Abstract
Differential expression of viral replication proteins is essential for successful infection. We report here that overexpression of the brome mosaic virus (BMV) 1a protein can repress viral RNA replication in a dosage-dependent manner. Using RNA replication-incompetent reporter constructs, repression of translation from BMV RNA1 and RNA2 was observed, suggesting that the effect on translation of the BMV RNA replication proteins is responsible for the decrease in RNA levels. Furthermore, repression of translation by 1a required the B box in the 5′-untranslated region (5′ UTR); BMV RNA3 that lacks a B box in its 5′ UTR is not subject to 1a-mediated translational inhibition. Mutations in either the methyltransferase or the helicase-like domains of 1a reduced the repression of replication and translation. These results suggest that in addition to its known functions in BMV RNA synthesis, 1a also regulates viral gene expression.
Viruses must produce their gene products in the proper amounts and with the appropriate timing. Failure to do so can lead to innate defense responses from the host that may act on RNA replication intermediates (47). A number of mechanisms can contribute to the differential expression of viral products, such as ribosome shunting, leaky scanning, frameshifting, functional recoding, and reinitiation (11, 12). For Sindbis virus and Tobacco mosaic virus, with single genomic RNAs, the expression of the RNA-dependent RNA polymerase is decreased by readthrough of leaky termination codons (27, 37, 44, 45). Translational control also plays an important role in regulating viral gene expression in negative-sense RNA viruses and retroviruses (19, 28, 31, 41, 49). Regulation of protein production from segmented plus-strand RNA viruses is less well understood.
Brome mosaic virus (BMV), a member of the alphavirus-like superfamily of RNA viruses, is a model segmented positive-strand RNA virus. The BMV genome consists of three capped, messenger-sense genomic RNAs which have a tRNA-like structure within the 3′-untranslated region (3′ UTR). Genomic RNA1 and RNA2 encode nonstructural proteins 1a and 2a, respectively, which direct RNA replication (33). Genomic RNA3 is a bicistronic RNA encoding the cell-to-cell movement protein (MP) and the coat protein (CP). The MP is translated from RNA3, whereas the 3′-proximal coat protein is translated from a subgenomic RNA4 that is made using minus-strand RNA3 as the template (33). In addition to replication in various plant species, BMV can replicate and transcribe its genome in Saccharomyces cerevisiae (20).
The multifunctional 1a protein contains two domains separated by a proline-rich sequence. The N-terminal domain contains activities for 7-methyl-guanosine methyltransferase and covalent GTP binding required for viral RNA capping (2, 23). The C-terminal domain contains all of the motifs of the DEAD box RNA helicase domain, with ATPase and GTPase activities (24, 48). 1a is the primary viral protein determinant for the subcellular localization of the BMV RNA replication complex (9, 39, 40). In yeast, 1a could recruit viral RNA2 and RNA3 to the replication complexes in a process that involves an RNA sequence, named the B box, in the intercistronic region of RNA3 and the 5′ UTR of RNA2 (7, 21). 1a could also interact with the N-terminal portion of the 2a RNA-dependent RNA polymerase to recruit it to the replication factory where RNA synthesis takes place (6, 8, 10, 22, 42, 43).
Recently, an Agrobacterium-mediated T-DNA gene delivery system was established to study BMV replication, gene expression, and RNA packaging in Nicotiana benthamiana (4, 14). The agroinfiltration system provides robust transient expression of viral proteins and allows careful dissection of the cis- and trans-acting requirements for BMV RNA replication in plants. Using this system, we made a preliminary observation that a green fluorescent protein (GFP) reporter expressed from BMV RNA1 and RNA2 was repressed by 1a in N. benthamiana (14). 1a was required and sufficient for this activity (14). How this activity relates to other functions of the 1a protein is not clear. In this report, we show that 1a can regulate BMV RNA replication through repression of the translation of RNA1 and RNA2 through a cis-acting sequence within their 5′ UTRs, i.e., the B box.
MATERIALS AND METHODS
Plasmid constructs.
The Agrobacterium-mediated gene delivery system to express the three BMV genomic RNAs for replication and all four of the BMV-encoded proteins independent of the viral RNAs was developed by Gopinath et al. (14). Constructs that express the hepatitis C virus (HCV) full-length NS3 and NS5B proteins were amplified from the HCV subgenomic replicon (29), using 5′ NcoI and 3′ XbaI restriction sites that were built into the primers (the sequences of all primers will be made available upon request). After digestion with NcoI and XbaI, the DNA fragments were subcloned into the corresponding sites of the binary pCB302 vector.
Construction of 1GFP1, 2GFP2, and R3MP/GFP was done by replacing the open reading frames of 1a, 2a, and MP in the pBR1, pBR2, and pBR3 plasmids, respectively, with the GFP coding sequence, as described by Gopinath et al. (14). To construct 3GFP3, which contains the RNA3 5′ UTR and 3′ UTR, two PCR products were amplified. One product, harboring the 5′ UTR, contained a BglII site at the 5′ end and an NcoI site at the 3′ end. The other PCR product, harboring the 3′ UTR, contained an XbaI site at the 5′ end and an ApaI site at the 3′ end. The GFP fragment cut from pRTL2-smGFP with NcoI and XbaI and the two PCR products digested with the corresponding enzymes were ligated and cloned into the binary pCB301 backbone cut with BglII and ApaI.
For construction of chimeric mutants 1GFP3 and 2GFP3, the DNA fragment containing the R3 3′ UTR, cut from plasmid 3GFP3 with XbaI and ApaI, was used to replace the corresponding sites of 1GFP1 and 2GFP2, respectively. To generate 3GFP1 and 3GFP2, the DNA fragment cut from 3GFP3 was used to replace the corresponding sites of 1GFP1 and 2GFP2, respectively. To construct deletion mutations in the 5′ UTRs of 1GFP1 and 2GFP2, mutant primers were used to amplify 5′ UTR DNA fragments. After digestion with BglII and NcoI, the DNA fragments were used to replace the corresponding sites of wild-type 1GFP1 and 2GFP2, respectively.
The frameshift mutants NcoIfs, ApaLIfs, and AatIIfs were made by digestion of the 1a expression plasmid at the unique NcoI, ApaL1, and AatII sites, respectively, filling in of the protruding ends with T4 DNA polymerase, and then religation with T4 DNA ligase. Site-directed mutagenesis to create the R136A and K691A mutants of 1a was performed with an Amersham QuickChange kit, using the protocol prescribed by the manufacturer. For construction of binary PK mutants, i.e., PK1, PK4, PK11, and PK15, the DNA fragments cut with ApaLI and AatII from PK mutant plasmids (24) were used to replace the corresponding sites of binary p1a. All constructs were sequenced to verify that they were correct and that no other mutations were introduced.
Agroinfiltration.
All of the plasmids were introduced into Agrobacterium tumefaciens C58C1 by electroporation. The cultures were grown and infiltrated into N. benthamiana plants as described by Gopinath et al. (14). The cultures expressing BMV RNA1, RNA2, and RNA3 were usually at a final concentration of 0.1 optical density units at 595 nm (OD595). The amounts of the reporter constructs and the 1a mutants could vary in each experiment, so the amounts infiltrated are stated in the figures or figure legends. The leaves were infiltrated by gently pressing the end of a 3-ml syringe loaded with the appropriate culture to the leaf and exerting gentle pressure to flood the interstitial areas within the leaf. For each sample tested, at least two independently infiltrated samples were analyzed.
RNA extraction and Northern blotting.
Total RNA was extracted from the leaf tissue by macerating the tissue with a disposable pestle made to fit into a microcentrifuge tube in the presence of lysis buffer (0.1 M glycine, pH 9.0, 40 mM EDTA, 100 mM NaCl, 2% sodium dodecyl sulfate [SDS], and 0.05% bentonite), extracting the tissue with an equal volume of phenol and chloroform, and precipitating the aqueous phase with an equal volume of isopropanol. Northern blots were performed with 5 μg glyoxylated RNA and strand-specific riboprobes, as described by Hema and Kao (15). For detection of GFP mRNA, a probe was made by in vitro transcription with an AmpliScribe T7 transcription kit, using a linearized GFP DNA fragment (cloned into the pGEM-T Easy vector) as the template. Key results of all of the experiments shown were confirmed in at least two additional, independent experiments.
Protein analysis and Western blotting.
Proteins were extracted by macerating the leaf tissue with a pestle in TB buffer (50 mM Tris-acetate, pH 7.4, 10 mM MgCl2, 5 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10% glycerol). The lysate was kept on ice for 20 min and centrifuged at 15,000 rpm for 30 min at 4°C to remove the plant cell debris and insoluble materials. The supernatant was subjected to SDS-polyacrylamide gel electrophoresis analysis. After gel electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane for Western blot analysis. Briefly, the membrane was blocked with 3% nonfat milk and then exposed to a mouse anti-GFP monoclonal antibody (1:5,000; Invitrogen). After incubation with goat anti-mouse immunoglobulin G coupled with horseradish peroxidase (1:5,000 dilution), the signal was detected by an enhanced chemiluminescence kit from Amersham Inc. (London, United Kingdom).
RESULTS
BMV 1a can repress viral RNA replication.
A useful feature of the Agrobacterium system is that the copy number of recombinant T-DNA integrated into the plant chromosome can be manipulated by varying the concentration of the infiltrated inoculuma. We used this property to explore the function of the BMV-encoded proteins on viral RNA replication. Agrobacterium cells expressing one of the following constructs were coinfiltrated into N. benthamiana leaves along with those expressing the three BMV RNAs: (i) the empty vector that serves as a control; (ii) the replication proteins NS5B and NS3 from the unrelated virus HCV, which should not affect BMV replication; and (iii) the BMV proteins, 1a, 2a, MP, or CP (Fig. 1A). Total RNAs were extracted at 44 h postagroinfiltration, a time at which we could observe robust (−)- and (+)-strand replication products (14). The expression of BMV MP, the HCV NS3 helicase, and the HCV NS5B polymerase had only modest effects on viral RNA replication, as detected by strand-specific riboprobes complementary to the conserved 3′ 200 nucleotides of BMV RNAs (Fig. 2A). However, BMV CP decreased the levels of all of the RNAs, while 2a specifically decreased RNA2 levels and increased the levels of the other RNAs (Fig. 2A, lanes 5, 6, 9, and 10). Iyer and Hall (18) reported that transgenic plants expressing the 2a protein could lead to the silencing of RNA2. Therefore, we did not pursue this result further.
FIG. 1.
Constructs used in this study. (A) Schematic representations of BMV cDNA constructs in pCB301 binary vector, named pR1, pR2, and pR3. These constructs were used to express the BMV viral RNAs. Large arrows denote the double cauliflower mosaic virus 35S promoter elements. The long rectangles represent the protein coding sequences, and the names of the proteins are within the rectangles. The cloverleaf structure represents the 3′ tRNA-like structure of BMV RNAs. The curved arrows represent the cis-cleaving ribozyme sequence. Constructs p1a, p2a, pMP, pCP, pNS3, and pNS5B in the pCB302 binary vector, with the tobacco etch virus (TEV) translational enhancer used to express the BMV proteins and hepatitis C virus NS3 and NS5B, are shown at the bottom. (B) Schematics of the reporter constructs used in this study. The open reading frames of BMV 1a and 2a in pR1 and pR2 were replaced with the GFP coding sequence, and the constructs were named 1GFP1 and 2GFP2, respectively. The flanking numbers in the names of these and other chimeric constructs denote the RNA origins of the 5′ and 3′ UTRs. 3GFP3 represents the GFP coding sequence flanked with the 5′ UTR and 3′ UTR of BMV RNA3. R3MP/GFP was constructed by replacing the MP open reading frame with the GFP coding sequence.
FIG. 2.
Expression of the 1a protein can inhibit BMV RNA replication. (A) (−)- and (+)-strand RNAs produced by BMV infection in N. benthamiana in the presence of the empty expression vector or additional viral protein. The infection was launched by a mixture of Agrobacterium cultures that expressed three BMV genomic RNAs, designated R1, R2, and R3 (all infiltrated at an OD595 of 0.1), and a culture expressing one of the following (infiltrated at an OD595 of 0.2): empty vector (Vec.), BMV MP, CP, replication protein 1a, or 2a, or the hepatitis C virus helicase (NS3) or polymerase (NS5B). The upper two panels are autoradiograms of the gel images probed with strand-specific riboprobes. The bottom image is from a slice of the denaturing agarose gel containing cellular RNAs (cRNAs) stained with ethidium bromide that serves as a loading control. Bands corresponding to the BMV RNAs are identified to the right of the gel images. (B) Quantification of the (−)- and (+)-strand BMV RNAs in the experiment shown in panel A as percentages of the wild-type RNAs. All samples were normalized to the BMV RNAs produced in the presence of the empty vector. (C) Effects of increasing the amount of the Agrobacterium culture expressing the BMV 1a protein. The density of the 1a culture infiltrated is noted above the gel images. The arrangement of the figure is similar to that for panel A. The quantifications shown are only for the (+)-strand BMV RNAs. (D) Demonstration that 1a production is needed for the observed inhibitory effect on BMV RNA accumulation. Quantifications of the (+)-strand BMV RNAs expressed in the presence of the empty vector, 1a, or 1a with a frameshift after the AUG codon (NcoIfs) are shown.
The most significant effect we observed was with the construct that expressed the 1a protein, which decreased both (−)- and (+)-strand BMV RNA accumulation. For (+)-strand RNA, RNA3 and subgenomic RNA4 levels dropped to 28 and 33%, respectively, of the results for the empty vector control (Fig. 2A, lanes 7 and 8, and B). To examine the repression more carefully, Agrobacterium cultures harboring the 1a construct were infiltrated into plants at concentrations ranging from 0.005 to 1.0 OD595 unit. At low concentrations of inocula, there was a modest increase in BMV RNA levels, but at 0.1 OD595 unit or higher, RNA accumulation was inhibited in a concentration-dependent manner (Fig. 2C). Increasing the ratio of Agrobacterium inoculum expressing RNA1 relative to the other RNAs also resulted in a concentration-dependent decrease in overall BMV RNA accumulation (data not shown).
To examine whether the effects observed with overexpression of 1a were due to induced RNA silencing, we added 4 bp to the NcoI site that is present immediately after the initiation codon of the 1a open reading frame, generating a construct containing a frameshift in 1a named NcoIfs. Since the majority of the RNA sequence is unaffected in NcoIfs, it should not affect RNA silencing, if it occurred. NcoIfs did not significantly decrease RNA levels, demonstrating that the 1a protein, not RNA silencing, is primarily responsible for the inhibition of BMV RNA replication (Fig. 2D).
Reporter constructs to assess the inhibitory activity of 1a.
Since both (−)- and (+)-strand RNA accumulation was affected, we hypothesize that 1a's inhibitory effect is through repressing translation from BMV RNAs. Since 1a expression in place of RNA1 can replicate RNA3 (14), the inhibitory effect is not through the production of a nonfunctional 1a protein. Instead, we posit that an overabundance of 1a down-regulates 2a levels and/or causes an imbalance of the ratio of 1a to 2a. To evaluate translation repression and to separate the requirements of translation from those of replication, we built a construct, named 2GFP2, that would express the GFP reporter protein in place of the 2a open reading frame (Fig. 1B). The left and right numbers in the names of this and other chimeric constructs denote the origins of the 5′ and 3′ UTRs, respectively. This reporter construct can express GFP but is not sufficient for RNA replication, even though viral replicase proteins are present and functional. In addition, 2GFP2 and other reporter constructs had no significant effects on the replication of the wild-type BMV RNAs (data not shown).
We infiltrated a mixture of Agrobacterium cultures that can express BMV RNA1, RNA2, RNA3, and 2GFP2 at ratios of 1:1:1:4, respectively, in N. benthamiana along with either the empty vector or a vector that can express 1a. Leaves expressing 1a had significantly lower GFP levels when the inoculum to express 1a increased, consistent with the inhibitory effects on viral RNA accumulation (Fig. 3B). However, the level of the 2GFP2 mRNA was not significantly affected (Fig. 3B), suggesting that 1a repressed GFP expression at the level of translation, not transcription. Similar translational inhibition was observed with 1GFP1, which has the 1a open reading frame in RNA1 replaced with the GFP coding sequence (Fig. 3A), showing that 1a can potentially regulate its own translation from RNA1. Since expression of 1a and 2a is necessary for viral RNA replication, repression of their translation from BMV RNA1 and RNA2 could be a mechanism for down-regulating BMV RNA replication.
FIG. 3.
BMV 1a protein can inhibit production of reporter protein from nonreplicating RNAs. (A) Effect of 1a expression on 1GFP1. A mixture of four Agrobacterium cultures was infiltrated into N. benthamiana to express BMV RNA1, RNA2, RNA3, and one of the chimeric RNAs, 1GFP1, 2GFP2, or 3GFP3 (at a ratio of 1:1:1:4), as labeled above the gel images. The levels of the BMV RNAs serve as an indicator of the effects of the 1a protein. The effects on BMV RNA3 relative to that in the reactions lacking overexpressed 1a are quantified below the gel images. The chimeric RNAs are named according to the origins of the 5′ and 3′ UTRs from the BMV RNAs. Panels labeled “GFP” are from Western blots probed with a monoclonal antibody to the GFP protein. The slice labeled “Rubisco” is from the Coomassie blue-stained SDS-PAGE gel with the protein lysates used for Western blots. The GFP mRNA and viral RNA panels are from Northern blots probed to detect the GFP-expressing RNAs and the BMV genomic (+)-strand RNAs. Lastly, the gel image labeled “cRNA” is from a denaturing agarose gel containing cellular RNAs stained with ethidium bromide to serve as a loading control. The BMV RNAs are identified to the left of the Northern blot results. (B) Effects of 1a expression on 2GFP2. (C) Effects of 1a expression on 3GFP3. (D) Effects of 1a expression on R3MP/GFP, which replaced the MP open reading frame with the GFP coding sequence. The formats of sections B to D are identical to that of section A.
Interestingly, 3GFP3 was not affected by 1a overexpression (Fig. 3C). In addition, a version of BMV RNA3 named R3MP/GFP that had the MP coding sequence replaced with GFP was also not regulated by 1a (Fig. 3D). Inhibition of expression from 1GFP1 and 2GFP2, but not from 3GFP3 or R3MP/GFP, demonstrates that this new activity of 1a is specific. Furthermore, the activity of 1a is not directly related to the effects of the 3′ UTR on BMV translation (5), since this sequence is shared in the three RNAs.
If the effect of 1a is through reducing 2a levels, we reasoned that overexpressing 2a should reverse the inhibition of RNA accumulation. To test this, we added 1a at a constant level that is inhibitory and sequentially increased the amount of the Agrobacterium culture that could produce 2a. The presence of additional 2a did overcome the inhibition of subgenomic RNA4 levels so that RNA4 accumulated to a higher level than that in the wild-type infection (Fig. 4). RNA3 was increased threefold from the inhibited state in the presence of excess 2a but did not increase to levels above the wild-type level. The RNA2 level was not rescued at all, while there was a modest increase in RNA1 levels upon 2a overexpression. The effects are thus complex and specific to each RNA. However, the obvious effects of 2a overexpression on RNA3 and RNA4 levels suggest that at least part of 1a's effect on RNA levels could be attributed to a decrease in the availability of the BMV replicase.
FIG. 4.
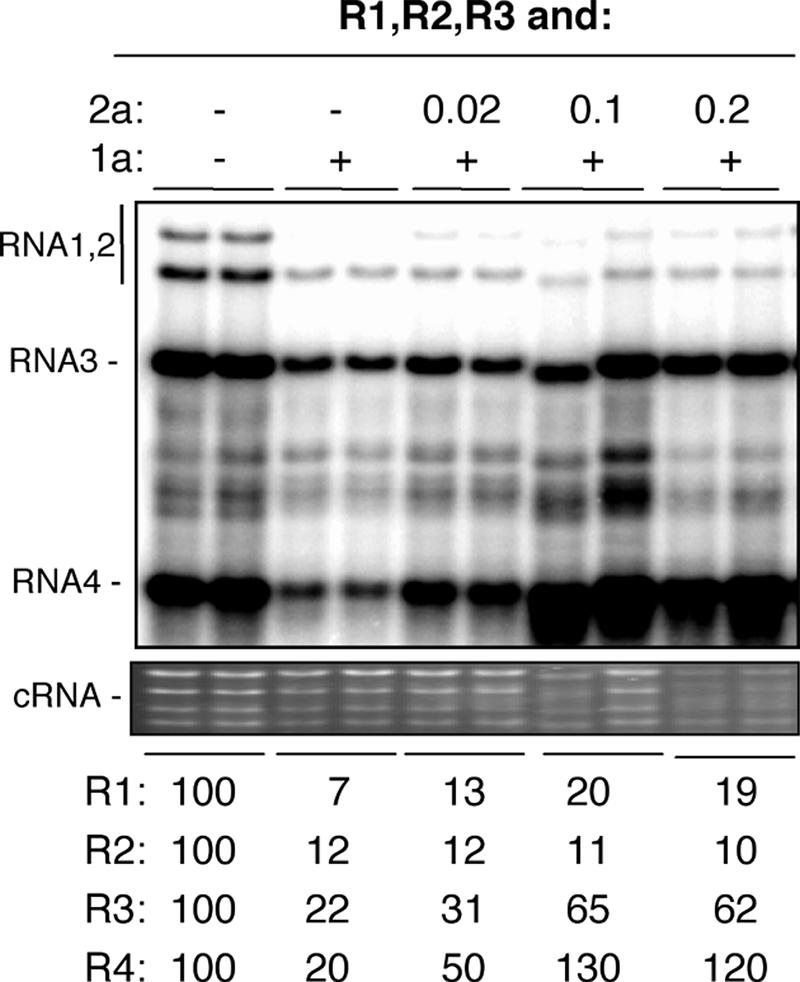
Coexpression of the BMV 2a protein can rescue BMV RNA3 and RNA4 production. Northern blots of the (+)-strand RNAs are shown, with the identities of the RNAs shown to the left of the gel image. All of the samples were agroinfiltrated to express the three BMV RNAs. Coinfiltration of the empty vector or 1a (both at 0.2 OD595 unit) is denoted with “+.” The amount of culture expressing 2a, where present, is noted with a number to denote the OD595. The images labeled “cRNA” are from a denaturing agarose gel containing cellular RNAs stained with ethidium bromide.
cis-acting sequence required for 1a inhibition of translation.
To identify the RNA elements required for 1a-dependent translational inhibition, chimeric RNAs containing swaps of the UTRs between RNA3 and RNA1 or RNA2 were tested in the context of the GFP reporter constructs (Fig. 5A). Since RNA3 is insensitive to 1a inhibition, we expect that it lacks the necessary cis-acting element. The chimeric RNAs were expressed in the presence of all three BMV RNAs and either the vector control or 1a in trans. The BMV genomic RNAs served as an internal control for the effects of 1a, and we observed significant reductions in all of the BMV RNA levels in these experiments (Fig. 5B and C). With the vector control, GFP was detected by microscopic examination of the plant cells for all of the chimeric constructs (Fig. 5A) and also in Western blots probed for GFP (left panels of Fig. 5B and C). In the presence of 1a, GFP expression decreased significantly in constructs 1GFP1, 1GFP3, 2GFP2, and 2GFP3 but not in 3GFP1 and 3GFP2 (Fig. 5A). These results were confirmed in Western blots detecting the GFP protein (right panels in Fig. 5B and C) and demonstrate that the cis-acting elements responsive to 1a-dependent inhibition lie within the 5′ UTRs of RNA1 and RNA2 but not RNA3.
FIG. 5.
cis-acting sequences in RNA1 and RNA2 that respond to 1a-mediated inhibition of translation. (A) Schematics of reporter constructs infiltrated into N. benthamiana, along with the effects on GFP expression in plant cells. A mixture of Agrobacterium cultures expressing BMV RNA1, RNA2, RNA3, and the chimeric RNAs diagrammed on the left were infiltrated into N. benthamiana leaves along with a culture expressing either the empty vector (Vec.) or the 1a protein. The microscopic images were taken with a fluorescein isothiocyanate filter. Magnification, ×10. GFP is normally seen in the cytoplasm, which appears in N. benthamiana cells as a thin band. (B) Further examination of the effects of 1a on chimeric RNAs made by swapping the UTRs of BMV RNA1 and RNA3. The chimeric reporter gene and the BMV RNAs infiltrated into plants are shown above the gel image. The concentration of the Agrobacterium cultures expressing the chimeric reporter was 0.5 OD595 unit, that of 1a was 0.75 OD595 unit, and that of the other constructs was 0.125 OD595 unit. The gel slices labeled “GFP” are from a Western blot of the total lysates prepared from the infiltrated leaves. Gel slices stained with Coomassie blue and labeled “Rubisco” served as a loading control for the Western blots. Northern blots of the (+)-strand RNAs, labeled “viral RNAs,” show the effects of 1a on BMV RNA accumulation. Quantifications of the RNA3 levels from the Northern blot results are shown below the gel images. The amount of each RNA in the right panel was normalized to the level of that RNA in the absence of the 1a protein in the right panel. The images of the cellular RNAs (cRNA) are intended to serve as a loading control. (C) Further examination of the effects of 1a on chimeric RNAs made by swapping the UTRs of BMV RNA2 and RNA3. The arrangement of the figure is identical to that in panel B.
The B boxes in the 5′ UTRs of BMV RNA1 and RNA2 are required for 1a-mediated translation inhibition.
The 5′ UTRs of BMV RNA1 and RNA2 share several secondary structures that can be distinguished from that of RNA3 (50). Both RNA1 and RNA2 contain a 5′-terminal hairpin named subdomain A that contains two internal loops (Fig. 6A and C). The apex of this structure contains the B box, which was determined to be critical in replicase assembly (7, 21) and (+)-strand RNA synthesis (38). RNA2, but not RNA1, contains a second stem-loop named subdomain B. Lastly, both RNA1 and RNA2 contain a polypyrimidine-rich sequence named subdomain C.
FIG. 6.
Subdomains in the RNA1 and RNA2 5′ UTRs required for 1a-dependent translational inhibition. (A) Mfold structure of the BMV RNA1 5′ UTR, from nucleotide 1 to the translation initiation codon (underlined) (50). Sequences within the boxes indicated by dotted lines are deleted in the constructs whose names are in parentheses. (B) Effects of deletions in the RNA1 5′ UTR on GFP production and BMV RNA levels in the absence or presence of exogenously provided 1a protein. The constructs tested are indicated above the gel slices. Western blots for GFP levels and Northern blots for the BMV RNAs are as described in the legend to Fig. 4B. The amount of each RNA in the right panel of the Northern blot was normalized to the level of that RNA in the absence of the 1a protein in the right panel. (C) Mfold structure of the BMV RNA2 5′ UTR, from nucleotide 1 to the translation initiation codon (underlined). Boxed areas denote the nucleotides deleted in the constructs shown in parentheses. For ΔA, the nucleotides above the dashed lines were also deleted. The sequences deleted in ΔB and ΔC are boxed with dotted lines. ΔBC deletes both the ΔB and ΔC sequences. (D) Effects of deletions in the RNA2 5′ UTR on GFP production and BMV RNA levels in the absence or presence of exogenously provided 1a protein.
To address which subdomains within the 5′ UTR of 1GFP1 are required for 1a-dependent translational inhibition, subdomains A and C were independently deleted, resulting in constructs ΔA and ΔC, respectively (Fig. 6A). RNA structure prediction by Mfold suggested that in the absence of each subdomain, the other can still exist (data not shown). Without coinfiltration of 1a, both ΔA and ΔC expressed detectable GFP in Western blots (Fig. 6B, left panel), although both had reduced GFP levels. In the presence of overexpressed 1a, GFP was observed only with ΔA, not with ΔC, indicating that subdomain A contains the element(s) required for 1a-dependent inhibition (Fig. 6B, right panel). To examine whether the B box in subdomain A is required for 1a-dependent inhibition, the 11-nucleotide region containing the B box was deleted, and the resultant construct, ΔBbox, was tested for 1a-dependent inhibition. ΔBbox was no longer subject to translational inhibition (Fig. 6B, right panel), confirming that the B box within subdomain A is the primary determinant needed for 1a inhibition.
The subdomains within the 5′ UTR of RNA2 were also examined for the cis-acting element(s) required for 1a inhibition in the context of 2GFP2. Deletion of subdomain A, B, or C did not prevent translation in the absence of excess 1a (Fig. 6D, left panels). However, deleting subdomain A or just the B box resulted in RNAs that were no longer inhibited by 1a, confirming that the B box is the primary determinant for 1a inhibition in both RNAs. A slight decrease in 1a-dependent inhibition was also observed with a deletion of subdomain B of 2GFP2, suggesting that the function of the cis-acting elements in RNA2 differs somewhat from the function of those in RNA1 (compare left panels of Fig. 6B and D). In yeast, the ΔB version of the RNA2 5′ UTR increased protein expression nearly threefold (32), but this increase was not obvious in N. benthamiana.
Both the methyltransferase and helicase domains of 1a are required to repress viral RNA replication.
Next, we sought to determine which domain in 1a is required to inhibit replication. A number of mutations targeting key motifs were tested (Fig. 7A), including a change of the conserved arginine 136 in the methyltransferase domain, a mutation of the conserved lysine 691 in helicase domain I (3), two truncations generated at restriction sites ApaLI and AatII that caused frameshifts after codons 122 and 716, respectively, and four mutants with insertions of two amino acids (PK mutants) that were previously characterized by Kroner et al. (24) for RNA replication in barley protoplasts and by O'Reilly et al. (35) for protein-protein interactions in yeasts. PK1 and PK4, with insertions after 1a residues 267 and 492, respectively, were viable for BMV replication, and PK11 and PK15, with insertions after residues 203 and 651, respectively, were unable to replicate (24). These PK mutants had similar stabilities to that of wild-type 1a (36). The two single amino acid substitutions, R136A and K691A, in the methyltransferase and helicase domains, respectively, were previously characterized for RNA replication, replicase assembly, and other 1a-associated activities (3, 36, 48).
FIG. 7.
Effects of mutations or truncations in the 1a construct on BMV RNA levels and protein synthesis from a reporter construct. (A) Schematic of the 1a protein and the locations and identities of the mutations made in 1a. The approximate locations of the core of the 1a methyltransferase domain and motifs I through VII in the helicase-like domain are marked and labeled. The names of the mutants are shown in parentheses. PK1 and PK4 had insertions of two amino acids in 1a that did not abolish BMV RNA replication. PK11 and PK15 had insertions of two amino acids that abolished BMV RNA replication in barley protoplasts (24). The parts of the proteins expressed in the two truncations of the 1a protein are shown as dark lines. (B) Effects of mutations on BMV RNA levels. Agrobacterium cultures expressed BMV RNA1, RNA2, and RNA3 and either the empty vector (Vec.), wild-type 1a, or mutant 1a, as indicated above the gel image. The cellular RNAs from the same gel (cRNA) served as a loading control. (C) Effects of mutant 1a constructs on translation from a GFP-expressing reporter construct, 2GFP2. GFP levels were detected by Western blotting, and the gel slices labeled “Rubisco” served as loading controls for the Western blots.
We first determined whether 1a mutations could affect the abundance of the BMV RNAs. Mutations in the key residues of the methyltransferase and helicase domains reduced 1a-mediated inhibition, indicating that both contribute to the inhibitory activity. However, the K691A mutant of helicase domain I retained partial inhibitory activity, suggesting that the inhibition does not absolutely require replication-competent 1a protein (Fig. 7B). The mutants causing frameshifts after codons 122 and 716 also released the 1a inhibition, demonstrating that the truncated methyltransferase domain alone is not sufficient to inhibit translation. In addition, PK11, which is incompetent for RNA replication (24), partially inhibited RNA replication compared to wild-type 1a (Fig. 7B).
We sought to confirm the effects of the 1a mutants by using the reporter GFP construct 2GFP2. The R136A and K691A mutants and the frameshift mutants reduced the ability to repress GFP translation from 2GFP2, whereas PK11 only maintained some ability to inhibit GFP translation compared to the wt 1a protein (Fig. 7C). These translation repression results are consistent with the inhibition of RNA replication, confirming that both the methyltransferase and helicase domains are required for 1a to repress RNA replication.
DISCUSSION
We found that overexpression of the 1a protein could repress BMV RNA replication, likely by inhibiting translation from RNA1 and RNA2. This inhibitory effect requires the B boxes in the 5′ UTRs of RNA1 and RNA2 and does not occur with RNA3. Given that the effect is RNA specific and does not occur with the helicase from hepatitis C virus, we suggest that overexpression revealed a natural regulatory property of the 1a molecule.
The B box is known to facilitate the transport of the BMV RNAs to the site of viral RNA replication, a process that is also mediated by 1a (7, 21). It is possible that 1a's inhibition of translation is related to replicase assembly. A simple scenario is that the site of replication is not accessible to the translational machinery. However, some features of 1a's roles in translation and replicase assembly are different. For example, the interaction between 1a and the B box that inhibits translation did not stimulate RNA replication, as was the case for replicase formation. In addition, PK11, which is incompetent for replication (24), retained a partial ability to inhibit translation. Lastly, while replicase formation has been demonstrated with both RNA3 and RNA2 (7, 21), we did not observe regulation of RNA3 at the translational level, even with construct R3MP/GFP, which contains the intercistronic region containing the B box. We do not expect the B box to regulate the translation of the CP since RNA4 does not contain a B box.
It is currently not clear whether 1a's inhibitory activity is through direct binding to the B box or is mediated by cellular factors. Several yeast proteins have been identified to regulate BMV replication and translation, such as Lsm1p-7p/Pat1p deadenylation-dependent mRNA-decapping factors and DED1. Mutations in these host proteins affected viral replication in yeast at the levels of both viral replication and gene expression (1, 17, 25, 26, 30, 32, 34, 46). If 1a represses translation through affecting cellular proteins, then these proteins are prime candidates for the B box binding proteins. The other possibility is that 1a overexpression might compete with a host translation initiation factor(s), such as eukaryotic initiation factor 4E, for access to the capped mRNA, thus prohibiting the ribosome small subunit from recruiting mRNA for translation initiation. Answers to these mechanistic questions will await further studies.
For positive-strand RNA virus replication, the viral genomic RNAs must serve first as the template for translation and then as the template for RNA replication. This transition from translation to replication must be regulated at a temporal level to accommodate both processes (13). We posit that 1a inhibition of translation could function as a temporal switch. Since 1a acts on RNA1, this could work as a regulatory loop to modulate the degree of inhibition. The 1a protein is thus the ultimate multitasking protein in BMV infection, with demonstrated roles in replication, RNA modification, replicase assembly, rapid repair of truncations in the BMV 3′ UTRs, and translation (2, 7, 16, 21, 23, 48). Since 1a repression of viral RNA replication can be partially reversed by overexpressing 2a (Fig. 4), we speculate that interaction with 2a, presumably leading to replicase formation, can regulate the translational inhibitory activity of 1a.
Acknowledgments
We thank the Texas A&M Cereal Killers for their helpful discussions and support during this work. We thank P. Ahlquist for the PK mutant plasmids.
Funding was provided by National Science Foundation grant 0332259.
Footnotes
Published ahead of print on 15 November 2006.
REFERENCES
- 1.Ahlquist, P., A. O. Noueiry, W. M. Lee, D. B. Kushner, and B. T. Dye. 2003. Host factors in positive-strand RNA virus genome replication. J. Virol. 77:8181-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahola, T., and P. Ahlquist. 1999. Putative RNA capping activities encoded by brome mosaic virus: methylation and covalent binding of guanylate by replicase protein 1a. J. Virol. 73:10061-10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahola, T., J. A. den Boon, and P. Ahlquist. 2000. Helicase and capping enzyme active site mutations in brome mosaic virus protein 1a cause defects in template recruitment, negative-strand RNA synthesis, and viral RNA capping. J. Virol. 74:8803-8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annamalai, P., and A. L. Rao. 2005. Replication-independent expression of genome components and capsid protein of brome mosaic virus in planta: a functional role for viral replicase in RNA packaging. Virology 338:96-111. [DOI] [PubMed] [Google Scholar]
- 5.Barends, S., J. Rudinger-Thirion, C. Florentz, R. Giege, C. W. A. Pleij, and B. Kraal. 2004. tRNA-like structure regulates translation of brome mosaic virus RNA. J. Virol. 78:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, J., and P. Ahlquist. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J., A. Noueiry, and P. Ahlquist. 2001. Brome mosaic virus protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J. Virol. 75:3207-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, J., A. Noueiry, and P. Ahlquist. 2003. An alternate pathway for recruiting template RNA to the brome mosaic virus RNA replication complex. J. Virol. 77:2568-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Den Boon, J., J. Chen, and P. Ahlquist. 2001. Identification of sequences in brome mosaic virus replicase protein 1a that mediate association with endoplasmic reticulum membranes. J. Virol. 75:12370-12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinant, S., M. Janda, P. A. Kroner, and P. Ahlquist. 1993. Bromovirus RNA replication and transcription require compatibility between the polymerase- and helicase-like viral RNA synthesis proteins. J. Virol. 67:7181-7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreher, T. W., and W. A. Miller. 2006. Translational control in positive strand RNA plant viruses. Virology 344:185-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gale, J. M., S. Tan, and M. G. Katze. 2000. Translational control of viral gene expression in eukaryotes. Microbiol. Mol. Biol. Rev. 64:239-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamarnik, A. V., and R. Andino. 1998. Switch from translation to RNA replication in a positive-strand RNA virus. Genes Dev. 12:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopinath, K., B. Dragnea, and C. C. Kao. 2005. Interaction between brome mosaic virus proteins and RNAs: effects on RNA replication, protein expression and RNA stability. J. Virol. 79:14222-14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hema, M., and C. C. Kao. 2004. Template sequence near the initiation nucleotide can modulate brome mosaic virus RNA accumulation in plant protoplasts. J. Virol. 78:1169-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hema, M., K. Gopinath, and C. C. Kao. 2005. Repair of the tRNA-like CCA sequence in a multipartite positive-strand RNA virus. J. Virol. 79:1417-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishikawa, M., J. Diez, M. Restrepo-Hartwig, and P. Ahlquist. 1997. Yeast mutations in multiple complementation groups inhibit brome mosaic virus RNA replication and transcription and perturb regulated expression of the viral polymerase-like gene. Proc. Natl. Acad. Sci. USA 94:13810-13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyer, L. M., and T. C. Hall. 2000. Virus recovery is induced in brome mosaic virus p2 transgenic plants showing synchronous complementation and RNA-2-specific silencing. Mol. Plant-Microbe Interact. 13:247-258. [DOI] [PubMed] [Google Scholar]
- 19.Jacamo, R., N. Lopez, M. Wilda, and M. T. Franze-Fernandez. 2003. Tacaribe virus Z protein interacts with the L polymerase protein to inhibit viral RNA synthesis. J. Virol. 77:10383-10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janda, M., and P. Ahlquist. 1993. RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell 72:961-970. [DOI] [PubMed] [Google Scholar]
- 21.Janda, M., and P. Ahlquist. 1998. Brome mosaic virus RNA replication protein 1a dramatically increases in vivo stability but not translation of viral genomic RNA3. Proc. Natl. Acad. Sci. USA 95:2227-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao, C. C., and P. Ahlquist. 1992. Identification of domains required for direct interaction of the helicase-like and polymerase-like RNA replication proteins of brome mosaic virus. J. Virol. 66:7293-7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kong, F., K. Sivakumaran, and C. C. Kao. 1999. The N-terminal half of the brome mosaic virus 1a protein has RNA capping-associated activities: specificity for GTP and S-adenosylmethionine. Virology 259:200-210. [DOI] [PubMed] [Google Scholar]
- 24.Kroner, P. A., B. M. Young, and P. Ahlquist. 1990. Analysis of the role of brome mosaic virus 1a protein domains in RNA replication, using linker insertion mutagenesis. J. Virol. 64:6110-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushner, D. B., B. D. Lindenbach, V. Z. Grdzelishvili, A. O. Noueiry, S. M. Paul, and P. Ahlquist. 2003. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc. Natl. Acad. Sci. USA 100:15764-15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, W. M., M. Ishikawa, and P. Ahlquist. 2001. Mutation of host delta9 fatty acid desaturase inhibits brome mosaic virus RNA replication between template recognition and RNA synthesis. J. Virol. 75:2097-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, G., and C. M. Rice. 1989. Mutagenesis of the in-frame opal termination codon preceding nsp4 of Sindbis virus: studies of translational readthrough and its effect on virus replication. J. Virol. 63:1326-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, Y., F. Horvath, J. A. Aligo, R. Wilson, and B. He. 2005. The role of simian virus 5 V protein on viral RNA synthesis. Virology 338:270-280. [DOI] [PubMed] [Google Scholar]
- 29.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 30.Mas, A., I. Alves-Rodrigues, A. Noueiry, P. Ahlquist, and J. Diez. 2006. Host deadenylation-dependent mRNA decapping factors are required for a key step in brome mosaic virus RNA replication. J. Virol. 80:246-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicot, C., M. Dundr, J. M. Johnson, J. R. Fullen, N. Alonzo, R. Fukumoto, G. Princler, D. Derse, T. Misteli, and G. Franchini. 2004. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 10:197-201. [DOI] [PubMed] [Google Scholar]
- 32.Noueiry, A. O., J. Chen, and P. Ahlquist. 2000. A mutant allele of essential, general translation initiation factor DED1 selectively inhibits translation of a viral mRNA. Proc. Natl. Acad. Sci. USA 97:12985-12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noueiry, A. O., and P. Ahlquist. 2003. Brome mosaic virus RNA replication: revealing the role of the host in RNA virus replication. Annu. Rev. Phytopathol. 41:77-98. [DOI] [PubMed] [Google Scholar]
- 34.Noueiry, A. O., J. Diez, S. P. Falk, J. Chen, and P. Ahlquist. 2003. Yeast Lsm1p-7p/Pat1p deadenylation-dependent mRNA-decapping factors are required for brome mosaic virus genomic RNA translation. Mol. Cell. Biol. 23:4094-4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Reilly, E. K., J. D. Paul, and C. C. Kao. 1997. Analysis of the interaction of viral RNA replication proteins by using the yeast two-hybrid assay. J. Virol. 71:7526-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly, E. K., Z. Wang, R. French, and C. C. Kao. 1998. Interactions between the structural domains of the RNA replication proteins of plant-infecting RNA viruses. J. Virol. 72:160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelham, H. R. B. 1978. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature 272:469-471. [DOI] [PubMed] [Google Scholar]
- 38.Pogue, G. P., and T. C. Hall. 1992. The requirement for a 5′ stem-loop structure in brome mosaic virus replication supports a new model for viral positive-strand RNA initiation. J. Virol. 66:674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Restrepo-Hartwig, M. A., and P. Ahlquist. 1996. Brome mosaic virus helicase- and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J. Virol. 70:8908-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restrepo-Hartwig, M. A., and P. Ahlquist. 1999. Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum. J. Virol. 73:10303-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reutter, G. L., C. Cortese-Grogan, J. Wilson, and S. A. Moyer. 2001. Mutations in the measles virus C protein that up regulate viral RNA synthesis. Virology 285:100-109. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz, M., J. Chen, W. M. Lee, M. Janda, and P. Ahlquist. 2004. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proc. Natl. Acad. Sci. USA 101:11263-11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skuzeski, J. M., L. M. Nichols, R. F. Gesteland, and J. F. Atkins. 1991. The signal for a leaky UAG stop codon in several plant viruses includes the two downstream codons. J. Mol. Biol. 218:365-373. [DOI] [PubMed] [Google Scholar]
- 45.Strauss, E. G., C. M. Rice, and J. H. Strauss. 1983. Sequence coding for the alphavirus nonstructural proteins is interrupted by an opal termination codon. Proc. Natl. Acad. Sci. USA 80:5271-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomita, Y., T. Mizuno, J. Diez, S. Naito, P. Ahlquist, and M. Ishikawa. 2003. Mutation of host dnaJ homolog inhibits brome mosaic virus negative-strand RNA synthesis. J. Virol. 77:2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voinnet, O. 2005. Induction and suppression of RNA silencing: insights from viral infections. Nat. Rev. Genet. 6:206-220. [DOI] [PubMed] [Google Scholar]
- 48.Wang, X., W. M. Lee, T. Watanabe, M. Schwartz, M. Janda, and P. Ahlquist. 2005. Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates. J. Virol. 79:13747-13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Younis, I., L. Khair, M. Dundr, M. D. Lairmore, G. Franchini, and P. L. Green. 2004. Repression of human T-cell leukemia virus type 1 and type 2 replication by a viral mRNA-encoded posttranscriptional regulator. J. Virol. 78:11077-11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]








