Summary
We have identified an Arabidopsis mutant that displays enhanced disease resistance (edr2) to the biotrophic powdery mildew pathogen Erysiphe cichoracearum. Inhibition of fungal growth on edr2 mutant leaves occurred at a late stage of the infection process and coincided with formation of necrotic lesions approximately 5 days after inoculation. Double-mutant analysis revealed that edr2-mediated resistance is suppressed by mutations that inhibit salicylic acid (SA)-induced defense signaling, including npr1, pad4 and sid2, demonstrating that edr2-mediated disease resistance is dependent on SA. However, edr2 showed normal responses to the bacterial pathogen Pseudomonas syringae pv. tomato strain DC3000. EDR2 appears to be constitutively transcribed in all tissues and organs and encodes a novel protein, consisting of a putative pleckstrin homology (PH) domain and a steroidogenic acute regulatory protein-related lipid-transfer (START) domain, and contains an N-terminal mitochondrial targeting sequence. The PH and START domains are implicated in lipid binding, suggesting that EDR2 may provide a link between lipid signaling and activation of programmed cell death mediated by mitochondria.
Keywords: defense responses, disease resistance, powdery mildew, programmed cell death, salicylic acid, senescence
Introduction
Powdery mildews are biotrophic pathogens that infect a large number of plant species, causing significant economic losses in crops such as grape, wheat, and barley (Schulze-Lefert and Vogel, 2000). In Arabidopsis, the process of infection with the powdery mildew pathogen Erysiphe cichoracearum has been described in detail (Adam and Somerville, 1996). When E. cichoracearum infects Arabidopsis plants, spores first produce appressorial germ tubes, which penetrate the host epidermal cells, and then the fungus forms a bag-like haustorium inside the epidermal cells of the host. Haustoria are surrounded by host cell plasma membrane and function as feeding structures for the fungus. The fungus then develops secondary hyphae that grow along the leaf surface, forming secondary haustoria in adjacent epidermal cells. Conidiophores (chains of asexual spores) are formed 4–7 days after infection. By day 7, abundant conidiation is apparent and the conidiophores produce a powdery appearance for which the disease is named. Analysis of Arabidopsis–E. cichoracearum interactions in various Arabidopsis genotypes is providing new insights into how plants combat infection by biotrophic pathogens (Schulze-Lefert and Vogel, 2000).
Resistance to powdery mildew among different Arabidopsis accessions is variable (Adam and Somerville, 1996; Adam et al., 1999). Among them, Arabidopsis accession Moscow-0 (Ms-0) is highly resistant. E. cichoracearum cannot grow on Ms-0 leaves, but instead induces small lesions on the leaves consistent with induction of a hypersensitive resistance (HR) response. The resistance to E. cichoracearum on Ms-0 is mediated by RPW8, a small basic protein with a putative N-terminal transmembrane domain and a coiled-coil domain (Xiao et al., 2001). Unlike most characterized disease resistance genes, RPW8 mediates a broad-spectrum resistance conferring resistance to all tested isolates of four species of powdery mildew pathogens. The RPW8-mediated powdery mildew resistance requires pathways induced by salicylic acid (SA), but does not require pathways induced by jasmonic acid (JA) and ethylene (Xiao et al., 2001, 2005). In contrast to Ms-0, Arabidopsis accession Columbia (Col-0) does not contain a functional RPW8 gene and is susceptible to E. cichoracearum, displaying abundant conidiophores on mature leaves 7 days after infection.
To investigate the interaction between E. cichoracearum and Arabidopsis plants, a number of Arabidopsis mutants displaying enhanced disease resistance to powdery mildew have been identified (Frye and Innes, 1998; Vogel and Somerville, 2000; Vogel et al., 2002, 2004). However, the mechanisms underlying these mutations mediating powdery mildew resistance appear to be very different. Among these mutants, the edr1 mutant develops necrotic lesions at the site of infection and displays almost no visible powder on the leaves 8 days after inoculation. The fungal growth is inhibited at a very late stage and resistance appears to be caused by an accelerated activation of host defenses, including programmed cell death (PCD), suggesting that EDR1 is a negative regulator of plant defense (Frye and Innes, 1998). Further characterization demonstrated that edr1-mediated disease resistance is dependent on SA but independent of JA and ethylene (Frye et al., 2001). Interestingly, the edr1 mutation enhances transcription of an RPW8.1 transgene in the Col-0 genetic background, and this enhanced expression correlates with spontaneous HR-like lesions (Xiao et al., 2005), further establishing a link between EDR1 and regulation of PCD.
EDR1 encodes a CTR1-like protein kinase, consisting of a putative regulatory N-terminal domain and a C-terminal kinase domain (Frye et al., 2001). The EDR1 kinase domain alone displays kinase activity in vitro and overexpression of an EDR1 kinase-deficient protein causes dominant negative phenotypes that mimic the edr1 mutant (Tang and Innes, 2002).
Unlike the edr1 mutant, several other resistant mutants including pmr1 to pmr6 do not display a powdery mildew-induced lesion phenotype (Frye et al., 2001; Vogel and Somerville, 2000; Vogel et al., 2002, 2004). PMR4 encodes a callose synthase responsible for producing callose in response to biotic and abiotic stresses (Nishimura et al., 2003). Although the pmr4 mutant is more resistant to powdery mildew, it produces less callose than wild-type (WT) Col-0 plants upon infection with E. cichoracearum (Nishimura et al., 2003). The pmr5- and pmr6-mediated powdery mildew resistance does not require the SA pathway and defense responses in pmr6 are not constitutively expressed (Vogel et al., 2002, 2004). PMR5 encodes a member of a large plant-specific gene family of unknown function, potentially involved in the regulation of cell wall composition (Vogel et al., 2004). PMR6 encodes a pectate lyase-like protein, which is thought to function as a susceptibility factor in Arabidopsis required for the growth of powdery mildew (Vogel et al., 2002).
Many plant defense responses are regulated by pathways induced by the plant hormones SA, JA and ethylene (Dong, 1998). Several genes associated with SA-induced defense responses have been identified by genetic approaches. Mutations in EDS1 (Falk et al., 1999; Parker et al., 1996) and PAD4 (Glazebrook et al., 1997; Jirage et al., 1999) affect SA accumulation. Mutations in NPR1/NIM1 (Cao et al., 1997; Delaney et al., 1995; Ryals et al., 1997) block SA-induced responses. Mutations in EDS5 (Nawrath et al., 2002; Rogers and Ausubel, 1997) and SID2 (Wildermuth et al., 2001) reduce SA production. All of these mutations compromise SA-induced defense responses against pathogen attack and enhance susceptibility to biotrophic pathogens. In contrast, mutants that constitutively accumulate high levels of SA are more resistant to a variety of biotrophic pathogens (Bowling et al., 1994, 1997; Clarke et al., 1998, 2000; Maleck et al., 2002).
Besides the SA pathway, some defense responses are controlled by ethylene and JA pathways (Alonso et al., 1999; Clarke et al., 2000; Penninckx et al., 1998; Staswick et al., 1998; Thomma et al., 1999). Among the mutants resistant to powdery mildew, edr1- and pmr4-mediated disease resistance requires SA but not ethylene, while pmr5- and pmr6-mediated resistance does not require SA, ethylene or JA (Nishimura et al., 2003; Vogel et al., 2002, 2004). Although both edr1- and pmr4-mediated resistance to powdery mildew are dependent on SA but independent of ethylene, their strategies for defense are largely different, as the edr1 mutant displays significant callose accumulation 3 days after infection (Frye and Innes, 1998) while the pmr4 mutant produces dramatically less callose in response to inoculation (Nishimura et al., 2003).
In an effort to characterize the signaling pathway regulated by EDR1, and to possibly identify substrates of the EDR1 kinase domain, we screened for additional edr1-like mutants that displayed E. cichoracearum-induced lesions and a reduction in formation of conidia.
Results
Isolation of Arabidopsis mutants resistant to E. cichoracearum
To identify Arabidopsis mutants with enhanced disease resistance, we inoculated ethyl methane-sulfonate mutagenized Col-0 plants with the UCSC strain of E. cichoracearum and scored for disease responses 8 days after inoculation. Plants displaying no visible powder were selected. Approximately 12 000 M2 Col-0 plants, derived from approximately 3000 M1 parents, were screened, and mutants that displayed enhanced disease resistance were selected. Three mutants were identified that displayed strongly enhanced disease resistance in the M3 generation. Here, we describe the characterization of one of these, edr2. Characterization of the other mutants will be described elsewhere.
Compared with Col-0 WT plants, edr2 mutant plants were much more resistant to E. cichoracearum. Figure 1a shows that visible necrotic lesions formed on edr2 leaves by 8 days after inoculation and little to no powder were produced. To further characterize the edr2-mediated resistance to powdery mildew, we monitored the development of E. cichoracearum and host cell death using trypan blue staining. Spores of E. cichoracearum germinated and produced appressorial germ tubes on edr2 leaves and WT leaves 1 day after inoculation (data not shown). By 3 days after inoculation, E. cichoracearum developed extensive branched hyphae on both Col-0 and edr2 leaves (data not shown). By 5 days, extensive hyphae nearly covered both Col-0 and edr2 leaves (Figure 1b,c). However, many conidiophores formed on Col-0 leaves while significantly fewer formed on edr2 leaves. By day 7, abundant conidiophores developed on Col-0 leaves while the number of conidiophores was largely reduced on the edr2 leaves (Figure 1d,e). These observations demonstrated that the growth of E. cichoracearum was affected at a late stage of the infection process on edr2 plants. Instead of producing abundant conidia, edr2 mutants displayed large patches of dead mesophyll cells 5 days after infection (Figure 1c) with cell death becoming dramatic by 7 days after infection (Figure 1e). This massive mesophyll cell death was not observed on Col-0 leaves (Figure 1d).
Figure 1.
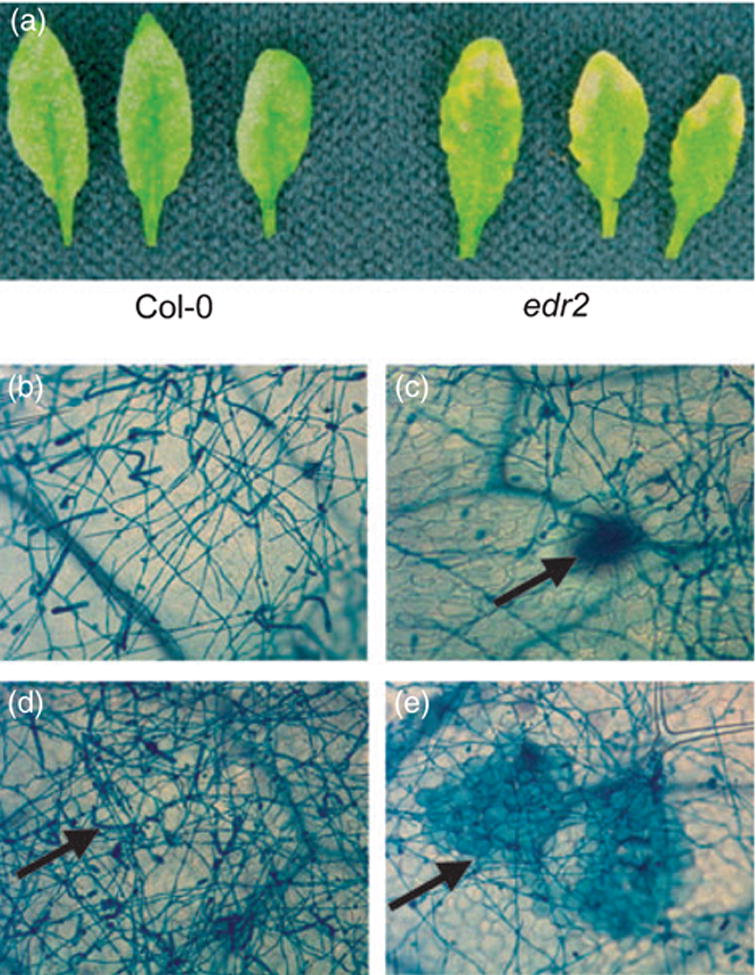
Response of Arabidopsis WT and edr2 mutant plants to E. cichoracearum.
(a) Col-0 and edr2 plants were inoculated with E. cichoracearum and leaves detached for photography 8 days after inoculation.
(b) Fungal hyphae growing on the surface of Col-0 leaves 5 days after infection, stained with trypan blue.
(c) Fungal hyphae growing on the surface of the edr2 mutant leaves 5 days after infection. Mesophyll cell death (arrow) is apparent at this stage.
(d) Extensive conidia (arrow) produced on Col-0 leaves 7 days after infection.
(e) Extensive mesophyll cell death (arrow) in edr2 mutant leaves 7 days after infection, but very few conidia.
The above phenotypes are highly similar to those of the edr1 mutant (Frye and Innes, 1998). F1 plants derived from a cross between the edr1 and edr2 mutant displayed WT susceptibility to E. cichoracearum, however, indicating that these mutations are in different genes. We therefore proceeded with detailed characterization of the edr2 mutant phenotype and isolation of the EDR2 gene.
The edr2 mutant displays normal responses to Pseudomonas syringae pv. tomato strain DC3000
To determine whether the edr2 mutation mediates a broad-spectrum disease resistance, we challenged edr2 mutant plants with the bacterial pathogen P. syringae pv. tomato strain DC3000 with or without the avirulence gene avrRpt2. Col-0 WT plants are susceptible to strain DC3000 and resistant to DC3000 (avrRpt2). We observed no significant differences in bacterial growth between WT and edr2 mutant plants, nor any differences in symptoms (data not shown). These data suggest that EDR2 does not play a role in regulating disease resistance against virulent or avirulent P. syringae strains.
PR-1 gene expression is enhanced in edr2 plants
To determine whether the edr2 mutation affects SA-induced gene expression, we monitored mRNA levels of the defense gene PR-1 at various time points after inoculation with E. cichoracearum. As shown in Figure 2, no or very little PR-1 transcript was detected prior to inoculation. PR-1 expression gradually increased after infection in both WT and edr2 mutant plants. By 3 days after inoculation, however, higher levels of PR-1 expression were observed in edr2 plants than in WT plants. The PR-1 expression was further increased at day 5 after inoculation in both WT and edr2 mutant plants; however, there was no observable difference between wild type and edr2. These data suggest that SA-induced defenses are more rapidly induced in the edr2 mutant than in WT Col-0 plants upon infection with E. cichoracearum.
Figure 2.
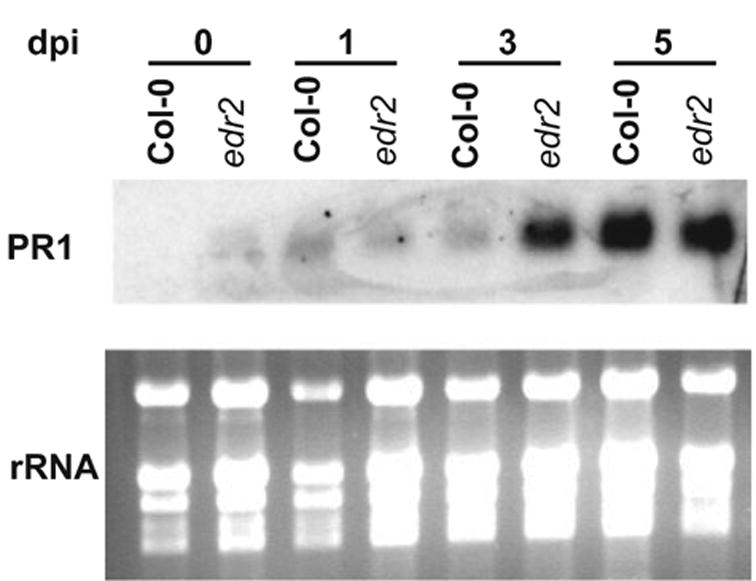
PR-1 transcripts accumulate more rapidly in the edr2 mutant than in WT plants after infection with E. cichoracearum. Top: an RNA gel-blot hybridized with a radiolabeled PR-1 cDNA probe. Bottom: the corresponding ethidium bromide-stained gel to show the relative amounts of RNA loaded in each lane.
Enhanced disease resistance mediated by edr2 is dependent on SA signaling, but not JA or ethylene signaling
To gain more insight into how EDR2 regulates defense responses in Arabidopsis, we assessed the roles of SA, ethylene and JA in edr2-mediated disease resistance using double-mutant analysis. A mutation in NPR1 (nim1-1), which reduces responsiveness to SA (Delaney et al., 1995), and mutations in PAD4 (pad4-1) and SID2 (sid2-2), which reduce levels of pathogen-induced SA (Nawrath and Metraux, 1999; Zhou et al., 1998), suppressed edr2-mediated enhanced resistance to powdery mildew (Figure 3), indicating that this phenotype is dependent on SA. In contrast, mutations in EIN2 (ein2-1) and COI1 (coi1-1), which block all known ethylene and JA responses, respectively (Alonso et al., 1999; Feys et al., 1994), did not suppress the edr2-mediated resistance (Figure 3), indicating that edr2-mediated resistance to powdery mildew does not require ethylene- and JA-induced defense responses.
Figure 3.
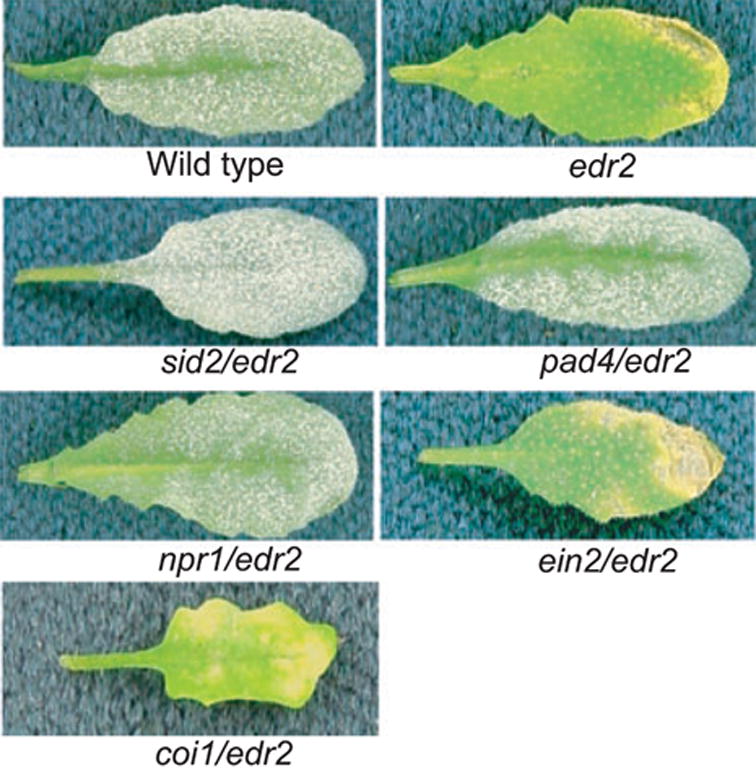
Resistance to powdery mildew mediated by the edr2 mutation is dependent on SA signaling, but not JA or ethylene signaling. The indicated mutants were inoculated with E. cichoracearum and disease phenotypes scored 8 days after infection. Single representative leaves were removed from intact plants for photography.
The edr2 mutant displays an enhanced ethylene-induced senescence phenotype
In addition to resistance to powdery mildew, the previously identified edr1 mutant also displays an enhanced ethylene-induced senescence phenotype (Frye et al., 2001). To test whether the edr2 mutant also has this trait, we exposed edr2 plants to ethylene (100 μl l−1) for 3 days. Interestingly, edr2 plants displayed an enhanced senescence phenotype indistinguishable from edr1 plants (Figure 4a). Ethylene induced visible chlorosis (yellowing) on the oldest two leaves of WT Col-0 plants after 3 days’ exposure to ethylene. However, in edr2 mutant plants, chlorosis occurred on much younger leaves (Figure 4a). Quantification of chlorophyll levels revealed significant differences between ethylene-treated WT Col-0 and edr2 mutant plants (Figure 4b).
Figure 4.
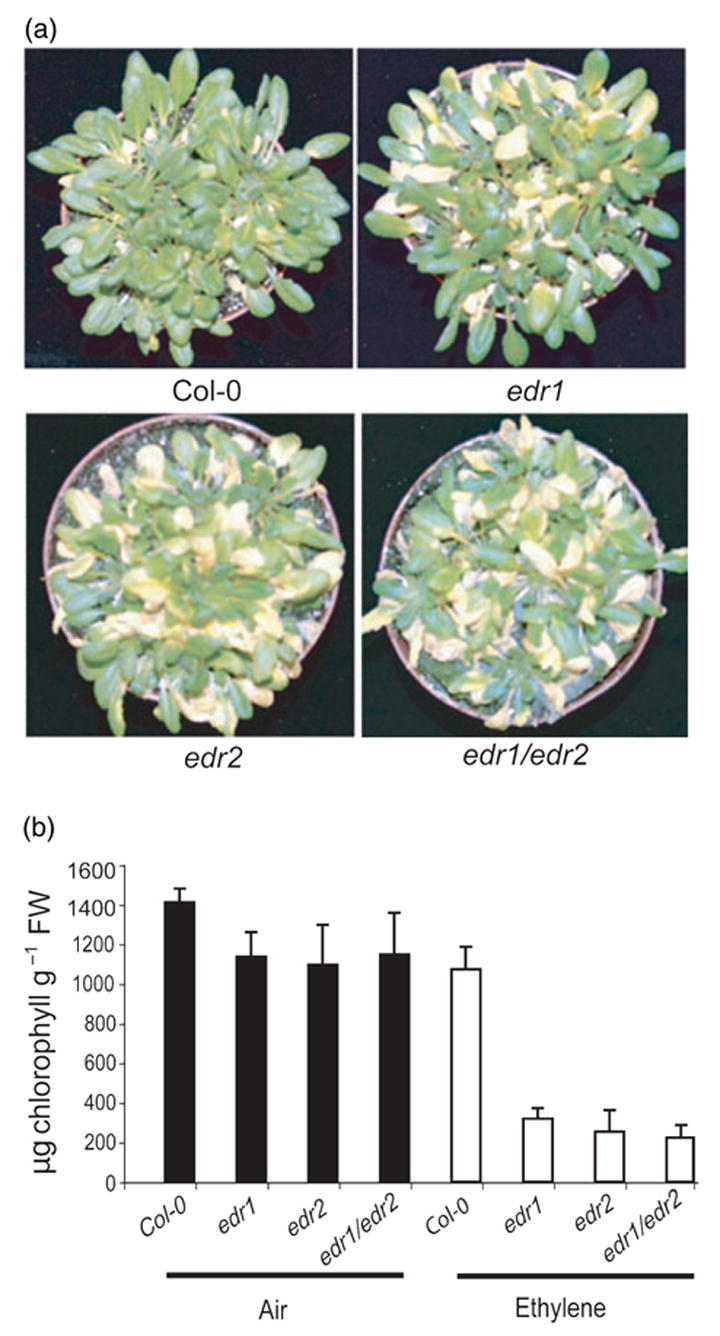
Enhanced ethylene-induced senescence in edr1 and edr2 mutant plants.
(a) Increased chlorosis after ethylene treatment. Plants were photographed after 3 days of exposure to 100 μl l−1 ethylene.
(b) The chlorophyll content in leaves five to eight (leaf one being the first true leaf) of the plants shown in (a). Bars represent the mean and standard deviation of values obtained from four plants.
The edr1 and edr2 mutations are not additive
The edr2 mutant phenotypes are very similar to those of the previously identified edr1 mutant in response to both E. cichoracearum and ethylene (Frye and Innes, 1998; Frye et al., 2001). To gain more insight into the relationship between the edr1 and edr2 mutations, we crossed edr1 with edr2 and characterized the phenotypes of the edr1/edr2 double mutant. The double mutant displayed a resistant phenotype similar to that of edr1 and edr2 single mutants when inoculated with E. cichoracearum, including a lack of conidia formation and development of similar necrotic lesions (data not shown). We also assayed the edr1/edr2 double mutant for its response to ethylene. Figure 4 shows that the double mutant displayed an enhanced ethylene-induced senescence phenotype similar to that of edr1 and edr2 single mutants. The similar phenotypes of edr1 and edr2 in response to both powdery mildew and ethylene and the observation that edr1 and edr2 do not display additive or synergistic effects suggest that EDR1 and EDR2 may function in the same signal transduction pathway.
Genetic mapping and identification of EDR2
Genetic mapping was accomplished using an F2 population derived from a cross between the edr2 mutant (Col-0 genotype) and Landsberg erecta (Ler). Genomic DNA was isolated from 46 resistant F2 plants and scored with published simple sequence length polymorphism (SSLP) markers. This initial mapping localized edr2 between molecular markers T6K21 and M4I22 on chromosome 4. We then developed our own molecular markers at intervals between these two markers using Monsanto Col-0 and Ler polymorphism data (http://www.arabidopsis.org/Cereon/index.jsp). Five hundred and four resistant F2 plants representing 1008 meioses were scored. Ultimately, edr2 was localized between bacterial artificial chromosome (BAC) end sequence F13C5 (GenBank accession AL021711) and an internal sequence of BAC clone T18B16 (GenBank accession AL021687) (Figure 5). This analysis defined a 120 kb region containing 28 predicted genes that co-segregated with edr2.
Figure 5.
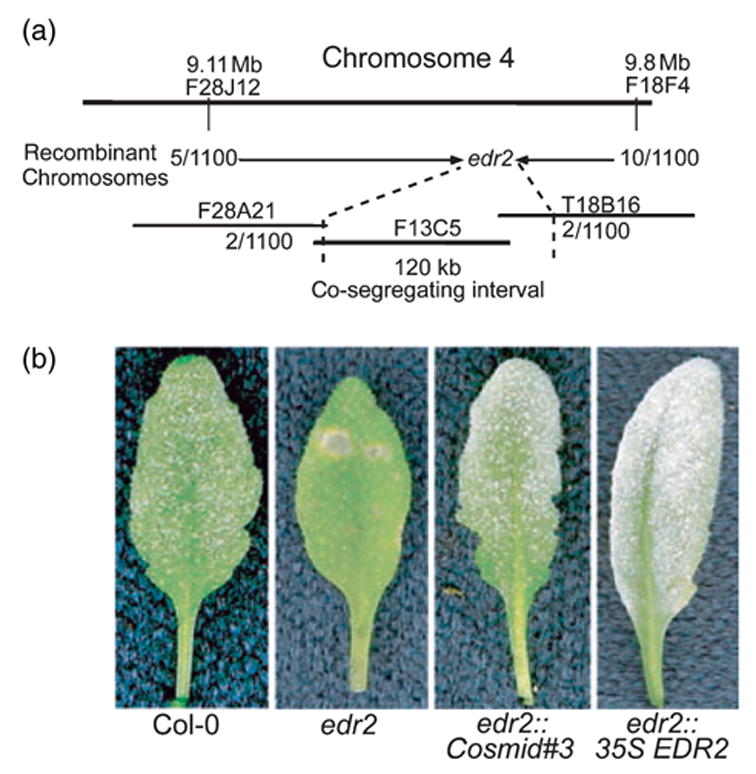
Positional cloning of the EDR2 gene.
(a) Genetic and physical map of the region flanking EDR2. Shorter horizontal lines indicate BAC clones spanning the region to which edr2 was mapped.
(b) Complementation of the edr2 mutation by Agrobacterium-mediated transformation. The indicated plants were inoculated with E. cichoracearum and representative leaves removed for photography 8 days after inoculation. Both a genomic cosmid clone (#3) and a cDNA clone (35S EDR2) containing the At4g19040 gene were able to complement the mutation.
To identify the EDR2 gene, we constructed a cosmid library using F13C5 BAC DNA. Twelve cosmid clones that overlapped and covered the whole BAC were identified and used to rescue the edr2 phenotype. Among the 12 clones, only cosmid clone 3 complemented the edr2 mutation (Figure 5b). This clone contained two full-length genes, At4g19030 (encodes a nodulin-26-like protein) and At4g19040 (encodes an unknown protein). No mutation was found in At4g19030, but a C → G transversion was found in At4g19040, which caused an early stop (P246stop) in the predicted open-reading frame (ORF). Taken together, these data indicated that EDR2 corresponds to At4g19040.
To confirm that At4g19040 is EDR2, and to identify more edr2 alleles, we obtained four T-DNA insertion lines (Alonso et al., 2003) from the Arabidopsis Biological Resource Center (ABRC, Ohio State University, Columbus, OH, USA). We identified homozygous plants for each T-DNA insertion line, confirmed the location of insertion sites by direct sequencing of flanking PCR products (Figure 6a), and then tested their resistance to E. cichoracearum. All four lines displayed the edr2-like phenotype, including a lack of visible powder and dramatic lesion production 8 days after inoculation (data not shown).
Figure 6.
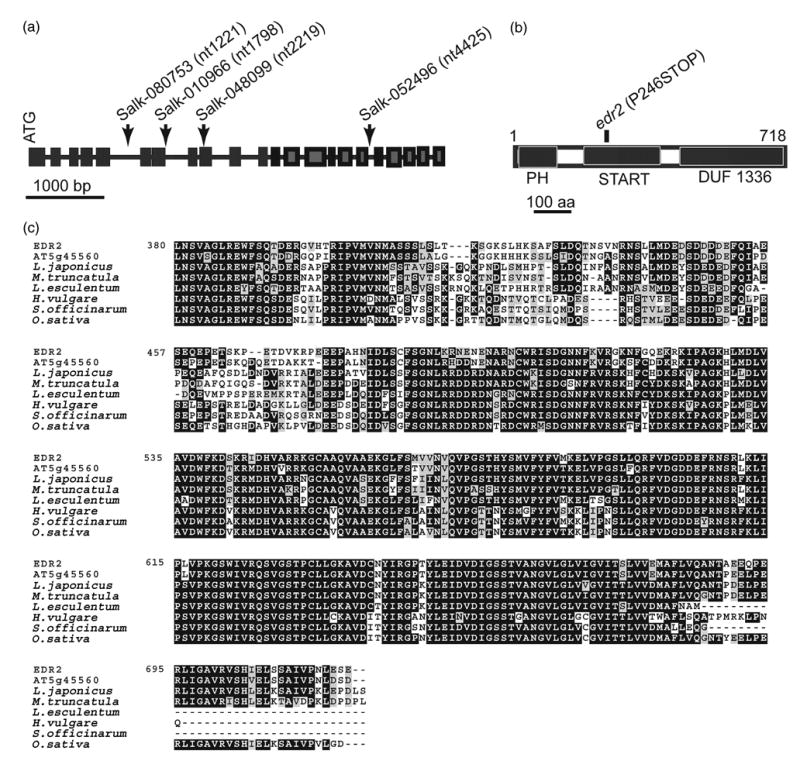
Characterization of EDR2.
(a) Structure of the EDR2 gene. Insertion sites in the Salk T-DNA lines are indicated by arrows. Numbers in parentheses indicate the nucleotide position in the genomic sequence relative to the start of the coding region.
(b) The predicted EDR2 protein contains 718 amino acids and includes a PH domain, a START domain and a C-terminal DUF1336 domain, which is highly conserved among homologs in other plant species.
(c) Alignment of the EDR2 C-terminal DUF1336 domain with the most similar Arabidopsis protein (At5g44560) and homologs from other plant species identified in the TIGR Plant Gene Indices database. Tentative consensus numbers for the homologs are Oryza sativa (rice), TC251404; Saccharum officinarum (sugarcane), TC58737; Hordeum vulgare (barley), TC150750; Lycopersicon esculentum (tomato), TC144758; Medicago truncatula (medicago), TC88033; Lotus japonicus (lotus), TC15086.
Overexpression of EDR2 complements the edr2 mutant phenotype
To gain more insight into how EDR2 regulates the defense responses of plants, we overexpressed a full-length EDR2 gene in both the WT and edr2 background under control of a cauliflower mosaic virus (CaMV) 35S promoter. Thirty independent T1 transgenic plants from WT or edr2 were inoculated with E. cichoracearum. There were no significant differences between WT plants and the WT plants carrying the 35S::EDR2 transgene. However, 27 out of 30 35S::EDR2 transgenic plants in the edr2 background displayed a WT-like phenotype. These transgenic plants were susceptible to E. cichoracearum and showed extensive conidiation and no necrotic lesions 8 days after infection, demonstrating that the 35S::EDR2 construct complemented the edr2 mutation (Figure 5b). The transgene did not cause any growth phenotype, as all transgenic lines were indistinguishable from WT plants prior to inoculation (data not shown).
Analysis of EDR2 expression
To gain insight into EDR2 expression, we searched Arabidopsis microarray data available through the AtGeneExpress Web interface (http://www.arabidopsis.org/info/expression/atgenexpress.jsp) for EDR2 expression. Microarray analyses showed that EDR2 is expressed in various tissues and organs at all developmental stages. To investigate EDR2 expression more directly, we expressed a GUS reporter gene in WT Arabidopsis plants under control of the EDR2 promoter, which was assumed to be contained within a 1040 base-pair fragment 5′ to the EDR2 start codon. We obtained a number of EDR2 ::GUS transformants and analyzed a total of 20 transgenic lines. Figure 7 shows typical GUS staining found in all plants analyzed. Consistent with the microarray data, GUS staining was observed in all tissues and organs tested, including leaves, roots, flowers, stems and siliques, demonstrating that EDR2 is ubiquitously expressed.
Figure 7.
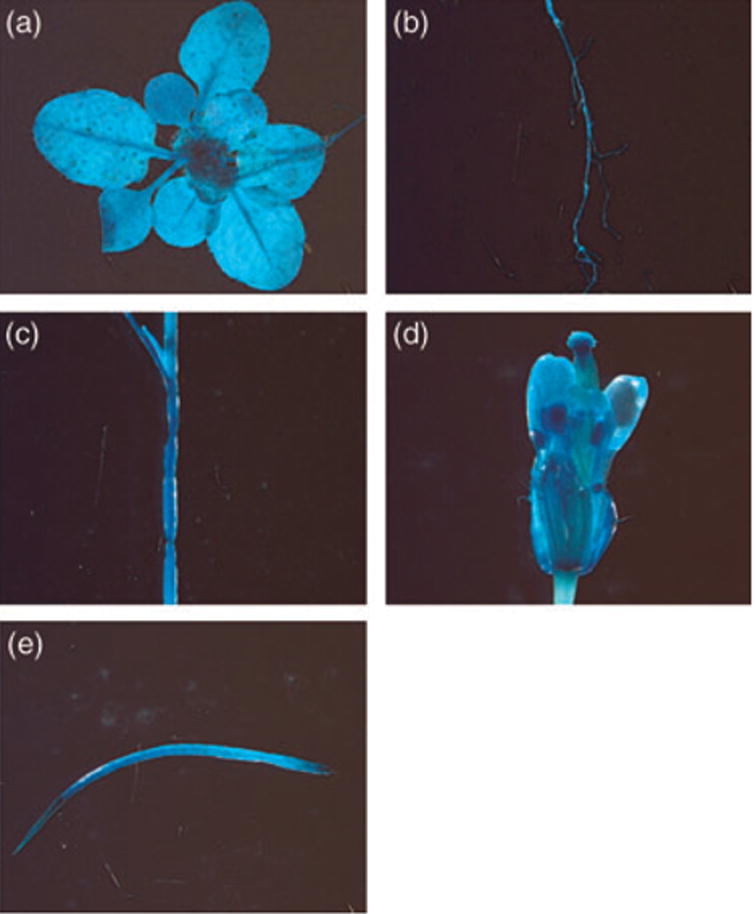
EDR2 is expressed in all tissues and organs examined. EDR2 promoter–GUS expression in (a) seedlings, (b) roots, (c) stem, (d) flower and (e) silique.
To investigate whether EDR2 is induced by E. cichoracearum, we conducted real-time RT-PCR analysis to examine EDR2 expression. The time course of EDR2 transcription was determined in Col-0 plants inoculated with E. cichoracearum. The levels of EDR2 transcription were not significantly affected by infection with powdery mildew (data not shown).
EDR2 encodes a novel protein containing a PH and a steroidogenic acute regulatory protein-related lipid-transfer (START) domain
EDR2 is predicted to encode an unknown protein of 718 amino acid residues with a mass of 77.6 kDa and an isoelectric point (pI) of 7.18. The annotation data predicted that EDR2 consists of 22 exons and 21 introns (Figure 6a). To confirm this prediction, we isolated RNA from WT Col-0 plants and performed RT-PCR followed by direct sequencing of the PCR product. The sequence data confirmed the predicted splice sites.
Sequence analysis indicates that EDR2 contains a pleckstrin homology domain (PH), a START domain, and a DUF1336 domain (PFAM accession nos PF00169, PF01852 and PF07059, respectively) (Figure 6b). The PH domain is approximately 120 amino acid residues and was first identified in pleckstrin (Haslam et al., 1993; Mayer et al., 1993). Several PH domains are known to function as lipid-binding domains, and facilitate membrane localization (Maffucci and Falasca, 2001).
The START domain is about 200 amino acids long and has also been implicated in lipid binding (Soccio and Breslow, 2003). It is found in many signaling proteins. The START domain is believed to have important roles in lipid transport, lipid metabolism and cellular signaling (Soccio and Breslow, 2003).
The DUF1336 domain is a plant-specific domain of unknown function and is approximately 250 amino acids in length. Four other Arabidopsis proteins, At5g45560, At3g54800, At2g18320 and At5g35180, contain PH, START and DUF1336 domains, but none of these proteins have a known function.
We also analyzed the EDR2 protein sequence for potential subcellular targeting signals. Both the iPSORT algorithm (Bannai et al., 2002; http://hc.ims.u-tokyo.ac.jp/ipsort/) and the TARGETP algorithm (Emanuelsson et al., 2000; http://www.cbs.dtu.dk/services/targetp/) identified a probable mitochondrial targeting peptide at the N-terminus of EDR2, suggesting that EDR2 may function inside mitochondria.
The C-terminal DUF1336 domain of EDR2 is highly conserved among homologs in other plant species
To determine whether EDR2 is conserved among plant species, we performed a BLAST search of the Institute for Genome Research (TIGR) plant gene indices database (http://www.tigr.org/tdb/tgi/ego/orth_search.shtml) using the full-length EDR2 protein sequence as a query. We identified highly similar proteins in many plant species, including several distantly related species such as rice and barley. Alignment of EDR2 with its homologs revealed that the EDR2 C-terminal DUF1336 domain is particularly well conserved (about 80% identical to the barley and rice homologs; Figure 6c), suggesting that this domain is critical to the function of EDR2-like proteins, and that EDR2 may play a fundamental and conserved role in the regulation of plant defense responses and cell death.
Discussion
Because loss-of-function mutations in the EDR2 gene confer enhanced disease resistance to powdery mildew, EDR2 probably functions as a negative regulator of powdery mildew resistance. Loss of EDR2 may lower the threshold of activation for host defenses such as PCD and PR gene expression. In this scenario, E. cichoracearum normally activates host defenses only weakly, but in the edr2 mutant defense responses are induced more rapidly and to a greater level.
The edr2 mutant phenotypes are very similar to edr1 mutants. Both display enhanced resistance to powdery mildew and enhanced ethylene-induced senescence. Furthermore, both edr1- and edr2-mediated resistances require pathways induced by SA, but not by JA or ethylene. In addition, the edr1/edr2 double mutant displays phenotypes indistinguishable from edr1 and edr2 single mutants. Combined, these observations strongly suggest that EDR1 and EDR2 may function in the same pathway(s) to regulate senescence and cell death.
EDR2 encodes a novel protein containing a PH and a START domain. The PH domain was first identified in pleckstrin, the major substrate of protein kinase C in platelets (Haslam et al., 1993; Mayer et al., 1993). The PH domain occurs in a wide range of proteins involved in intracellular signaling, cytoskeletal organization, membrane transport and modification of phospholipids (Lemmon et al., 2002; Rebecchi and Scarlata, 1998). In the human genome, 252 different PH domain-containing proteins have been found (Consortium, 2001). In Saccharomyces cerevisiae, 33 different proteins with PH domains have been identified (Yu et al., 2004). To date, a number of PH domain structures have been solved by nuclear magnetic resonance and X-ray crystallography. Despite the low sequence similarity among different PH domains, the three-dimensional structure of the PH domain is remarkably conserved (Maffucci and Falasca, 2001).
Although a large number of PH domains have been identified in different genomes, the function of PH domains is not yet clear and may vary from one protein to another (Yu et al., 2004). When it was first identified, the PH domain was thought to be a protein-binding domain (Maffucci and Falasca, 2001), and several protein ligands have been identified, such as the beta/gamma subunit of heterotrimeric G proteins, WD40 repeat-containing proteins and tyrosine kinase (Maffucci and Falasca, 2001). The best-known feature of PH domains, however, is their ability to bind to phospholipids, such as phosphoinositides or inositol phosphates (Lemmon, 2003). Phospholipid binding is believed to play an important role in targeting PH domain-containing proteins to cellular membranes. To date, the best-characterized PH domains are from the phospholipase C (PLC) family of proteins. PLC enzymes hydrolyze phosphatidylinositol 4,5- bisphosphate (PIP2), a key regulator of several cellular processes. The products of the hydrolysis are two second messengers 1,4,5-trisphosphate and diacylglycerol, which regulate release of Ca2+ from intracellular stores and activate protein kinase C (Philip et al., 2002). By analogy to these proteins, the PH domain of EDR2 may function in subcellular localization of EDR2 via lipid-binding or protein– protein interactions.
In addition to the PH domain, EDR2 contains a START domain. The START domain is a lipid/sterol-binding domain first found in StAR (steroidogenic acute regulatory protein), which transfers cholesterol to the inner mitochondrial membrane in steroid-hormone-producing cells (Stocco, 2001). Proteins with START domains can bind various ligands such as sterols (StAR protein) and phosphatidylcholine (PC-TP) (Ponting and Aravind, 1999; Soccio and Breslow, 2003). In multiple-domain proteins, ligand binding by the START domain can regulate the activities of other domains that co-occur with the START domain, such as Rho-gap, the homeodomain and the thioesterase domain. In the human and mouse genomes, 15 genes have been identified that encode START domains (Soccio and Breslow, 2003). In Arabidopsis, there are 35 START domain-containing genes, 21 of which are fused to homeodomains, suggesting important roles for these START-domain containing proteins in plant development (Ponting and Aravind, 1999; Schrick et al., 2004).
Proteins containing both a PH domain and START domain are rare; however, the human ceramide transport protein CERT contains a PH and a START domain (Hanada et al., 2003). CERT mediates the intermembrane transfer of ceramide from the endoplasmic reticulum to the Golgi apparatus. The START domain of CERT specifically binds ceramide, while the PH domain targets the Golgi apparatus by binding to phosphatidylinositol-4-monophosphate (PtdIns4p). In addition, the remaining middle region of CERT contains a motif for targeting to the endoplasmic reticulum (Loewen et al., 2003). Ceramide has been shown to regulate various cellular processes (Hannun and Luberto, 2000; Mathias et al., 1998). Interestingly, a mutation in a ceramide kinase, ACD5, in Arabidopsis leads to spontaneous cell death, indicating that ceramide plays an important role in modulating PCD in Arabidopsis (Liang et al., 2003). By analogy, EDR2 may function in a way similar to CERT, mediating the intermembrane transfer of a lipid signal molecule such as ceramide to regulate defense responses and cell death.
Many studies have implicated lipid signaling in disease resistance. For instance, the EDS1 and PAD4 proteins, two positive regulators of SA signaling, contain lipase-like domains (Falk et al., 1999; Jirage et al., 1999). Significantly, mutations in EDS1 and PAD4 compromise edr1-mediated resistance to powdery mildew (Frye et al., 2001). In addition, a mutation in DIR1, which encodes a putative apoplastic lipid transfer protein, abolishes induction of systemic acquired resistance, although the dir1 mutant exhibits WT local resistance, indicating that a lipid signal may be involved in systemic acquired resistance (Maldonado et al., 2002). Furthermore, a mutation in SSI2, which encodes a stearoyl-ACP desaturase, suppresses the npr1 mutation and displays constitutive PR gene expression, spontaneous lesions and enhanced resistance to Peronospora parasitica (Kachroo et al., 2001; Shah et al., 2001). Several of these ssi2-mediated phenotypes, including constitutive PR gene expression, are suppressed by mutations in the FAD6 or SFD1 genes. FAD6 encodes a plastidic ω6-desaturase that is involved in the synthesis of lipids containing polyunsaturated fatty acids (Nandi et al., 2003), while SFD1 encodes a putative dihydroxyacetone phosphate reductase and may play an important role in glycerol lipid metabolism (Nandi et al., 2004). In another report, both SA- and JA-mediated phenotypes of ssi2 plants are restored by a mutation in glycerol-3-phosphate dehydrogenase (Kachroo et al., 2004). These findings demonstrate that lipid signaling may interact with the SA pathway and play an important role in defense responses and PCD. EDR2 may be an important component that connects SA and lipid signaling.
Both iPSORT and TARGETP predict that mitochondria are the target for EDR2. If true, this would provide an intriguing functional link between lipid signaling, mitochondria and PCD in plants. In animals, mitochondria play a central role in integrating cellular stress signals in the activation of PCD (Ferri and Kroemer, 2001). Release of cytochrome c from mitochondria leads to the activation of caspases, a family of cysteine proteases that serve as the essential switch for most forms of PCD in animal cells (Green, 2000). Release of cytochrome c from mitochondria in animal cells is regulated by several different proteins including Bax, which associates with the outer mitochondrial membrane and modifies its permeability (Ferri and Kroemer, 2001). Although plants do not contain a recognizable homolog of Bax, expression of murine Bax in plant cells induces PCD and this PCD is correlated with targeting of Bax to mitochondria (Lacomme and Santa Cruz, 1999), suggesting that release of mitochondrial proteins and/or loss of mitochondrial membrane potential may be key activators of PCD in plant cells. Indeed, loss of mitochondrial membrane potential has recently been shown to be an early indicator of PCD in Arabidopsis protoplasts induced by diverse stimuli, including ceramide (Yao et al., 2004). It will be interesting to determine whether the edr2 mutation affects mitochondrial function, particularly the permeability of the mitochondrial outer membrane.
Regardless of the mechanism of edr2-mediated disease resistance, the EDR2 gene identified in this study may serve as an important entry point for understanding the function of plant PH and START domains and possible links between lipid signaling, mitochondria and the activation of PCD in plants.
Experimental procedures
Plant growth
Plants (Arabidopsis thaliana) were grown in growth rooms under 9 h light/15 h dark cycles at 22–24°C as described previously (Frye and Innes, 1998).
Powdery mildew infections
Erysiphe cichoracearum strain UCSC1 was maintained by growing on hyper-susceptible pad4-1 mutant plants. To inoculate plants, diseased pad4-1 plants (8–10 days after inoculation) were used to brush healthy 4–6-week-old plants to pass conidia (asexual spores) onto new plants. The disease phenotype was scored 8 days after inoculation. Fungal structures and dead plant cells were stained using alcoholic trypan blue (Koch and Slusarenko, 1990). Samples were observed and photographed using a Nikon e800 microscope.
Mutant screening
Mutagenized Col-0 plants (M2 generation) were inoculated with E. cichoracearum and scored for disease responses 8 days after inoculation. Plants displaying no visible powder were selected and allowed to set seeds. Approximately 12 000 M2 plants from ethyl methylsulfonate-mutagenized Col-0 plants, derived from 3000 M1 parents, were screened.
P. syringae infections
Plants were grown in a growth room under 9 h light/15 h dark cycles (150 μmol m−2 sec−1 of light) at 22–24°C. Plants that were 4–6 weeks old were inoculated by dipping leaves in a suspension of 2 × 108 colony-forming units ml−1 of strain DC3000 or DC3000 carrying avrRpt2 suspended in 10 mM MgCl2 supplemented with 250 μl l−1 L77 Silwet (OSI Specialties; Danbury, CT, USA). Inoculated plants were covered with a dome for 2 days. Disease symptoms were scored 3 days after inoculation. To monitor bacterial growth inside plant leaves, leaf samples were removed from plants using a number 2 cork borer (three discs per sample) and macerated in 200 μl of 10 mM MgCl2. Dilutions were made in 10 mM MgCl2 and plated on trypticase soy agar containing 50 mg l−1 kanamycin sulfate. Colonies were counted 48 h after incubation at 30°C.
Ethylene-induced senescence assay
Five-week-old plants were placed in a sealed chamber containing 100 μl l−1 ethylene for 3 days. Leaves five to eight (leaf one being the oldest true leaf) were removed and chlorophyll was extracted and measured as previously described (Frye et al., 2001).
Cosmid library construction
Bacterial artificial chromosome clone F13C5 (GenBank accession AL021711) was obtained from the ABRC. The binary vector pCLD04541 (Bancroft et al., 1997) and F13C5 BAC DNA were isolated using the Hi-Speed kit from Qiagen (Valencia, CA, USA) following the manufacturer’s protocol. F13C5 BAC DNA was partially digested with the restriction enzyme Sau3A and ligated to BamHI-digested pCLD04541. The ligation mix was packaged using a GigapackIII XL packaging extract (Stratagene, La Jolla, CA, USA) and transfected into Escherichia coli strain DH5α. Positive clones were selected on Luria–Bertani (LB) agar medium with 10 mg l−1 tetracycline.
Assembly of cosmid contigs
Overlapping cosmid clones were identified by PCR-based library screening with specific primer pairs derived from internal sequences of BAC F13C5. A total of 12 cosmid clones that covered all of BAC F13C5 were selected. These cosmids were purified using a plasmid miniprep kit (Qiagen) and transformed into Agrobacterium tumefaciens strain GV3101 by electroporation. Positive Agrobacterium clones were selected on 50 mg l−1 kanamycin and further confirmed by PCR using specific primer pairs.
Complementation of the edr2 mutation
Arabidopsis plants were transformed using the floral dip method (Clough and Bent, 1998). Transgenic plants were selected by growing on half-strength Murashige and Skoog salts plus 0.8% agar and 50 mg l−1 kanamycin. Transformants were transplanted to soil 7 days after germination and were inoculated with E. cichoracearum when 5 weeks old. Disease resistance was scored 8 days after inoculation. The cosmid that complemented the edr2 mutant phenotype was analyzed by sequencing of the junctions between the insert and vector using the flanking primers T3 and T7. Sequencing reactions were performed using the BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) and analyzed on an ABI 3730 automated sequencer.
DNA sequence analysis of Arabidopsis EDR2
We amplified the intact EDR2 ORF by PCR using an Arabidopsis Col-0 cDNA library (Frye et al., 2001) as template. The PCR product was sequenced directly using the ABI BigDye Terminator Cycle Sequencing Kit. DNA sequences were assembled using the SEQUENCHER program (Gene Codes; Ann Arbor, MI, USA). Our experimentally determined cDNA sequence was identical to the predicted full-length coding sequence for EDR2 (GenBank accession NM118022).
Construction of double mutants
Double mutants were created by standard genetic crosses. The edr1, edr2, pad4-1, sid2-2 and ein2-1 mutations were all in the Col-0 genotype of Arabidopsis, while npr1 (nim1-1) was in the Ws genotype and coi1-1 was in the Col-6 genotype. The double mutant of npr1/edr2 was identified by PCR-based molecular marker screening of F2 progeny. To identify edr1/edr2, pad4-1/edr2, sid2-2/edr2, coi1- 1/edr2, ein2-1/edr2 double mutants, we used PCR to amplify the respective genes followed by direct sequencing to identify plants that were homozygous for the mutations. All double mutants were verified to contain the edr2 mutation using a cleaved amplified polymorphic sequence (CAPS) marker designed to detect the edr2 mutation. CAPS primers (5′-AGACAAGAACCATCATTATAGTGCTA- 3′ and 5′-AACAACACAACTTCACAGAAAGAGCA-3′) were used for PCR amplification and the PCR product was digested by BsmAI to detect the edr2 mutation.
Identification of EDR2 T-DNA insertion mutants
Four T-DNA insertion lines were ordered from the ABRC (Salk-010966, Salk-048099, Salk-052496 and Salk-080753). Seeds from each line were sowed and 5-week-old plants were inoculated with E. cichoracearum. Defense responses were scored 8 days after infection. All four lines segregated for resistant and susceptible phenotypes. To confirm the phenotype, three plants from each line displaying a resistant phenotype were selected and self-fertilized, and the self-progeny tested for resistance to powdery mildew. T-DNA insertion sites, shown in Figure 7a, were confirmed by PCR amplification using a gene-specific primer and a T-DNA left border primer followed by direct DNA sequencing of the PCR products.
RNA gel blot hybridization
Total RNA was isolated from Arabidopsis leaf tissue using an RNeasy Mini Kit (Qiagen). A total of 5 μg of RNA was separated on a denaturing formaldehyde–agarose gel and transferred to Hybond N nylon membranes (Amersham Pharmacia Biotech, Buckinghamshire, UK). RNA gel blots were hybridized with a [32P]-labeled PR-1 DNA probe and washed at 65°C using Church buffer (Ashfield et al., 1998).
RT-PCR analysis
Plants were grown and inoculated with E. cichoracearum as described above. Leaves were removed from plants at different time points. Total RNA was isolated using the RNeasy kit. First strand cDNA from 2 μg of total RNA was synthesized using a SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). RT-PCR was performed using EDR2-specific primers (5′-ATGTCTAAGGTAGTGTACGAAGG- 3′ and 5′-GTCCTCTTCATCCTCTGCCGCA- 3′). A tubulin gene (At5g19770) was used as a control for normalizing the amount of cDNA using the following primers: 5′-GCGAGAAATCATAAGCAT-3′ and 5′-ACCATCAAACCTCAAAGA-3′.
Overexpression of EDR2
EDR2 full-length cDNA was amplified by PCR using primers that incorporated restriction sites for NheI and XhoI. The PCR products were cloned into a pGEM-T Easy vector (Promega, Madison, WI, USA). The EDR2 cDNA was then excised from pGEM-T Easy and inserted into XbaI- and XhoI-digested pBI1.4t vector, which contains a modified 35S CaMV promoter (Leister et al., 1996). The construct was verified by sequencing and transformed into Agrobacterium strain GV3101 by electroporation. Plant transformation, transgenic plant selection and phenotyping were performed as described above.
Construction of the EDR2 promoter::GUS reporter and GUS activity assay
A 1040 bp promoter fragment of EDR2 was amplified by PCR from genomic DNA of WT Col-0 using primers containing XbaI and BamHI restriction sites: (5′-AAGGTCTAGACAAAACCCAAATCCTCTGTCCAAT- 3′ and 5′-ACTTGGATCCCTGTCCCCAGAAATTACAAAAAATCT- 3′). The PCR product was digested with XbaI and BamHI and inserted into the pCB308 vector (Xiang et al., 1999). The clone was verified by sequencing and transformed into Agrobacterium strain GV3101 by electroporation. Plant transformation was conducted as described above. GUS activity analysis was performed as described (Jefferson et al., 1987). Samples were observed and photographed using a Nikon SMZ1500-dissecting microscope.
Protein sequence alignments
EDR2 protein homologs were identified by searching the GenBank and TIGR databases using the BLASTP program (Altschul et al., 1997). Putative EDR2 orthologs were identified in the TIGR website (http://www.tigr.org/tdb/tgi/ego/orth_search.shtml). Protein alignments were performed using CLUSTAL X with manual corrections (Thompson et al., 1997).
Acknowledgments
We thank J. G. Turner for providing coi1-1 seeds, F. M. Ausubel for providing sid2-2 seeds, J. Parker for providing pad4-1 seeds and T. Delaney for nim1-1 seeds. We also thank the ABRC for providing the F13C5 BAC clone, ein2-1 seeds and Salk T-DNA insertion lines. This work was supported by The National Institutes of Health, grant number R01 GM63761 to R.W.I.
References
- Adam L, Somerville SC. Genetic characterization of five powdery mildew disease resistance loci in Arabidopsis thaliana. Plant J. 1996;9:341–356. doi: 10.1046/j.1365-313x.1996.09030341.x. [DOI] [PubMed] [Google Scholar]
- Adam L, Ellwood S, Wilson I, Saenz G, Xiao S, Oliver RP, Turner JG, Somerville S. Comparison of Erysiphe cichoracearum and E. cruciferarum and a survey of 360 Arabidopsis thaliana accessions for resistance to these two powdery mildew pathogens. Mol Plant Microbe Interact. 1999;12:1031–1043. doi: 10.1094/MPMI.1999.12.12.1031. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfield T, Danzer JR, Held D, Clayton K, Keim P, Saghai Maroof MA, Webb PM, Innes RW. Rpg1, a soybean gene effective against races of bacterial blight, maps to a cluster of previously identified disease resistance genes. Theor Appl Genet. 1998;96:1013–1021. [Google Scholar]
- Bancroft I, Love K, Bent E, Sherson S, Lister C, Cobbett C, Goodman HM, Dean C. A strategy involving the use of high redundancy YAC subclone libraries facilitates the contiguous representation in cosmid and BAC clones of 1.7 Mb of the genome of the plant Arabidopsis thaliana. Weeds World. 1997;4ii:1–9. [Google Scholar]
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. Extensive feature detection of N-terminal protein sorting signals. Bioinformatics. 2002;18:298–305. doi: 10.1093/bioinformatics/18.2.298. [DOI] [PubMed] [Google Scholar]
- Bowling SA, Guo A, Cao H, Gordon AS, Klessig DF, Dong X. A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell. 1994;6:1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dong X. The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1- independent resistance. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Liu Y, Klessig DF, Dong X. Uncoupling PR gene expression from NPR1 and bacterial resistance: characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell. 1998;10:557–569. doi: 10.1105/tpc.10.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JD, Volko SM, Ledford H, Ausubel FM, Dong X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell. 2000;12:2175–2190. doi: 10.1105/tpc.12.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Consortium IHG. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Friedrich L, Ryals JA. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci USA. 1995;92:6602–6606. doi: 10.1073/pnas.92.14.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. SA, JA, ethylene, and disease resistance in plants. Curr Opin Plant Biol. 1998;1:316–323. doi: 10.1016/1369-5266(88)80053-0. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol. 2000;300:1005–1016. doi: 10.1006/jmbi.2000.3903. [DOI] [PubMed] [Google Scholar]
- Falk A, Feys BJ, Frost LN, Jones JD, Daniels MJ, Parker JE. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci USA. 1999;96:3292–3297. doi: 10.1073/pnas.96.6.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Mitochondria-the suicide organelles. BioEssays. 2001;23:111–115. doi: 10.1002/1521-1878(200102)23:2<111::AID-BIES1016>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Innes RW. An Arabidopsis mutant with enhanced resistance to powdery mildew. Plant Cell. 1998;10:947–956. doi: 10.1105/tpc.10.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Tang D, Innes RW. Negative regulation of defense responses in plants by a conserved MAPKK kinase. Proc Natl Acad Sci USA. 2001;98:373–378. doi: 10.1073/pnas.98.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Zook M, Mert F, Kagan I, Rogers EE, Crute IR, Holub EB, Hammerschmidt R, Ausubel FM. Phytoalexin-deficient mutants of Arabidopsis reveal that PAD4 encodes a regulatory factor and that four PAD genes contribute to downy mildew resistance. Genetics. 1997;146:381–392. doi: 10.1093/genetics/146.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426:803–809. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- Haslam RJ, Koide HB, Hemmings BA. Pleckstrin domain homology. Nature. 1993;363:309–310. doi: 10.1038/363309b0. [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA. 1999;96:13583–13588. doi: 10.1073/pnas.96.23.13583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo P, Shanklin J, Shah J, Whittle EJ, Klessig DF. A fatty acid desaturase modulates the activation of defense signaling pathways in plants. Proc Natl Acad Sci USA. 2001;98:9448–9453. doi: 10.1073/pnas.151258398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Venugopal SC, Lapchyk L, Falcone D, Hildebrand D, Kachroo P. Oleic acid levels regulated by glycerolipid metabolism modulate defense gene expression in Arabidopsis. Proc Natl Acad Sci USA. 2004;101:5152–5157. doi: 10.1073/pnas.0401315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell. 1990;2:437–445. doi: 10.1105/tpc.2.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacomme C, Santa Cruz S. Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acad Sci USA. 1999;96:7956–7961. doi: 10.1073/pnas.96.14.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister RT, Ausubel FM, Katagiri F. Molecular recognition of pathogen attack occurs inside of plant cells in plant disease resistance specified by the Arabidopsis genes RPS2 and RPM1. Proc Natl Acad Sci USA. 1996;93:15497–15502. doi: 10.1073/pnas.93.26.15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM, Abrams CS. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 2002;513:71–76. doi: 10.1016/s0014-5793(01)03243-4. [DOI] [PubMed] [Google Scholar]
- Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramides modulate programmed cell death in plants. Genes Dev. 2003;17:2636–2641. doi: 10.1101/gad.1140503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen CJ, Roy A, Levine TP. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 2003;22:2025–2035. doi: 10.1093/emboj/cdg201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffucci T, Falasca M. Specificity in pleckstrin homology (PH) domain membrane targeting: a role for a phosphoinositide-protein co-operative mechanism. FEBS Lett. 2001;506:173–179. doi: 10.1016/s0014-5793(01)02909-x. [DOI] [PubMed] [Google Scholar]
- Maldonado AM, Doerner P, Dixon RA, Lamb CJ, Cameron RK. A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature. 2002;419:399–403. doi: 10.1038/nature00962. [DOI] [PubMed] [Google Scholar]
- Maleck K, Neuenschwander U, Cade RM, Dietrich RA, Dangl JL, Ryals JA. Isolation and characterization of broad-spectrum disease-resistant Arabidopsis mutants. Genetics. 2002;160:1661–1671. doi: 10.1093/genetics/160.4.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S, Pena LA, Kolesnick RN. Signal transduction of stress via ceramide. Biochem J. 1998;335 (Pt 3):465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer BJ, Ren R, Clark KL, Baltimore D. A putative modular domain present in diverse signaling proteins. Cell. 1993;73:629–630. doi: 10.1016/0092-8674(93)90244-k. [DOI] [PubMed] [Google Scholar]
- Nandi A, Krothapalli K, Buseman CM, Li M, Welti R, Enyedi A, Shah J. Arabidopsis sfd mutants affect plastidic lipid composition and suppress dwarfing, cell death, and the enhanced disease resistance phenotypes resulting from the deficiency of a fatty acid desaturase. Plant Cell. 2003;15:2383–2398. doi: 10.1105/tpc.015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi A, Welti R, Shah J. The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene suppressor of fatty acid desaturase deficiency1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell. 2004;16:465–477. doi: 10.1105/tpc.016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Metraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Heck S, Parinthawong N, Metraux JP. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell. 2002;14:275–286. doi: 10.1105/tpc.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science. 2003;301:969–972. doi: 10.1126/science.1086716. [DOI] [PubMed] [Google Scholar]
- Parker JE, Holub EB, Frost LN, Falk A, Gunn ND, Daniels MJ. Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell. 1996;8:2033–2046. doi: 10.1105/tpc.8.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IA, Thomma BP, Buchala A, Metraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip F, Guo Y, Scarlata S. Multiple roles of pleckstrin homology domains in phospholipase C-beta function. FEBS Lett. 2002;531:28–32. doi: 10.1016/s0014-5793(02)03411-7. [DOI] [PubMed] [Google Scholar]
- Ponting CP, Aravind L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci. 1999;24:130–132. doi: 10.1016/s0968-0004(99)01362-6. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Annu Rev Biophys Biomol Struct. 1998;27:503–528. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM. Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell. 1997;9:305–316. doi: 10.1105/tpc.9.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals J, Weymann K, Lawton K, et al. The Arabidopsis NIM1 protein shows homology to the mammalian transcription factor inhibitor I kappa B. Plant Cell. 1997;9:425–439. doi: 10.1105/tpc.9.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrick K, Nguyen D, Karlowski WM, Mayer KF. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 2004;5:R41. doi: 10.1186/gb-2004-5-6-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lefert P, Vogel J. Closing the ranks to attack by powdery mildew. Trends Plant Sci. 2000;5:343–348. doi: 10.1016/s1360-1385(00)01683-6. [DOI] [PubMed] [Google Scholar]
- Shah J, Kachroo P, Nandi A, Klessig DF. A recessive mutation in the Arabidopsis SSI2 gene confers SA- and NPR1-independent expression of PR genes and resistance against bacterial and oomycete pathogens. Plant J. 2001;25:563–574. doi: 10.1046/j.1365-313x.2001.00992.x. [DOI] [PubMed] [Google Scholar]
- Soccio RE, Breslow JL. StAR-related lipid transfer (START) proteins: mediators of intracellular lipid metabolism. J Biol Chem. 2003;278:22183–22186. doi: 10.1074/jbc.R300003200. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Yuen GY, Lehman CC. Jasmonate signaling mutants of Arabidopsis are susceptible to the soil fungus Pythium irregulare. Plant J. 1998;15:747–754. doi: 10.1046/j.1365-313x.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- Stocco DM. StAR protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Tang D, Innes RW. Overexpression of a kinase-deficient form of the EDR1 gene enhances powdery mildew resistance and ethylene-induced senescence in Arabidopsis. Plant J. 2002;32:975–983. doi: 10.1046/j.1365-313x.2002.01482.x. [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF. Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol. 1999;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J, Somerville S. Isolation and characterization of powdery mildew-resistant Arabidopsis mutants. Proc Natl Acad Sci USA. 2000;97:1897–1902. doi: 10.1073/pnas.030531997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Schiff C, Somerville SC. PMR6, a pectate lyase-like gene required for powdery mildew susceptibility in Arabidopsis. Plant Cell. 2002;14:2095–2106. doi: 10.1105/tpc.003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Raab TK, Somerville CR, Somerville SC. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004;40:968–978. doi: 10.1111/j.1365-313X.2004.02264.x. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Xiang C, Han P, Lutziger I, Wang K, Oliver DJ. A mini binary vector series for plant transformation. Plant Mol Biol. 1999;40:711–717. doi: 10.1023/a:1006201910593. [DOI] [PubMed] [Google Scholar]
- Xiao S, Ellwood S, Calis O, Patrick E, Li T, Coleman M, Turner JG. Broad-spectrum mildew resistance in Arabidopsis thaliana mediated by RPW8. Science. 2001;291:118–120. doi: 10.1126/science.291.5501.118. [DOI] [PubMed] [Google Scholar]
- Xiao S, Calis O, Patrick E, Zhang G, Charoenwattana P, Muskett P, Parker JE, Turner JG. The atypical resistance gene, RPW8, recruits components of basal defence for powdery mildew resistance in Arabidopsis. Plant J. 2005;42:95–110. doi: 10.1111/j.1365-313X.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- Yao N, Eisfelder BJ, Marvin J, Greenberg JT. The mitochondrion – an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J. 2004;40:596–610. doi: 10.1111/j.1365-313X.2004.02239.x. [DOI] [PubMed] [Google Scholar]
- Yu JW, Mendrola JM, Audhya A, Singh S, Keleti D, DeWald DB, Murray D, Emr SD, Lemmon MA. Genome-wide analysis of membrane targeting by S. cerevisiae pleckstrin homology domains. Mol Cell. 2004;13:677–688. doi: 10.1016/s1097-2765(04)00083-8. [DOI] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J. PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell. 1998;10:1021–1030. doi: 10.1105/tpc.10.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]


