Abstract
Understanding of the natural history and basic biology of hepatitis B virus (HBV) has increased greatly in recent years. In view of this, the following are reviewed here: (a) recent advances in HBV biology pertinent to the rheumatic disease population; (b) the risks of HBV reactivation in patients with rheumatic disease undergoing immunosuppression; and (c) potential strategies to manage these risks.
Keywords: hepatitis B virus , biologic therapy, immunosuppression
Hepatitis B virus (HBV) infection is by far the most common chronic viral infection affecting the liver in the world, with over 400 million subjects infected, and it is the leading cause of cirrhosis and hepatocellular carcinoma.1 Reactivation of HBV replication in patients undergoing immunosuppressive therapy is a well recognised and frequently reported complication of considerable clinical importance.2,3 Not surprisingly, most of these reports have come from the fields of oncology and transplantation, but there have been a growing number of cases reported in patients with rheumatic disease undergoing immunosuppressive therapy as well.4 Recent reports of HBV reactivation leading to serious complications have been described in patients with rheumatic disease undergoing treatment with biological agents, including tumour necrosis factor (TNF) inhibitors5,6,7 and anti‐B cell therapy.8,9,10 These reports further emphasise the growing scope and complexity of this problem.
Over the past decade remarkable strides have been made in furthering our understanding of the natural history and basic biology of HBV and strategies have been proposed to assess and manage risks of reactivation in oncology and transplant recipients.11 In view of these events it would appear timely to review the following: (a) recent advances in HBV biology pertinent to the rheumatic disease population; (b) the risks of HBV reactivation in patients with rheumatic disease undergoing immunosuppression; and (c) potential strategies to manage these risks.
Hepatitis B virus infection: natural history and pathogenesis
Natural history
HBV, an enveloped double stranded DNA virus that belongs to the Hepadnaviridae family of viruses, infects predominantly hepatocytes, although detection of viral DNA in other sites such as mononuclear cells, pancreas, and kidneys has been documented.12 The HBV virion is a 42 nm circulating double shelled particle that is composed of an outer glycoprotein envelope containing the hepatitis B surface proteins (HBsAg), and an inner nucleocapsid or core (HBcAg) that encloses the viral DNA (approximately 3.2 kb in size) and the HBV DNA polymerase.
After entry to the circulation, HBV replicates in hepatocytes and encodes for a number of viral proteins, including the surface, core, polymerase, and X protein. HBeAg is a soluble circulating protein that is derived from the core gene after intracellular modification and is presumed to have a significant role in the establishment of persistent infection. So far, seven genotypes of HBV, designated A to G, have been identified.
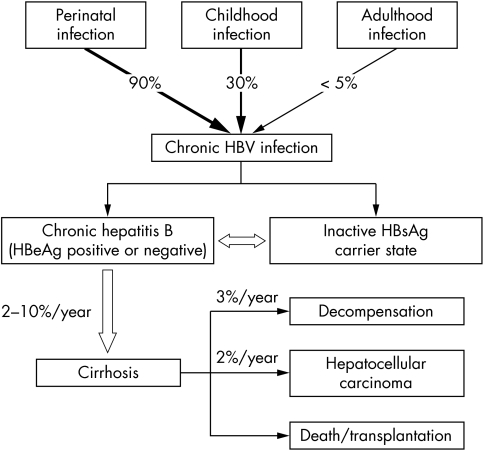
The natural course of HBV infection is influenced by a number of factors, among which the age of the initial encounter with the virus appears to be the most important (fig 1).12,13 The vast majority of chronic infection is due to vertical or perinatal transmission from HBV infected mothers. Person to person transmission during the first 5 years of life (mainly through intrafamilial spread) is another significant mode of transmission leading to chronic infection. Adulthood infection mainly occurs through injection drug use and high risk sexual behaviour and is rarely associated with chronicity (<5%). In one third of cases, acute icteric hepatitis develops that rarely can follow a fulminant course (0.5–1%).12,13
Figure 1 Natural course of HBV infection. Modified from reference 13.
“The natural course of HBV infection is influenced by the age of the person infected”
Acute hepatitis B is diagnosed by the presence of increased aminotransferases (alanine aminotransferase (ALT) and aspartate aminotransferase), HBsAg, and high titres of IgM antibodies against the core antigen (IgM anti‐HBc).12,13 Early in the course of acute infection, HBeAg is also detectable. After the resolution of acute HBV infection (within 4–6 months), HBsAg and HBeAg disappear from the circulation and antibodies against HBsAg (anti‐HBs, indicative of protective immunity) and HBeAg (anti‐HBe) are detected in the serum of infected subjects (resolved HBV infection).12,13
Chronic HBV infection is defined by the persistence of the virus in the circulation for more than 6 months (HBsAg positive).13 A number of epidemiological and clinical observation studies over the past 20 years have indicated that chronic HBV infection follows a dynamic course, running through successive phases.12,13,14
The first or immunotolerant phase is characterised by the presence of HBeAg, high serum HBV DNA levels, and normal or near normal aminotransferase levels. For infections acquired perinatally this period lasts for decades until adulthood, whereas for infections acquired in early childhood this period ends earlier during adolescence.
During the second or immune clearance (immunoactive) phase, immunity against the replicating virus develops, leading to liver inflammation, an increase of aminotransferases and decrease of HBV DNA levels. This period usually leads to HBeAg clearance and development of anti‐HBe antibodies (HBeAg seroconversion). Factors that have been associated with a high rate of HBeAg clearance include older age, female sex, increased ALT levels, and possibly, HBV genotype B. The rate of HBeAg loss has been estimated as about 50% at 5 years and 70% at 10 years, respectively. Patients who are unable to clear HBeAg and achieve HBeAg seroconversion despite active immune response develop chronic liver necroinflammation—that is, HBeAg positive chronic hepatitis B (see table 1).
Table 1 Definitions and terms used in hepatitis B virus infection.
| Definition | Diagnosis | |
|---|---|---|
| Acute hepatitis B | Acute hepatic injury that develops within 6 months after exposure to the virus and resolves within 6 months after onset of symptoms | HBsAg (+) ↑↑↑ ALT/AST ↑↑↑ IgM anti‐HBc |
| Chronic hepatitis B | Chronic necroinflammatory disease of the liver caused by HBV | HBsAg (+) for >6 months Serum HBV DNA (+) (>105 copies/ml) ↑ ALT/AST (persistently or intermittently) Chronic hepatitis in liver biopsy (necroinflammatory score ⩾ 4)* HBeAg ±† |
| Inactive HBsAg carrier state | Chronic HBV infection characterised by: | HBsAg (+) for > 6 months HBeAg (−)/Anti‐HBe (+) Persistently normal ALT/AST (serial testing for 1 year) Serum HBV DNA levels (<105 copies/ml) (serial testing for 1 year) Absent or minimal liver necroinflammation in liver biopsy (necroinflammatory score < 4)* |
| Resolved HBV infection | HBsAg (−) Serum HBV DNA (−) Normal ALT levels Known history of acute/chronic hepatitis B or anti‐HBc (+)/anti‐HBs± | |
| Occult HBV infection | HBsAg (−) HBV DNA (+) in serum/liver Anti‐HBc ±, Anti‐HBs± |
Adapted from Lok and McMahon and de Franchis et al.12,13
*Optional; †HBeAg (+) = HBeAg positive chronic hepatitis B or HBeAg (−) = HBeAg negative chronic hepatitis B.
HBsAg, hepatitis B surface antigen; ALT, alanine aminotransferase; AST, aspartate aminotransferase; anti‐HBc, antibody to hepatitis B core antigen; HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; anti‐HBe, antibody to hepatitis B e antigen.
HBeAg seroconversion highlights the transition to the third or low/non‐replicative phase of chronic HBV infection. This phase is characterised by normal or near normal ALT levels, absent or barely detectable serum HBV DNA levels (<105 copies/ml), and the absence of significant liver necroinflammation and fibrosis. This phase is also termed inactive HBsAg carrier state.12,13,14 During this period, a proportion of patients can spontaneously clear the virus at a rate ranging from 0.05% to 2% a year. This occurs more often in patients who acquire infection later in life, women, and older carriers. The incidence of cirrhosis and hepatocellular carcinoma (HCC) is particularly low in this group of patients, who represent the majority of patients with chronic HBV infection in the daily clinical practice world wide.
In 20–30% of chronic HBsAg carriers, immediately after or, more commonly, years after HBeAg seroconversion, viral replication recurs, leading to host immune response and chronic hepatitis. This form of hepatitis is called HBeAg negative chronic hepatitis B.14 Analysis of the replicating HBV strains has shown that most of them possess certain mutations that abolish or significantly reduce the rate of HBeAg synthesis and secretion. A number of animal and human studies have shown that these strains emerge and predominate under the host immune pressure during the HBeAg seroconversion period.14
Chronic hepatitis B (either HBeAg positive or negative) is defined by raised ALT and HBV DNA (>105 copies/ml) levels and liver necroinflammation (documented by liver biopsy, see table 1).13 Regardless of the HBeAg status, it is associated with a number of complications, including cirrhosis, hepatic decompensation (ascites, variceal bleeding, jaundice), HCC, and death (see fig 1).13 The rate of progression to cirrhosis varies between 2% and 10% a year, while the annual rate of hepatic decompensation is about 3% (fig 1). Development of HCC occurs at an annual rate of 0.2–0.6% in non‐cirrhotic patients, but this rate increases to 2% a year in cirrhotic patients. The mortality of chronic hepatitis B reaches 20% in cirrhotic patients and 70–85% in patients with hepatic decompensation at 5 years, respectively.
In recent years, patients with occult HBV infection who are persistently HBsAg negative, but with evidence of HBV DNA in the serum and/or liver tissue, have been increasingly reported.15,16 These patients belong to two major groups: the first group includes patients with a prior history of HBV infection (recovered) identified by the concomitant presence of anti‐HBs and the second group, patients with chronic HBV infection. Chronically infected patients either possess HBsAg escape mutants that are not recognised by the commercially available HBsAg assays or have very low levels of viraemia with undetectable HBsAg. Some of these patients with chronic HBV infection have anti‐HBc as the sole marker of HBV infection (anti‐HBc only) or can be completely negative for any serological marker of HBV infection (except HBV DNA).
Pathogenesis of HBV reactivation
HBV‐induced liver inflammation is predominantly immune mediated.17,18 The basic hypothesis is that HBV replication and expression of viral epitopes in infected hepatocytes is followed by a variable host immune response, leading to acute or chronic liver necroinflammation. The cells that are actively involved in this process are the cells of the adaptive immune response, predominantly CD8+ T lymphocytes, which recognise specific viral antigenic epitopes on the surface of infected hepatocytes.17,18 The role of CD4+ T cells is also crucial in this process through the provision of the required help for the production of specific antibodies against the HBV proteins (anti‐HBc, anti‐HBe, anti‐HBs) and the local/systemic production of cytokines with antiviral activity such as interferon (IFN) γ and TNFα. Non‐cytolytic mechanisms of hepatocyte viral clearance have been shown to operate in animal models of acute HBV infection through the localised action of cytokines.18,19 IFNγ is the major cytokine involved in this process, produced mainly by CD4 and CD8 T cells, macrophages, and NK T cells (a cell population particularly enriched in the liver microenvironment).
Longitudinal studies in patients with acute or chronic hepatitis B have shown that increases in serum HBV DNA are followed by a peak in ALT levels, indicating active immune response and hepatocyte lysis.2,14 This is particularly the case in patients with HBeAg negative chronic hepatitis B, where spontaneous intermittent rises in ALT levels preceded by increases in serum HBV DNA are common, leading to chronic necroinflammation and fibrosis.14
“Immunosuppressive drugs may precipitate flares of chronic HBV infection”
Among the factors that have been shown to precipitate acute flares of chronic HBV infection is the administration of immunosuppressive drugs. The bulk of available data comes from patients who have received immunosuppression for defined periods of time for haematological or oncological diseases and as long term prophylaxis after bone marrow or solid organ transplantation.2,3,20 In vitro and in vivo studies clearly indicated that immunosuppression leads to increased HBV replication, assessed by different methods (serum HBV DNA, HbsAg, and HBV DNA polymerase titres). This enhanced replication is attributed to two mechanisms. Firstly, in vitro studies have demonstrated a direct stimulatory effect of these agents on HBV replication.2 This is particularly the case for corticosteroids, because a corticosteroid responsive element is present in HBV DNA and is responsible for increased HBV DNA transcriptional activity and viraemia in patients receiving corticosteroids.21 In a recent carefully designed prospective study, increases in HBV DNA titres were noted in half of the patients within 2 weeks of the start of chemotherapy, before the development of neutropenia, indicating a potential direct stimulatory effect on HBV DNA transcription.22 Further studies are needed to confirm these preliminary findings. Secondly, an indirect immunosuppressive effect on the host immune response can be responsible for the enhanced HBV viraemia. Unopposed viral replication, followed by an exaggerated host immune response after withdrawal of immunosuppression, is the presumed pathogenetic mechanism of liver injury induced by immunosuppression.2
The rate of HBV reactivation in HBsAg positive patients receiving chemotherapy for haematological diseases or solid tumours ranges between 14% and 72%.3 These HBV exacerbations can occur either during the first cycles of chemotherapy (range 1–7 cycles) or after chemotherapy has been completed.3,20 As is the case with chronic hepatitis B, a rise in HBV DNA precedes increases in ALT by 7–10 days, strongly suggesting an immune mediated mechanism of liver injury.22,23,24 Similar observations have been made in patients receiving autologous haematopoietic stem cell transplantation, where immune reconstitution coincides with the exacerbation of hepatitis B (about 4 months after transplantation).25 In most cases (∼85%), viral reactivation is followed by development of clinical hepatitis.22,23 Clinically these exacerbations can be quite severe, leading to fulminant hepatitis and even death.
Despite these findings, limited information is available on the effect of the different immunosuppressive regimens given for longer periods of time in lower doses in patients with chronic HBV infection. Data from the long term administration of immunosuppressive drugs in HBsAg positive renal transplant recipients have shown a high incidence of hepatitis and liver related mortality (10–30%).20 There is a paucity of information for other diseases where long term administration of immunosuppression is required—for example, patients with inflammatory bowel diseases, severe asthma, etc.26 The data on patients with rheumatic diseases will be analysed below.
Reactivation of HBV replication or clinical hepatitis, or both, has been also demonstrated in patients with occult HBV infection after the administration of immunosuppressive drugs.8 Although the real magnitude of the problem has not been adequately estimated, a number of clinical observations indicate that is probably not large. In HBsAg negative patients with serological markers of HBV infection (anti‐HBc or anti‐HBs) receiving chemotherapy for haematological diseases fewer than 5% developed HBV reactivation,27 while in a recent study of HBsAg (−)/anti‐HBc (+) renal transplant recipients the respective incidence was <1%.28
HBV reactivation in patients with rheumatic disease
Table 2 summarises reports of patients with rheumatic disease and HBV reactivation associated with non‐biological immunosuppressive therapies.29,30,31,32,33,34
Table 2 Published reports of HBV reactivation in patients with rheumatic disease treated with non‐biological immunosuppressive drugs*.
| Patient No | Reference | Diagnosis | Age/sex | Immunosuppressive regimen | Time to flare | Treatment and outcome |
|---|---|---|---|---|---|---|
| 1 | 31 | RA | 72/F | MTX, 4 mg/week 2 years | 60 days† | IFN, GC, CsA |
| PSL, 5 mg/day | Died | |||||
| 2 | 32 | RA | 75/F | MTX, 7.5 mg/week | 15 days† | Plasmapheresis, IFN |
| PSL, 5 mg/day | Died | |||||
| 3 | 34 | RA | 67/M | MTX, 7.5 mg/week | 21 days† | GC |
| PSL, 5 mg/day | Died | |||||
| 4 | 30 | RA | 57/F | MTX, 7.5–10 mg/week | 41 days† | Liver transplant |
| PSL, 5 mg/day | Alive | |||||
| 5 | 4 | RA | 58/F | MTX, 15 mg/week | 2 years chronic treatment | LAM |
| PSL, 7.5 mg/day | Alive | |||||
| 6 | 33 | PM | 57/F | PSL, 40 mg/day | 40 days chronic treatment | IFN, CsA |
| Recovered | ||||||
| 7 | 29 | Behçet's disease | 43/M | Cyclo, IVMP | 2 years chronic treatment; 10 days† | GC |
| Died |
* Baseline serology in each case was HBsAg+ HBeAg−; †indicates time between discontinuation or reduction of immunosuppressive therapy and hepatitis B flare.
RA, rheumatoid arthritis; PM, polymyositis; F, female; M, male; MTX, methotrexate; PSL, prednisone or prednisolone; Cyclo, cyclophosphamide; IVMP, intravenous methyprednisolone; IFN, interferon; GC, glucocorticoids; CsA, ciclosporin A; LAM, lamivudine.
As can be seen, in five of the seven reports HBV reactivation was seen briefly (15–60 days) after decrease or interruption of the immunosuppressive regimen, while in two reports reactivation was noted during chronic therapy. All patients were HBsAg positive and HBeAg negative. The outcome was severe with four patients dying and one requiring liver transplantation. The other two patients were helped with antiviral therapy. Table 3 describes the published experience of HBV infected patients with rheumatic disease who have been treated with anti‐TNF based therapies.4,5,6,7,35
Table 3 Published experience of patients with rheumatic disease and underlying HBV infection treated with biological agents.
| Patient No | Reference | Diagnosis | Age/sex | Biological immunosuppressive therapy | Baseline serology | Treatment and outcome |
|---|---|---|---|---|---|---|
| 1 | 6 | AS | 32/M | Infliximab 5 mg/kg IV × 3 (0, 2, 6 weeks) | HBsAg+ HBeAg:NR | Pre‐emptive LAM before infliximab × 1 year, well tolerated |
| 2 | 7 | RA | 49/M | Infliximab 6mg/kg IV every 2 months, MTX 10 mg/week × 18 months, prednisone 8 mg/day | HBsAg+ HBeAg− Anti‐HBe+ | Flare of HBV, infliximab and MTX stopped, treated successfully with LAM |
| 3 | 5 | AOSD | 28/F | Infliximab 5 mg/kg IV × 2 (0, 2 weeks) | HBsAg+ HBeAg− Anti‐HBe+ | Acute hepatitis with severe liver decompensation without evidence of HBV reactivation in serum or liver, successful liver transplantation |
| 4 | 35 | SpA | 35/F | Infliximab 5 mg/kg IV ×3 (0, 2, 6 weeks) and then every 8 weeks for 4 months | HBsAg+ HBeAg− | HBV reactivation, successful treatment with LAM, restarted infliximab without incident |
| 5 | 4 | RA | 58/F | Infliximab 3 mg/kg IV every 8 weeks then etanercept 25 mg SC twice weekly | HBsAg+ HBeAg− Anti‐HBe+ | Previously flared while receiving MTX treatment; treated pre‐emptively with LAM; infliximab and etanercept well tolerated |
AS, ankylosing spondylitis; M, male; IV, intravenously; NR, not reported; LAM, lamivudine; RA, rheumatoid arthritis; MTX, methotrexate; AOSD, adult onset Still's disease; F, female; SpA, spondyloarthropathy; SC, subcutaneously.
These cases are particularly intriguing because HBV reactivation has also been recently reported in several patients treated with infliximab for Crohn's disease, including one patient who was considered an inactive HBsAg carrier before treatment.36,37 Table 3 includes three patients treated pre‐emptively with lamivudine, who had no reactivation when given infliximab despite a history of reactivation with conventional immunosuppressant drugs in one.4,6,35 Another patient was successfully treated by discontinuing the immunosuppressive drugs, including infliximab, then giving lamivudine treatment.7 The final patient5 required a liver transplant and this case is the most difficult to understand from a pathogenic perspective, for despite the progression to end stage liver disease and unlike all other cases, there was no evidence of HBV in serum or liver. It is interesting to note that at the time of writing, no cases of HBV reactivation with etanercept or adalimumab had been reported. Finally, HBV reactivation should be expected in rheumatology patients treated with ever more complex immunosuppressive regimens which include biological agents. Rituximab, for example, an agent in therapeutic trials in numerous rheumatic disorders, has been repeatedly associated with HBV reactivation in oncology treatment.8,9,10 Continued vigilance and enhanced measures to screen and prevent a flare appear prudent.
Considerations for assessment, prevention, and treatment of HBV reactivation in patients with rheumatic disease
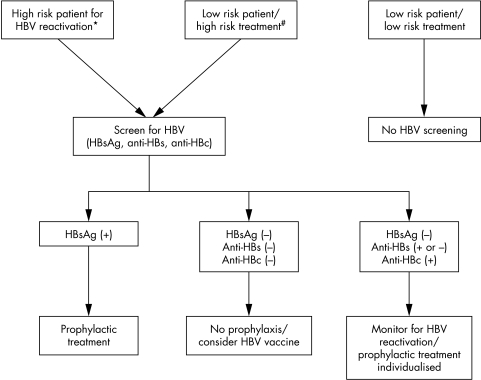
The field of rheumatology has no consensus guidelines for screening or treatment strategies for the prevention of HBV exacerbation in patients with rheumatological disorders undergoing immunosuppressive therapy. In the absence of such guidance we present our preferred approach based on our own clinical experience and the limited published reports (fig 2). Many of these recommendations are derived from experience gathered from patients with malignant disorders undergoing chemotherapy, or from solid organ transplant recipients.
Figure 2 Algorithm for assessment and prevention of HBV reactivation in patients with rheumatic disease. *Please refer to high risk groups mentioned in the text. #Includes methotrexate, leflunomide, high dose glucocorticoids, anti‐TNFα agents, other biological agents (rituximab).
Reactivation of HBV infection in patients undergoing immunosuppressive therapy is characterised clinically by an increase in serum HBV DNA and ALT level. In most instances the flare of HBV is asymptomatic, although icteric flares and hepatic decompensation leading to death or requiring liver transplantation have been well documented.24 Furthermore, small studies have shown that prophylactic treatment with antiviral agents can reduce the rate of reactivation and the mortality associated with flares.20,25 Given the potentially poor outcome of HBV reactivation and effectiveness of prophylaxis in patients receiving immunosuppressive drugs, it is important to identify those at risk and implement appropriate preventive strategies.
Who should be screened?
Three strategies for HBV screening deserve consideration, including (a) universal screening; (b)screening of high risk patients; and (c) screening of those being considered for high risk treatments. No studies have examined the cost effectiveness of universal screening for HBV infection in all patients in rheumatological practice in countries where the prevalence of HBV is low and universal vaccination has been successfully implemented (North America and most of Western Europe). However, universal screening for HBV is commonly practised in these countries, especially for those being assessed for organ transplantation. In the absence of data suggesting a more prevalent problem (that is, HBV reactivation), universal screening cannot be supported.
In contrast, screening for those at high risk for HBV infection will probably prove to be an effective strategy and one that is economically viable. At risk patients include people born in endemic areas, men who have sex with men, injection drug users, renal dialysis patients, HIV infected patients, and household or sexual contacts of HBV infected patients. Identification of those at‐risk subjects requires careful and detailed medical history before initiation of immunosuppressive treatment.
“Screening of high risk patients is a more effective strategy than universal screening”
Lastly, HBV screening should be considered for all patients going onto immunosuppressive regimens which have the potential to induce reactivation of HBV infection. At present the only formal recommendations in rheumatology are those to screen high risk patients starting treatment with methotrexate or leflunomide.38 Given the recent reports of serious even fatal cases of HBV reactivation with the drug infliximab,6,35,36,37 it would appear prudent to consider screening all patients who are to be treated with a member of the TNF inhibiting class.
Finally, although an absolute minimal level of immunosuppressive/immunomodulatory activity below which HBV reactivation is no longer a risk cannot be defined at present, it appears unlikely to occur in patients given low dose glucocorticoid treatment (<7.5 mg of prednisone or equivalent a day), with or without such agents as antimalarial drugs, sulfasalazine, and gold compounds, because to date there are no such reports. Alternatively, for patients who are to receive high dose glucocorticoid treatment, with or without a cytotoxic drug (that is, an alkylator or antimetabolite) or a calcineurin antagonist, screening for HBV should be strongly considered.
A decision about screening is more difficult for all patients (not just those at high risk) who are to be treated with methotrexate or leflunomide based regimens. The data in table 2 show that reports of severe, even fatal, reactivation of HBV have been documented when these drugs are used and, although rare, suggest that serious consideration should be given to screening all patients treated with these agents. Screening of patients merely at high risk will lead to missing one in four infected patients who lack identifiable risks.
What test should be used for screening?
HBsAg testing of all at‐risk subjects, should be carried out. Patients with detectable serum HBsAg should receive prophylaxis before immunosuppressive treatment, as discussed later in this manuscript. Whether a more detailed serological testing including anti‐HBc and anti‐HBe is needed for the screening of patients at risk remains unknown. HBV reactivation can occur in people who are HBsAg negative but anti‐HBs and anti‐HBc positive.11,24,27 Similarly, HBV reactivation is well documented in solid organ transplant recipients of an anti‐HBc positive donor, and prophylaxis with antiviral agents (short term) has been recommended for those patients.39 Given these data, we recommend screening of patients at risk for HBV reactivation with a serological profile that would include HBsAg, anti‐HBs and anti‐HBc.
Who should receive prophylactic therapy?
Treatment of all HBsAg positive patients should be started with prophylactic antiviral drugs before they receive immunosuppressive therapy including corticosteroids. For HBsAg negative, anti‐HBs, and/or anti‐HBc positive patients, prophylactic therapy may not be necessary routinely and the decision can be made individually based on the likelihood of reactivation (type of immunosuppressive therapy, length of treatment, etc.). If no antiviral prophylactic treatment is given in this group of patients, periodic follow up of ALT and HBV DNA is recommended to identify flares at an early asymptomatic stage, at which time treatment of HBV can be started before the onset of jaundice or liver failure.
Strategies for prophylaxis
IFNα has no role in the prophylaxis against HBV reactivation for those undergoing immunosuppressive therapeutic regimens. Similarly, the use of hepatitis B immunoglobulin (HBIG) prophylaxis should be limited to patients with an actively replicating HBV infection (HBsAg positive) undergoing liver transplantation and has no role in prophylaxis in patients undergoing immunosuppressive therapy, such as patients with cancer or patients with rheumatological disorders.
Although no randomised clinical trials have been completed, most reports to date have demonstrated the benefit of lamivudine given prophylactically (100 mg daily) to patients with cancer undergoing chemotherapy.23,40,41 It has been suggested that lamivudine should be given throughout the course of treatment and extended for 6 months after completion of the chemotherapy regimen because HBV flares may occur days or weeks after chemotherapy has stopped.27,42 Short term lamivudine is safe and, usually free of toxicity with a risk‐benefit ratio that favours prophylaxis. A randomised study showed that lamivudine given 1 week before administration of chemotherapy was better than deferred lamivudine treatment given at the time of viral reactivation.23
In a small number of patients with rheumatic disease who had reactivation of HBV during an immunosuppressive regimen, lamivudine was successfully employed to suppress HBV replication, allowing successful reinstitution of treatment.4,35 The benefit versus risk of prophylactic antiviral therapy to prevent HBV flares is less certain in those requiring an extended course of immunosuppressive therapy. A longer course of lamivudine prophylaxis may potentially be associated with emergence of lamivudine resistant HBV strains (through mutation in the YMDD motif of the HBV polymerase). This is of particular concern in rheumatic disorders where immunosuppressive regimens including corticosteroids may be given over an extended period of time or sometimes for life. The annual risk of developing the YMDD mutation increases with the duration of treatment in immunocompetent patients with chronic hepatitis B (HBeAg positive or negative). The rate of virological breakthrough due to YMDD mutant strains has been estimated as 15–30% at year 1, 40% at year 2, 50% at year 3, and 60% at year 4 of treatment.43,44 The clinical course of patients with lamivudine resistant HBV strains is variable and may rarely lead to hepatic decompensation.45 This is particularly the case for patients with advanced fibrosis or cirrhosis.43 However, most patients with the YMDD mutation are asymptomatic or may have mild exacerbation of hepatitis.
Limited information is available on the rate of lamivudine resistance in immunosuppressed subjects. Long term immunosuppression in kidney transplant recipients has been associated with a number of mutations in different areas of the HBV genome,46 but whether the rate of YMDD mutations is higher in these patients is unclear. A recent meta‐analysis of lamivudine treatment in renal transplant recipients with chronic HBV infection showed a rate of lamivudine resistance that ranged between 10% and 42%.47 In a study by Chan et al, where long term follow up was available (approximately 2 ½ years), the rate of YMDD resistance was 7% and 37% after 1 and 2 years of treatment, respectively.48 These rates are similar to the rates seen in immunocompetent HBV patients. Nevertheless, increased vigilance with frequent monitoring of HBV DNA and ALT levels is imperative in these patients, especially when advanced liver fibrosis is present.
Adefovir dipivoxil, an alternative antiviral drug, has not been studied in the setting of prophylaxis during immunosuppressive therapy. Adefovir dipivoxil has been shown to be effective in the treatment of patients with chronic hepatitis B49,50 and in those developing YMDD mutation during lamivudine treatment.51,52,53 The recommended dose of adefovir dipivoxil in adults is 10 mg daily given orally. So far, long term administration of adefovir dipivoxil has been associated with a much lower rate of drug resistance than lamivudine (∼15% after 4 years of treatment).54 Combination therapy with lamivudine and adefovir has been proposed for patients with advanced liver disease in order to avoid severe decompensation in cases where resistance to lamivudine monotherapy develops. Whether such an approach should be implemented in patients with HBV cirrhosis who will receive immunosuppressive therapy is unclear. Other antiviral agents are currently under development and may potentially provide alternative treatments to prevent HBV reactivation.44
Abbreviations
ALT - alanine aminotransferase
anti‐HBc - antibody to hepatitis B core antigen
anti‐HBs - antibody to hepatitis B surface antigen
HBcAg - hepatitis B core antigen
HBeAg - hepatitis B e antigen
HBsAg - hepatitis B surface antigen
HHBV - hepatitis B virus
HCC - hepatocellular carcinoma
IFN - interferon
TNF - tumour necrosis factor
References
- 1.Lai C L, Ratziu V, Yuen M F, Poynard T. Viral hepatitis B. Lancet 20033622089–2094. [DOI] [PubMed] [Google Scholar]
- 2.Perrillo R P. Acute flares in chronic hepatitis B: the natural and unnatural history of an immunologically mediated liver disease. Gastroenterology 20011201009–1022. [DOI] [PubMed] [Google Scholar]
- 3.Vento S, Cainelli F, Longhi M S. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol 20023333–340. [DOI] [PubMed] [Google Scholar]
- 4.Calabrese L H, Zein N, Vassilopoulos D. Safety of antitumour necrosis factor (anti‐TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis 200463(suppl 2)ii18–ii24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset still's disease. J Rheumatol 2003301624–1625. [PubMed] [Google Scholar]
- 6.Oniankitan O, Duvoux C, Challine D, Mallat A, Chevalier X, Pawlotsky J M.et al Infliximab therapy for rheumatic diseases in patients with chronic hepatitis B or C. J Rheumatol 200431107–109. [PubMed] [Google Scholar]
- 7.Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S. Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 200362686–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dervite I, Hober D, Morel P. Acute hepatitis B in a patient with antibodies to hepatitis B surface antigen who was receiving rituximab. N Engl J Med 200134468–69. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumi Y, Kawamura T, Saitoh S, Yamada M, Obara S, Miura T.et al Hepatitis B virus reactivation in a case of non‐Hodgkin's lymphoma treated with chemotherapy and rituximab: necessity of prophylaxis for hepatitis B virus reactivation in rituximab therapy. Leuk Lymphoma 200445627–629. [DOI] [PubMed] [Google Scholar]
- 10.Westhoff T H, Jochimsen F, Schmittel A, Stoffler‐Meilicke M, Schafer J H, Zidek W.et al Fatal hepatitis B virus reactivation by an escape mutant following rituximab therapy. Blood 20031021930. [DOI] [PubMed] [Google Scholar]
- 11.Keeffe E B, Dieterich D T, Han S H, Jacobson I M, Martin P, Schiff E R.et al A treatment algorithm for the management of chronic hepatitis B virus infection in the United States. Clin Gastroenterol Hepatol 2004287–106. [DOI] [PubMed] [Google Scholar]
- 12.Lok A S, McMahon B J. Chronic hepatitis B. Hepatology 2001341225–1241. [DOI] [PubMed] [Google Scholar]
- 13.de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N.et al EASL International Consensus Conference on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol 200339(suppl 1)S3–25. [PubMed] [Google Scholar]
- 14.Hadziyannis S J, Vassilopoulos D. Hepatitis B e antigen‐negative chronic hepatitis B. Hepatology 200134617–624. [DOI] [PubMed] [Google Scholar]
- 15.Allain J P. Occult hepatitis B virus infection: implications in transfusion. Vox Sang 20048683–91. [DOI] [PubMed] [Google Scholar]
- 16.Torbenson M, Thomas D L. Occult hepatitis B. Lancet Infect Dis 20022479–486. [DOI] [PubMed] [Google Scholar]
- 17.Chisari F V, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 19951329–60. [DOI] [PubMed] [Google Scholar]
- 18.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 20055215–229. [DOI] [PubMed] [Google Scholar]
- 19.Guidotti L G, Rochford R, Chung J, Shapiro M, Purcell R, Chisari F V. Viral clearance without destruction of infected cells during acute HBV infection. Science 1999284825–829. [DOI] [PubMed] [Google Scholar]
- 20.Rossi G. Prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with hemato‐oncological neoplasias treated with chemotherapy. Leuk Lymphoma 200344759–766. [DOI] [PubMed] [Google Scholar]
- 21.Tur‐Kaspa R, Burk R D, Shaul Y, Shafritz D A. Hepatitis B virus DNA contains a glucocorticoid‐responsive element. Proc Natl Acad Sci USA 1986831627–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng A L, Hsiung C A, Su I J, Chen P J, Chang M C, Tsao C J.et al Steroid‐free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV‐carriers with lymphoma. Hepatology 2003371320–1328. [DOI] [PubMed] [Google Scholar]
- 23.Lau G K, Yiu H H, Fong D Y, Cheng H C, Au W Y, Lai L S.et al Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 20031251742–1749. [DOI] [PubMed] [Google Scholar]
- 24.Yeo W, Chan P K, Chan H L, Mo F K, Johnson P J. Hepatitis B virus reactivation during cytotoxic chemotherapy‐enhanced viral replication precedes overt hepatitis. J Med Virol 200165473–477. [PubMed] [Google Scholar]
- 25.Lau G K, Leung Y H, Fong D Y, Au W Y, Kwong Y L, Lie A.et al High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood 2002992324–2330. [DOI] [PubMed] [Google Scholar]
- 26.Biancone L, Del Vecchio B G, Pallone F, Castiglione F, Bresci G, Sturniolo G. Immunomodulatory drugs in Crohn's disease patients with hepatitis B or C virus infection. Gastroenterology 2002122593–594. [DOI] [PubMed] [Google Scholar]
- 27.Lok A S, Liang R H, Chiu E K, Wong K L, Chan T K, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology 1991100182–188. [DOI] [PubMed] [Google Scholar]
- 28.Berger A, Preiser W, Kachel H G, Sturmer M, Doerr H W. HBV reactivation after kidney transplantation. J Clin Virol 200532162–165. [DOI] [PubMed] [Google Scholar]
- 29.Bird G L, Smith H, Portmann B, Alexander G J, Williams R. Acute liver decompensation on withdrawal of cytotoxic chemotherapy and immunosuppressive therapy in hepatitis B carriers. Q J Med 198973895–902. [PubMed] [Google Scholar]
- 30.Flowers M A, Heathcote J, Wanless I R, Sherman M, Reynolds W J, Cameron R G.et al Fulminant hepatitis as a consequence of reactivation of hepatitis B virus infection after discontinuation of low‐dose methotrexate therapy. Ann Intern Med 1990112381–382. [DOI] [PubMed] [Google Scholar]
- 31.Hagiyama H, Kubota T, Komano Y, Kurosaki M, Watanabe M, Miyasaka N. Fulminant hepatitis in an asymptomatic chronic carrier of hepatitis B virus mutant after withdrawal of low‐dose methotrexate therapy for rheumatoid arthritis. Clin Exp Rheumatol 200422375–376. [PubMed] [Google Scholar]
- 32.Ito S, Nakazono K, Murasawa A, Mita Y, Hata K, Saito N.et al Development of fulminant hepatitis B (precore variant mutant type) after the discontinuation of low‐dose methotrexate therapy in a rheumatoid arthritis patient. Arthritis Rheum 200144339–342. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi K, Ishikawa M, Nakauchi M, Sakurai A, Doi K, Taniguchi Y. Antibody to hepatitis B e positive hepatitis induced by withdrawal of steroid therapy for polymyositis: response to interferon‐alpha and cyclosporin A. Intern Med 199837519–522. [DOI] [PubMed] [Google Scholar]
- 34.Narvaez J, Rodriguez‐Moreno J, Martinez‐Aguila M D, Clavaguera M T. Severe hepatitis linked to B virus infection after withdrawal of low dose methotrexate therapy. J Rheumatol 1998252037–2038. [PubMed] [Google Scholar]
- 35.Wendling D, Auge B, Bettinger D, Lohse A, Le Huede G, Bresson‐Hadni S.et al Reactivation of a latent precore mutant hepatitis B virus related chronic hepatitis during infliximab treatment for severe spondyloarthropathy. Ann Rheum Dis 200564788–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Esteve M, Saro C, Gonzalez‐Huix F, Suarez F, Forne M, Viver J M. Chronic hepatitis B reactivation following infliximab therapy in Crohn's disease patients: need for primary prophylaxis. Gut 2004531363–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valle Garcia‐Sanchez M, Gomez‐Camacho F, Poyato‐Gonzalez A, Iglesias‐Flores E M, Dios‐Vega J F, Sancho‐Zapatero R. Infliximab therapy in a patient with Crohn's disease and chronic hepatitis B virus infection. Inflamm Bowel Dis 200410701–702. [DOI] [PubMed] [Google Scholar]
- 38.Anonymous Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum 200246328–346. [DOI] [PubMed] [Google Scholar]
- 39.Vargas H E, Dodson F S, Rakela J. A concise update on the status of liver transplantation for hepatitis B virus: the challenges in 2002. Liver Transpl 200282–9. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri F, Ermacora A, Sperotto A, Patriarca F, Zaja F, Damiani D.et al Lamivudine allows completion of chemotherapy in lymphoma patients with hepatitis B reactivation. Br J Haematol 2000108394–396. [DOI] [PubMed] [Google Scholar]
- 41.Al‐Taie O H, Mork H, Gassel A M, Wilhelm M, Weissbrich B, Scheurlen M. Prevention of hepatitis B flare‐up during chemotherapy using lamivudine: case report and review of the literature. Ann Hematol 199978247–249. [DOI] [PubMed] [Google Scholar]
- 42.Simpson N D, Simpson P W, Ahmed A M, Nguyen M H, Garcia G, Keeffe E B.et al Prophylaxis against chemotherapy‐induced reactivation of hepatitis B virus infection with Lamivudine. J Clin Gastroenterol 20033768–71. [DOI] [PubMed] [Google Scholar]
- 43.Di Marco V, Marzano A, Lampertico P, Andreone P, Santantonio T, Almasio P L.et al Clinical outcome of HBeAg‐negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 200440883–891. [DOI] [PubMed] [Google Scholar]
- 44.Papatheodoridis G V, Hadziyannis S J. Review article: current management of chronic hepatitis B. Aliment Pharmacol Ther 20041925–37. [DOI] [PubMed] [Google Scholar]
- 45.Dienstag J L, Goldin R D, Heathcote E J, Hann H W, Woessner M, Stephenson S L.et al Histological outcome during long‐term lamivudine therapy. Gastroenterology 2003124105–117. [DOI] [PubMed] [Google Scholar]
- 46.Preikschat P, Gunther S, Reinhold S, Will H, Budde K, Neumayer H H.et al Complex HBV populations with mutations in core promoter, C gene, and pre‐S region are associated with development of cirrhosis in long‐term renal transplant recipients. Hepatology 200235466–477. [DOI] [PubMed] [Google Scholar]
- 47.Fabrizi F, Dulai G, Dixit V, Bunnapradist S, Martin P. Lamivudine for the treatment of hepatitis B virus‐related liver disease after renal transplantation: meta‐analysis of clinical trials. Transplantation 200477859–864. [DOI] [PubMed] [Google Scholar]
- 48.Chan T M, Fang G X, Tang C S, Cheng I K, Lai K N, Ho S K. Preemptive lamivudine therapy based on HBV DNA level in HBsAg‐positive kidney allograft recipients. Hepatology 2002361246–1252. [DOI] [PubMed] [Google Scholar]
- 49.Marcellin P, Chang T T, Lim S G, Tong M J, Sievert W, Shiffman M L.et al Adefovir dipivoxil for the treatment of hepatitis B e antigen‐positive chronic hepatitis B. N Engl J Med 2003348808–816. [DOI] [PubMed] [Google Scholar]
- 50.Hadziyannis S J, Tassopoulos N C, Heathcote E J, Chang T T, Kitis G, Rizzetto M.et al Adefovir dipivoxil for the treatment of hepatitis B e antigen‐negative chronic hepatitis B. N Engl J Med 2003348800–807. [DOI] [PubMed] [Google Scholar]
- 51.Perrillo R, Hann H W, Mutimer D, Willems B, Leung N, Lee W M.et al Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology 200412681–90. [DOI] [PubMed] [Google Scholar]
- 52.Peters M G, Hann H H, Martin P, Heathcote E J, Buggisch P, Rubin R.et al Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine‐resistant chronic hepatitis B. Gastroenterology 200412691–101. [DOI] [PubMed] [Google Scholar]
- 53.Schiff E R, Lai C L, Hadziyannis S, Neuhaus P, Terrault N, Colombo M.et al Adefovir dipivoxil therapy for lamivudine‐resistant hepatitis B in pre‐ and post‐liver transplantation patients. Hepatology 2003381419–1427. [DOI] [PubMed] [Google Scholar]
- 54.Locarnini S, Qi X, Arterbrun S, Snow A, Brosgart C L, Currie G.et al Incidence and predictors of emergence of adefovir resistant HBV during four years of adefovir dipivoxil (ADV) therapy for patients with chronic hepatitis B (CHB) [abstract]. J Hepatol 200542(suppl 2)17 [Google Scholar]