyme (ICE) [27], caspase-3/CPP32β [28], caspase-6 [32], caspase-8 [30], Fas associated protein with death domain (FADD) [29], and Bax [31]. The main concern of these strategies is about safety, what if apoptosis is also induced in normal brain cells surrounding the tumor? It is necessary to regulate the induction of apoptosis to occur only in malignant cells as described below.
Antisense therapy for telomerase
Our group developed a system to inhibit human telomerase RNA (hTER) with 2-5A-linked antisense [25, 33, 35]. 2-5A or 2′ , 5′-oligoadenylate is a pathway of interferon actions [65, 66]. It activates RNase L that is ubiquitous in the cells and degrades RNA randomly. Chimeric combination of an antisense and 2-5A enables us to degrade specific RNA [67]. We designed an antisense and synthesized 2-5A antisense molecule to target hTER (2-5A-anti-hTER) [33]. It degrades hTER specifically and effectively, resulting in the inhibition of telomerase. Interestingly, 2-5A-anti-hTER induced apoptosis massively and inhibited tumor growth in the subcutaneous and intracranial tumor models [33, 35]. 2-5A-anti-hTER induced apoptosis unexpectedly early (within 4 days, in vitro) compared to the treatment with the cDNA vector for hTER (about one month) [34].
Oncolytic viruses
Oncolytic viruses are designed to replicate selectively in and lyse tumor cells. These viruses are more effective in infecting tumor cells compared to the viruses that are constructed as replication-defective. Oncolytic viruses increase in number in tumor cells and lyse the cells directly, not by transducing specific genes. A recombinant herpes simplex virus with some deletions, designated DM33, inhibited growth of intracranial tumors and prolonged the survival of tumor-bearing animals [38]. The tumor-killing effect was even better with the following injection of ganciclovir [40]. Adenovirus ONYX-015 targets tumors with mutant p53 and its clinical studies are ongoing for head and neck cancer. Recently, it was shown that ONYX-015 is effective in malignant gliomas regardless of their p53 status [39]. Fueyo et al constructed Delta 24, a tumor-selective adenovirus with a deletion in the EIA region responsible for binding Rb protein [36]. This virus targets tumor cells with Rb alteration. They showed that Delta 24 inhibited the growth of glioma cells implanted subcutaneously. Ansardi et al constructed oncolytic RNA-based vectors derived from poliovirus and termed replicons [37]. They showed that replicons suppressed tumor growth and extended the survival of the animals with intracranial tumors.
Immunomodulation
Central nervous system (CNS) had been considered as immune-privileged site. The important factors of tolerance for activated host immune are the presence of the blood-brain barrier and the absence of a lymphatic drainage system [68]. However, lymphocytic infiltration has been observed frequently in malignant gliomas and the degree of infiltration seems to correlate with survival [69]. Moreover, disability to elicit the immune reaction implies that the oncogenesis of primary glioma cells occurs without interaction to immune cells. Therefore, glioma cells derived from brain parenchyma may be more immunogenic than tumors derived from peripheral system and the immunogene therapy is very attractive for therapy of malignant glioma.
IL-2
Interleukin (IL)-2, a cytokine produced by activated T cells, can promote immune reactions. Fibroblasts, genetically engineered to secrete IL-2, suppress tumor growth and induce antitumor immunity to murine gliomas in vivo [41]. Clinically, patients with malignant glioma were subcutaneously immunized with autologous glioma cells and received IL-2 secreting fibroblasts [42]. Posttreatment with MRI revealed the marked tumor necrosis, but the tumor did not disappear. As shown in vivo immunotherapy model using IL-2, the combination of immunization in peripheral tissues and intracerebral transplantation of IL-2-producing cells is necessary to eliminate established brain tumors [43].
IL-4
IL-4 is a multifunctional lymphokine produced by helper T cells and has a broad range of activities on both B and T lymphocytes. When IL-4-transduced glioma cells were injected into animals, eosinophil infiltration and inhibition of tumor angiogenesis were observed in athymic mice [45] and T-cell infiltration and humoral response were shown in immunocompetent rats [46]. Furthermore, retroviral packaging cells producing IL-4 were produced [44]. When these cells were injected into established intracranial tumors, tumors were completely eradicated and inhibition of tumor angiogenesis and infiltration of T cells and macrophages were revealed.
IL-12
IL-12 is secreted by antigen-presenting cells such as dendritic cells, macrophages or microglia [70]. Among cytokines, it has been demonstrated that IL-12 possesses particularly potent antitumor properties [71]. It is because IL-12 plays a critical role in mediating inflammatory and immune responses in host defense, IL-12 exerts a variety of biological effects on T cells and natural killer (NK) cells [72], including induction of interferon (IFN)-γ production [71], enhancement of proliferation and cytolytic function of T cells and NK cells [73], and promotion of the Th1-type immune response [74]. In addition to stimulatory effects on the immune system, IL-12 is also a potent antiangiogenic factor [75]. Local delivery of IL-12 by genetically engineered cells significantly prolongs the survival time in animals with brain tumor [47]. Moreover, a single intratumoral treatment of nude mice with a vaccinia virus (VV) expressing IL-12 induced significant tumor growth inhibition [48]. However, most animals injected with high doses of recombinant viruses (105 to 107 pfu) showed signs of cytokine toxicity.
Interferon (IFN)
IFNs are produced by activated immune cells including T cells, NK cells, and monocyte lineage cells. Glioma cells transfected with the human β-interferon gene by liposomes, elicit systemic immune reactions and inhibit the tumor growth [49]. In addition, IFNs directly stimulate cell differentiation and apoptosis via signals from interferon receptors on glioma cells [50].
TNF-α
Tumor necrosis factor (TNF)-α was initially supposed to be a promising cancer therapeutic reagent. However, recent investigation shows that TNF-α does not kill most types of cancer cells partly due to the activation of an anti-apoptotic gene, NF-κB [76]. Therefore, suppressing NF-κB is expected to potentiate TNF-α-induced apoptosis. Recently, it has been demonstrated that combination of TNF-α with other cytokines [51], radiation [52], chemotherapy [53], or hyperthermia [54] is more effective in therapy for glioma models than single treatments.
Others
Angiogenesis inhibitors
Based on the observation that gliomas are among the most angiogenic tumors, therapeutic strategies aimed to inhibit angiogenesis are theoretically attractive. Angiostatin, an internal peptide fragment of plasminogen, has recently been shown to potently inhibit endothelial proliferation in vitro and tumor growth in vivo [77]. The AAV (adeno-associated virus) vector has been able to deliver sustained and high-level gene expression in vivo. Intratumoral [55] or intramuscular [56] injection of a high-titer AAV-angiostatin vector has rendered efficacious tumor suppression and resulted in long-term survival. However, recombinant angiostatin, a peptide fragment, might be unstable in vivo. A tricistronic retroviral vector, expressing natural antiangiogenic factors, inhibits angiogenesis in vitro, but is not able to block tumor progression in vivo [57]. A sustained in vivo protein delivery is required to achieve the therapeutic effects. Endostatin, internal peptide fragment of 18 collagen, is also an angiogenic inhibitor. Engineered C6 cells that endogenously express mouse endostatin reduced tumor growth in vivo [58]. However, complete tumor inhibition was not observed in either the athymic or immunocompetent tumor models. Antiangiogenetic therapy using these peptides might be developed as an adjuvant gene therapy for the effective treatment of malignant gliomas.
Neural stem cells
Gene therapy of glioblastomas is limited because viral vectors usually are unable to survive for long time, continue to express proteins, and hardly reach glioblastoma cells infiltrating the brain parenchyma. Neural stem cells, implanted distant from brain tumor lesion into experimental intracranial gliomas in vivo, migrated brain parenchyma towards brain tumor site, chasing the infiltrating tumors [59]. This migratory cell delivery method has the potential to expand the range of delivery of HSV-1 vectors to tumor cells in the brain [60]. Genetically engineering neural stem cells expressing IL-4 elicited the systemic immune response and inhibited tumor growth [61]. Moreover, supernatant of neural stem cells inhibited the proliferation of glioma cells [61]. Therefore, neural stem cells may be an ideal vehicle to overcome the above difficulties in gene therapy of malignant gliomas.
VECTORS
Vector development is an important field of study, because efficacy of gene transfer depends mostly on the ability of vectors to be incorporated into tumor cells. Vectors for gene therapy can be divided into classes of viral and nonviral systems (Table 2). Viruses are effective vehicles for gene delivery as they can enter human cells and express their genes specifically and efficiently. The main device for viral vector development is improving the targeting efficiency of viruses, while abrogating their ability to cause disease. Modifying the viral genome to remove sequences necessary for viral replication and pathogenecity makes it possible to achieve these goals. The removed viral coding sequence can be replaced with exogenous therapeutic genes. Such genetically engineered viruses theoretically keep wild-type viral cellular tropism and ensure transgene expression in the target cell population without causing harmful diseases. Efforts to alter the natural tropism of viruses by manipulating the viral components that mediate cell binding and internalization represent a means of leading viruses specifically to chosen target cells.
Table 2.
Vectors.
Viral vectors
Retrovirus
Retrovirus and adenovirus have been used for a wide variety of gene therapy applications. Retrovirus is a single strand RNA virus. Retrovirus enters cells by binding surface envelope protein, encoded by the env gene, to specific cellular receptors. After entering cells, the viral enzyme reverse transcriptase, encoded by the pol gene, transcribes the viral genome into a double-strand DNA copy. Double-strand DNA can enter the nucleus of dividing cells and become integrated randomly into the host genome. This event occurs preferentially in dividing cells, meaning the virus does not enter neurons or other normal brain cells. Retroviruses used in gene therapy protocols are designed to be replication-deficient by removing their gag, pol, and env genes. Therefore, infectious but replication-deficient retrovirus particles are produced in packaging cells that express retrovirus gag, pol, and env genes from plasmids lacking the packaging sequence. A great variety of in vivo experiments [13, 44, 57] and clinical trials [8, 15] using retroviral vectors for gene therapy for malignant glioma have been performed. There are some drawbacks to use retroviruses as vectors. Retrovirus packaging cells produce relatively low titers. The retrovirus genome is small, which limits the size of genetic constructs they can carry. Random integration into the host genome may disrupt cellular genes by insertional mutagenesis.
Adenovirus
Adenovirus is a double-strand DNA virus. Adenovirus enters cells by binding to the adenoviral receptor, which promotes interaction of viral arginine-glycine-aspartate sequences with cellular integrins. After internalization, the virus escapes from cellular endosomes, partially disassembles and translocates to the nucleus, where viral gene expression begins. They can produce in high titers and infect non-dividing cells. Gene expression occurs without integration into the host genome and gutless adenovirus offers the opportunity to develop vectors with expanded capacity for therapeutic transgenes. Transduction efficiency of adenovirus is better than that of retrovirus. One of the drawbacks is that the induced genomes decrease by cell divisions. The administration is limited only once, as it possesses high antigenicity. Sandmair et al compared the effect of HSV-tk gene therapy combined with ganciclovir medication in malignant gliomas between retrovirus-packaging cell and adenovirus gene therapy [14]. Although the results are from a small number of patients and should be interpreted cautiously, adenoviral HSV-tk gene therapy seems to be beneficial as shown by magnetic resonance imaging (MRI)-determined tumor regrowth, 3 months after gene therapy, and the survival of patients [14].
Adeno-associated virus
Enhanced gene delivery has been demonstrated in preclinical studies using adeno-associated viruses (AAV) vectors targeting malignant glioma cells [9, 56]. AAV is a native human parvovirus that does not cause any known human disease. They enter cells after binding to heparan sulfate but need coinfection with a helper virus (adenovirus or herpes virus) to replicate. Without coinfection of helper virus, AAV infection leads to latency, in which the viral genome remains in an integrated form or as episomal DNA. Subsequent infection of the cell with a virus capable of providing the necessary helper functions allows replication to proceed. AAV vectors have a number of potential advantages over retroviral and adenoviral vectors. They can infect a wide range of host cells independent of their cell cycling status and are stably integrated and maintained in the host genome in which transient transgene expression may be adequate. The drawbacks of AAV as vectors for gene therapy are limited packaging capacity of approximately 5 kbp and gene expression that may be slow to reach its peak. In addition, production requires helper viruses, which may contaminate preparations for preclinical and clinical use.
Nonviral vectors
Gene transfer using nucleic acid therapeutics or nonviral vectors is much less immunogenic and cytotoxic than viral-vector systems. Although major weakness of nonviral vectors is that transduction efficiency is significantly lower than viral vectors, it can be overcome by frequent intratumoral injection or application of osmotic minipumps or polymer/microsphere system as described below.
Antisense oligonucleotide
Over the last two decades, cloning and sequencing of the critical genes in tumorigenecity have made progress remarkably. Many target genes attractive for antisense therapy have been identified. Antisense oligonucleotides are designed to bind to a certain sequence of specific mRNA and degrade it, providing a powerful tool for the cancer therapy. We have recently selected telomerase, a ribonucleoprotein enzyme, as a target for the therapy of malignant gliomas [25, 33, 34]. Telomerase is considered as a potential target of cancer therapy because malignant gliomas are predominantly telomerase-positive, while normal brain tissues do not express the enzyme [78, 79]. We have shown that a 19-mer antisense oligonucleotide against human telomerase RNA linked to a 2′, 5′-oligoadenylate (2-5A) inhibited malignant tumors growth in subcutaneous or brain tumor models in mice [25, 33, 34]. Interestingly, inhibition of telomerase activity resulted in apoptotic cell death. Angiogenic factors are also potentially optimal targets for antisense oligonucleotide because malignant gliomas are highly angiogenic. The antisense therapy against vascular endothelial growth factor (VEGF) was useful in down-regulation of VEGF expression, resulting in inhibition of growth of malignant glioma cells in vivo [80, 81].
Naked DNA plasmid
Gene transfer with naked DNA plasmid in the presence of the modified liposomes such as cationic liposome or the hemagglutinating virus of Japan (HVJ) liposome has been developed and applied to the treatment of malignant tumors. Multiple intratumoral administrations of liposomes containing the murine IFN-β gene resulted in the reduction of tumors in the brains of mice and elicited cytotoxic T lymphocytes without side effects [49]. Intramuscular injection of DNA plasmid encoding murine IFN-α leads to potent antitumor effects in mice bearing subcutaneous glioma cells [82]. Moreover, this gene transfer system has been applied to the treatment using the suicide gene [83], apoptosis inducible-genes [32], because this delivery system can be repeated.
DELIVERY SYSTEMS
The transfer of therapeutic genes into malignant brain tumors is the subject of experimental models and clinical trial of gene therapy (Table 3). Most approaches have used direct intratumoral placement of a variety of vectors and genes, such as retroviruses [8, 13, 15, 44, 57], replication-defective adenoviruses [5, 14], replication-competent or-conditioned oncolytic viruses [36, 37, 38, 39, 40], antisense oligonucleotides [25, 33, 34, 80, 81], and naked plasmid vectors [32, 49, 82, 83]. These approaches have shown the efficiency of gene therapy. However, these approaches need to repeat injections or are unable to keep continuous gene expression.
Table 3.
Delivery systems.
Using a minipump combined with stereotaxic techniques allows continuous delivery of therapeutic genetic materials into the brain. Continuous intracerebral delivery of liposome-mediated HSV-tk gene complexes using an osmotic minipump led to tumor regression in the treated animal [83]. Polymer microspheres can encapsule antisense oligonucleotide, naked DNA plasmid, viral particle, or monoclonal antibody, and release them. This system not only decreases treatment frequency, but also reduces the potent immune response by sequestering the content from antibody exposure, leading to improvement of in vivo efficacy [84, 85, 86]. However, the above systems are unable to effectively distribute the genetic materials into the target cell population. As described above (Table 1), neural stem cells may have the potential to chase the infiltrating tumor cells [59, 60, 61].
TARGETING
Gene transfer vectors will dramatically increase the safety and effectiveness of cancer gene therapy, if they can restrict expression of the therapeutic products to the target tumors. Substances that are overexpressed in tumor cells but not in normal cells are good targets for gene therapy (Table 4). Although no specific marker is known for malignant gliomas, four targets including us came up with expression regulating system using specific promoters. First, we used the promoter of the human telomerase reverse transcriptase (hTERT) gene and developed expression vectors of caspase-6 [32], caspase-8 [87], or FADD [88] under the control of the promoter, respectively. The activity of telomerase is tightly regulated at the transcriptional level of the hTERT gene [89]. Since about 75% of malignant gliomas have telomerase activity while normal brain tissues do not have the enzyme [78, 79], we can restrict the expression of apoptosis-inducing proteins to malignant glioma cells. We showed that the growth of subcutaneous tumors was inhibited due to induction of apoptosis after the treatment [32, 87, 88]. Second, Shinoura et al used the myelin basic protein (MBP) promoter to regulate the expression of Bax and caspase-8 [30, 31]. They showed that the growth of tumors in the animal model was suppressed. Third, epidermal growth factor receptor (EGFR) is often highly expressed in tumor but not in normal brain. EGFR may be a good target to increase the selectivity of delivering genes to tumor cells [90, 91]. Forth, stress can also be a target for tumor specific expression of therapeutic genes. The presence of hypoxic cells in human brain tumor is an important factor leading to resistance to radiation therapy. However, this physiological difference between tumor and normal tissues also provides the potential for designing cancer-specific gene therapy [92]. When the gene expression is triggered by heat stress, combined therapeutic effects of hyperthermia and gene therapy may be promising [93].
Table 4.
Targeting.
CONCLUSIONS
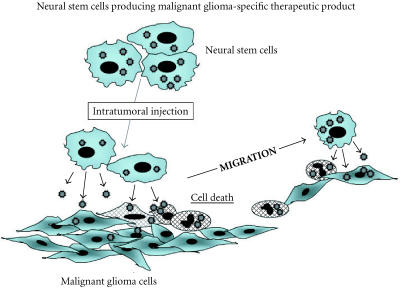
In recent years, many neuro-oncologists have focused on evolving gene therapy as a new therapeutic modality for malignant gliomas. However, clinical success has been limited due to insufficient gene transfer or limited spread of therapeutic genes. These obstacles may be overcome by neural stem cell-guided gene therapy (Figure 1). Neural stem cells are expected to migrate extensively into malignant gliomas in the brain, although further investigation is necessary. Therefore, the application of neural stem cells producing tumor-specific therapeutic product will offer a means of accessing invasive tumor cells. If we can engineer neural stem cells to produce oncolytic virus as tumor-specific therapeutic product, this approach may be significantly enhanced. It is expected that replication-conditional or competent oncolytic virus will significantly increase the extent of gene transfer into tumors compared to replication-defective virus. However, issues such as controlling virus replication and keeping normal cells intact should be confirmed. The use of tumor-specific promoter such as hTERT promoter system in the brain will increase the safety of oncolytic virus produced by neural stem cells.
Figure 1.
Prospective gene therapy for malignant gliomas.
Acknowledgments
ACKNOWLEDGMENT
This study was supported in part by NIH Grants 1R01 CA80233 and CA88936 awarded by the National Cancer Institute (S Kondo), by a start-up fund from The University of Texas M. D. Anderson Cancer Center (S Kondo), and by a generous donation from the Anthony D. Bullock III Foundation (S Kondo).
References
- Schoenberg B S. The epidemiology of central nervous system tumors. In: Walker M D, editor. Oncology of the nervous system. Martinus Nijhoff; Boston: 1983. pp. 1–30. [Google Scholar]
- Mahaley M S. Jr, Mettlin C, Natarajan N, Laws E R. Jr, Peace B B. National survey of patterns of care for brain-tumor patients. J Neurosurg. 1989;71(6):826–836. doi: 10.3171/jns.1989.71.6.0826. [DOI] [PubMed] [Google Scholar]
- Deen D F, Chiarodo A, Grimm E A, et al. Brain Tumor Working Group Report on the 9th International Conference on Brain Tumor Research and Therapy. Organ System Program, National Cancer Institute. J Neurooncol. 1993;16(3):243–272. doi: 10.1007/BF01057041. [DOI] [PubMed] [Google Scholar]
- Culver K W, Ram Z, Wallbridge S, Ishii H, Oldfield E H, Blaese R M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Trask T W, Trask R P, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1(2):195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- Somia N, Verma I M. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1(2):91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- Colombo B M, Benedetti S, Ottolenghi S, et al. The “bystander effect”: association of U-87 cell death with ganciclovir-mediated apoptosis of nearby cells and lack of effect in athymic mice. Hum Gene Ther. 1995;6(6):763–772. doi: 10.1089/hum.1995.6.6-763. [DOI] [PubMed] [Google Scholar]
- Izquierdo M, Martin V, de Felipe P, et al. Human malignant brain tumor response to herpes simplex thymidine kinase (HSVtk)/ganciclovir gene therapy. Gene Ther. 1996;3(6):491–495. [PubMed] [Google Scholar]
- Okada H, Miyamura K, Itoh T, et al. Gene therapy against an experimental glioma using adeno-associated virus vectors. Gene Ther. 1996;3(11):957–964. [PubMed] [Google Scholar]
- Klatzmann D, Valery C A, Bensimon G, et al. A phase I/II study of herpes simplex virus type 1 thymidine kinase “suicide” gene therapy for recurrent glioblastoma. Study Group on Gene Therapy for Glioblastoma. Hum Gene Ther. 1998;9(17):2595–2604. doi: 10.1089/hum.1998.9.17-2595. [DOI] [PubMed] [Google Scholar]
- Shand N, Weber F, Mariani L, et al. A phase 1-2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther. 1999;10(14):2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- Dewey R A, Morrissey G, Cowsill C M, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5(11):1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- Kruse C A, Lamb C, Hogan S, Smiley W R, Kleinschmidt-Demasters B K, Burrows F J. Purified herpes simplex thymidine kinase retroviral particles. II. Influence of clinical parameters and bystander killing mechanisms. Cancer Gene Ther. 2000;7(1):118–127. doi: 10.1038/sj.cgt.7700097. [DOI] [PubMed] [Google Scholar]
- Sandmair A M, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11(16):2197–2205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- Floeth F W, Shand N, Bojar H, et al. Local inflammation and devascularization—in vivo mechanisms of the “bystander effect” in VPC-mediated HSV-Tk/GCV gene therapy for human malignant glioma. Cancer Gene Ther. 2001;8(11):843–851. doi: 10.1038/sj.cgt.7700382. [DOI] [PubMed] [Google Scholar]
- Moriuchi S, Wolfe D, Tamura M, et al. Double suicide gene therapy using a replication defective herpes simplex virus vector reveals reciprocal interference in a malignant glioma model. Gene Ther. 2002;9(9):584–591. doi: 10.1038/sj.gt.3301693. [DOI] [PubMed] [Google Scholar]
- Martin V, Cortes M L, de Felipe P, Farsetti A, Calcaterra N B, Izquierdo M. Cancer gene therapy by thyroid hormone-mediated expression of toxin genes. Cancer Res. 2000;60(12):3218–3224. [PubMed] [Google Scholar]
- Badie B, Drazan K E, Kramar M H, Shaked A, Black K L. Adenovirus-mediated p53 gene delivery inhibits 9L glioma growth in rats. Neurol Res. 1995;17(3):209–216. doi: 10.1080/01616412.1995.11740314. [DOI] [PubMed] [Google Scholar]
- Kock H, Harris M P, Anderson S C, et al. Adenovirus-mediated p53 gene transfer suppresses growth of human glioblastoma cells in vitro and in vivo. Int J Cancer. 1996;67(6):808–815. doi: 10.1002/(SICI)1097-0215(19960917)67:6<808::AID-IJC9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Badie B, Kramar M H, Lau R, Boothman D A, Economou J S, Black K L. Adenovirus-mediated p53 gene delivery potentiates the radiation-induced growth inhibition of experimental brain tumors. J Neurooncol. 1998;37(3):217–222. doi: 10.1023/a:1005924925149. [DOI] [PubMed] [Google Scholar]
- Broaddus W C, Liu Y, Steele L L, et al. Enhanced radiosensitivity of malignant glioma cells after adenoviral p53 transduction. J Neurosurg. 1999;91(6):997–1004. doi: 10.3171/jns.1999.91.6.0997. [DOI] [PubMed] [Google Scholar]
- Cirielli C, Inyaku K, Capogrossi M C, Yuan X, Williams J A. Adenovirus-mediated wild-type p53 expression induces apoptosis and suppresses tumorigenesis of experimental intracranial human malignant glioma. J Neurooncol. 1999;43(2):99–108. doi: 10.1023/a:1006289505801. [DOI] [PubMed] [Google Scholar]
- Lang F F, Yung W K, Sawaya R, Tofilon P J. Adenovirus-mediated p53 gene therapy for human gliomas. Neurosurgery. 1999;45(5):1093–1104. doi: 10.1097/00006123-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Li H, Alonso-Vanegas M, Colicos M A, et al. Intracerebral adenovirus-mediated p53 tumor suppressor gene therapy for experimental human glioma. Clin Cancer Res. 1999;5(3):637–642. [PubMed] [Google Scholar]
- Komata T, Kondo Y, Koga S, Ko S C, Chung L W, Kondo S. Combination therapy of malignant glioma cells with 2-5A-antisense telomerase RNA and recombinant adenovirus p53. Gene Ther. 2000;7(24):2071–2079. doi: 10.1038/sj.gt.3301327. [DOI] [PubMed] [Google Scholar]
- Wang T J, Huang M S, Hong C Y, Tse V, Silverberg G D, Hsiao M. Comparisons of tumor suppressor p53, p21, and p16 gene therapy effects on glioblastoma tumorigenicity in situ. Biochem Biophys Res Commun. 2001;287(1):173–180. doi: 10.1006/bbrc.2001.5565. [DOI] [PubMed] [Google Scholar]
- Kondo S, Barna B P, Morimura T, et al. Interleukin-1 β-converting enzyme mediates cisplatin-induced apoptosis in malignant glioma cells. Cancer Res. 1995;55(24):6166–6171. [PubMed] [Google Scholar]
- Kondo S, Tanaka Y, Kondo Y, et al. Retroviral transfer of CPP32β gene into malignant gliomas in vitro and in vivo. Cancer Res. 1998;58(5):962–967. [PubMed] [Google Scholar]
- Kondo S, Ishizaka Y, Okada T, et al. FADD gene therapy for malignant gliomas in vitro and in vivo. Hum Gene Ther. 1998;9(11):1599–1608. doi: 10.1089/hum.1998.9.11-1599. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Koike H, Furitu T, et al. Adenovirus-mediated transfer of caspase-8 augments cell death in gliomas: implication for gene therapy. Hum Gene Ther. 2000;11( 8):1123–1137. doi: 10.1089/10430340050015185. [DOI] [PubMed] [Google Scholar]
- Shinoura N, Saito K, Yoshida Y, et al. Adenovirus-mediated transfer of bax with caspase-8 controlled by myelin basic protein promoter exerts an enhanced cytotoxic effect in gliomas. Cancer Gene Ther. 2000;7(5):739–748. doi: 10.1038/sj.cgt.7700158. [DOI] [PubMed] [Google Scholar]
- Komata T, Kondo Y, Kanzawa T, et al. Treatment of malignant glioma cells with the transfer of constitutively active caspase-6 using the human telomerase catalytic subunit (human telomerase reverse transcriptase) gene promoter. Cancer Res. 2001;61(15):5796–5802. [PubMed] [Google Scholar]
- Kondo S, Kondo Y, Li G, Silverman R H, Cowell J K. Targeted therapy of human malignant glioma in a mouse model by 2-5A antisense directed against telomerase RNA. Oncogene. 1998;16(25):3323–3330. doi: 10.1038/sj.onc.1201885. [DOI] [PubMed] [Google Scholar]
- Kondo S, Tanaka Y, Kondo Y, et al. Antisense telomerase treatment: induction of two distinct pathways, apoptosis and differentiation. FASEB J. 1998;12(10):801–811. doi: 10.1096/fasebj.12.10.801. [DOI] [PubMed] [Google Scholar]
- Mukai S, Kondo Y, Koga S, Komata T, Barna B P, Kondo S. 2-5A antisense telomerase RNA therapy for intracranial malignant gliomas. Cancer Res. 2000;60(16):4461–4467. [PubMed] [Google Scholar]
- Fueyo J, Gomez-Manzano C, Yung W K, et al. Overexpression of E2F-1 in glioma triggers apoptosis and suppresses tumor growth in vitro and in vivo. Nat Med. 1998;4(6):685–690. doi: 10.1038/nm0698-685. [DOI] [PubMed] [Google Scholar]
- Ansardi D C, Porter D C, Jackson C A, Gillespie G Y, Morrow C D. RNA replicons derived from poliovirus are directly oncolytic for human tumor cells of diverse origins. Cancer Res. 2001;61(23):8470–8479. [PubMed] [Google Scholar]
- Samoto K, Perng G C, Ehtesham M, et al. A herpes simplex virus type 1 mutant deleted for gamma34.5 and LAT kills glioma cells in vitro and is inhibited for in vivo reactivation. Cancer Gene Ther. 2001;8(4):269–277. doi: 10.1038/sj.cgt.7700306. [DOI] [PubMed] [Google Scholar]
- Geoerger B, Grill J, Opolon P, et al. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62(3):764–772. [PubMed] [Google Scholar]
- Samoto K, Ehtesham M, Perng G C, et al. A herpes simplex virus type 1 mutant with gamma 34.5 and LAT deletions effectively oncolyses human U87 glioblastomas in nude mice. Neurosurgery. 2002;50(3):599–605. doi: 10.1097/00006123-200203000-00031. [DOI] [PubMed] [Google Scholar]
- Glick R P, Lichtor T, Mogharbel A, Taylor C A, Cohen E P. Intracerebral versus subcutaneous immunization with allogeneic fibroblasts genetically engineered to secrete interleukin-2 in the treatment of central nervous system glioma and melanoma. Neurosurgery. 1997;41(4):898–906. doi: 10.1097/00006123-199710000-00025. [DOI] [PubMed] [Google Scholar]
- Sobol R E, Fakhrai H, Shawler D, et al. Interleukin-2 gene therapy in a patient with glioblastoma. Gene Ther. 1995;2(2):164–167. [PubMed] [Google Scholar]
- Iwadate Y, Yamaura A, Sato Y, Sakiyama S, Tagawa M. Induction of immunity in peripheral tissues combined with intracerebral transplantation of interleukin-2-producing cells eliminates established brain tumors. Cancer Res. 2001;61(24):8769–8774. [PubMed] [Google Scholar]
- Saleh M, Wiegmans A, Malone Q, Stylli S S, Kaye A H. Effect of in situ retroviral interleukin-4 transfer on established intracranial tumors. J Natl Cancer Inst. 1999;91(5):438–445. doi: 10.1093/jnci/91.5.438. [DOI] [PubMed] [Google Scholar]
- Saleh M, Davis I D, Wilks A F. The paracrine role of tumour-derived mIL-4 on tumour-associated endothelium. Int J Cancer. 1997;72(4):664–672. doi: 10.1002/(sici)1097-0215(19970807)72:4<664::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Giezeman-Smits K M, Okada H, Brissette-Storkus C S, et al. Cytokine gene therapy of gliomas: induction of reactive CD4+ T cells by interleukin-4-transfected 9L gliosarcoma is essential for protective immunity. Cancer Res. 2000;60(9):2449–2457. [PubMed] [Google Scholar]
- DiMeco F, Rhines L D, Hanes J, et al. Paracrine delivery of IL-12 against intracranial 9L gliosarcoma in rats. J Neurosurg. 2000;92(3):419–427. doi: 10.3171/jns.2000.92.3.0419. [DOI] [PubMed] [Google Scholar]
- Chen B, Timiryasova T M, Haghighat P, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001;24(1):46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Natsume A, Mizuno M, Ryuke Y, Yoshida J. Antitumor effect and cellular immunity activation by murine interferon-beta gene transfer against intracerebral glioma in mouse. Gene Ther. 1999;6(9):1626–1633. doi: 10.1038/sj.gt.3300990. [DOI] [PubMed] [Google Scholar]
- Yagi K, Ohishi N, Hamada A, et al. Basic study on gene therapy of human malignant glioma by use of the cationic multilamellar liposome-entrapped human interferon beta gene. Hum Gene Ther. 1999;10(12):1975–1982. doi: 10.1089/10430349950017338. [DOI] [PubMed] [Google Scholar]
- Harada K, Yoshida J, Mizuno M, Sugita K, Kurisu K, Uozumi T. Growth inhibition of subcutaneously transplanted human glioma by transfection-induced tumor necrosis factor-alpha and augmentation of the effect by gamma-interferon. J Neurooncol. 1994;22(3):221–225. doi: 10.1007/BF01052922. [DOI] [PubMed] [Google Scholar]
- Staba M J, Mauceri H J, Kufe D W, Hallahan D E, Weichselbaum R R. Adenoviral TNF-alpha gene therapy and radiation damage tumor vasculature in a human malignant glioma xenograft. Gene Ther. 1998;5(3):293–300. doi: 10.1038/sj.gt.3300594. [DOI] [PubMed] [Google Scholar]
- Walther W, Stein U, Pfeil D. Gene transfer of human TNF alpha into glioblastoma cells permits modulation of mdr1 expression and potentiation of chemosensitivity. Int J Cancer. 1995;61(6):832–839. doi: 10.1002/ijc.2910610615. [DOI] [PubMed] [Google Scholar]
- Gridley D S, Li J, Kajioka E H, et al. Lymphocyte activation with localized pGL1-TNF-alpha gene therapy in a glioma model. Oncology. 2002;62(1):66–77. doi: 10.1159/000048249. [DOI] [PubMed] [Google Scholar]
- Ma H I, Guo P, Li J, et al. Suppression of intracranial human glioma growth after intramuscular administration of an adeno-associated viral vector expressing angiostatin. Cancer Res. 2002;62(3):756–763. [PubMed] [Google Scholar]
- Ma H I, Lin S Z, Chiang Y H, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9(1):2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- Ciafre S A, Barillari G, Bongiorno-Borbone L, Wannenes F, Izquierdo M, Farace M G. A tricistronic retroviral vector expressing natural antiangiogenic factors inhibits angiogenesis in vitro, but is not able to block tumor progression in vivo. Gene Ther. 2002;9(4):297–302. doi: 10.1038/sj.gt.3301652. [DOI] [PubMed] [Google Scholar]
- Peroulis I, Jonas N, Saleh M. Antiangiogenic activity of endostatin inhibits C6 glioma growth. Int J Cancer. 2002;97(6):839–845. doi: 10.1002/ijc.10115. [DOI] [PubMed] [Google Scholar]
- Aboody K S, Brown A, Rainov N G, et al. From the cover: neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlinger U, Woiciechowski C, Sena-Esteves M, et al. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1(4):347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Pirola B, Pollo B, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6(4):447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- Sidransky D, Mikkelsen T, Schwechheimer K, Rosenblum M L, Cavanee W, Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992;355(6363):846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Fults D, Brockmeyer D, Tullous M W, Pedone C A, Cawthon R M. p53 mutation and loss of heterozygosity on chromosomes 17 and 10 during human astrocytoma progression. Cancer Res. 1992;52(3):674–679. [PubMed] [Google Scholar]
- Bold R J, Termuhlen P M, McConkey D J. Apoptosis, cancer and cancer therapy. Surg Oncol. 1997;6(3):133–142. doi: 10.1016/s0960-7404(97)00015-7. [DOI] [PubMed] [Google Scholar]
- Clemens M J, Williams B R. Inhibition of cell-free protein synthesis by pppA2′p5′A2′p5′A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Zhou A, Hassel B A, Silverman R H. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72(5):753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- Maran A, Maitra R K, Kumar A, et al. Blockage of NF-kappa B signaling by selective ablation of an mRNA target by 2-5A antisense chimeras. Science. 1994;265(5173):789–792. doi: 10.1126/science.7914032. [DOI] [PubMed] [Google Scholar]
- Miller D W. Immunobiology of the blood-brain barrier. J Neurovirol. 1999;5(6):570–578. doi: 10.3109/13550289909021286. [DOI] [PubMed] [Google Scholar]
- Hitchcock E R, Morris C S. Mononuclear cell infiltration in central portions of human astrocytomas. J Neurosurg. 1988;68(3):432–437. doi: 10.3171/jns.1988.68.3.0432. [DOI] [PubMed] [Google Scholar]
- Lamont A G, Adorini L. IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17(5):214–217. doi: 10.1016/0167-5699(96)30011-x. [DOI] [PubMed] [Google Scholar]
- Brunda M J, Luistro L, Warrier R R, et al. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med. 1993;178(4):1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrzak J A, Brunda M J. Interleukin-12. Biologic activity, therapeutic utility, and role in disease. Lab Invest. 1995;72(6):619–637. [PubMed] [Google Scholar]
- Gately M K, Desai B B, Wolitzky A G, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor) J Immunol. 1991;147(3):874–882. [PubMed] [Google Scholar]
- Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Voest E E, Kenyon B M, O'Reilly M S, Truitt G, D'Amato R J, Folkman J. Inhibition of angiogenesis in vivo by interleukin 12. J Natl Cancer Inst. 1995;87(8):581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- Manna S K, Mukhopadhyay A, Aggarwal B B. IFN-alpha suppresses activation of nuclear transcription factors NF-kappa B and activator protein 1 and potentiates TNF-induced apoptosis. J Immunol. 2000;165(9):4927–4934. doi: 10.4049/jimmunol.165.9.4927. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Langford L A, Piatyszek M A, Xu R, Schold S C. Jr, Shay J W. Telomerase activity in human brain tumours. Lancet. 1995;346(8985):1267–1268. doi: 10.1016/s0140-6736(95)91865-5. [DOI] [PubMed] [Google Scholar]
- Le S, Zhu J J, Anthony D C, Greider C W, Black P M. Telomerase activity in human gliomas. Neurosurgery. 1998;42(5):1120–1124. doi: 10.1097/00006123-199805000-00099. [DOI] [PubMed] [Google Scholar]
- Saleh M, Stacker S A, Wilks A F. Inhibition of growth of C6 glioma cells in vivo by expression of antisense vascular endothelial growth factor sequence. Cancer Res. 1996;56(2):393–401. [PubMed] [Google Scholar]
- Im S A, Gomez-Manzano C, Fueyo J, et al. Antiangiogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999;59(4):895–900. [PubMed] [Google Scholar]
- Horton H M, Anderson D, Hernandez P, Barnhart K M, Norman J A, Parker S E. A gene therapy for cancer using intramuscular injection of plasmid DNA encoding interferon alpha. Proc Natl Acad Sci USA. 1999;96(4):1553–1558. doi: 10.1073/pnas.96.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang L, Hanisch U K, Felgner P L, Reszka R. A continuous intracerebral gene delivery system for in vivo liposome-mediated gene therapy. Gene Ther. 1996;3(6):472–476. [PubMed] [Google Scholar]
- Davidson B L, Hilfinger J M, Beer S J. Extended release of adenovirus from polymer microspheres: potential use in gene therapy for brain tumors. Adv Drug Deliv Rev. 1997;27(1):59–66. [PubMed] [Google Scholar]
- Beer S J, Matthews C B, Stein C S, Ross B D, Hilfinger J M, Davidson B L. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5(6):740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- Thorsen F, Read T A, Lund-Johansen M, Tysnes B B, Bjerkvig R. Alginate-encapsulated producer cells: a potential new approach for the treatment of malignant brain tumors. Cell Transplant. 2000;9(6):773–783. doi: 10.1177/096368970000900604. [DOI] [PubMed] [Google Scholar]
- Komata T, Kondo Y, Kanzawa T, et al. Caspase-8 gene therapy using the human telomerase reverse transcriptase promoter for malignant glioma cells. Hum Gene Ther. 2002;13(9):1015–1025. doi: 10.1089/104303402753812421. [DOI] [PubMed] [Google Scholar]
- Komata T, Koga S, Hirohata S, et al. A novel treatment of human malignant gliomas in vitro and in vivo: FADD gene transfer under the control of the human telomerase reverse transcriptase gene promoter. Int J Oncol. 2001;19(5):1015–1020. doi: 10.3892/ijo.19.5.1015. [DOI] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, et al. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59(3):551–557. [PubMed] [Google Scholar]
- Grill J, Van Beusechem V W, Van Der Valk P, et al. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin Cancer Res. 2001;7(3):641–650. [PubMed] [Google Scholar]
- Van Beusechem V W, Grill J, Mastenbroek D C, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J Virol. 2002;76(6):2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan H, Su H, Hu L, Lamborn K R, Kan Y W, Deen D F. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001;3(3):255–263. doi: 10.1038/sj.neo.7900157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Shinkai M, Honda H, Kobayashi T. Heat-inducible TNF-alpha gene therapy combined with hyperthermia using magnetic nanoparticles as a novel tumor-targeted therapy. Cancer Gene Ther. 2001;8(9):649–654. doi: 10.1038/sj.cgt.7700357. [DOI] [PubMed] [Google Scholar]