Abstract
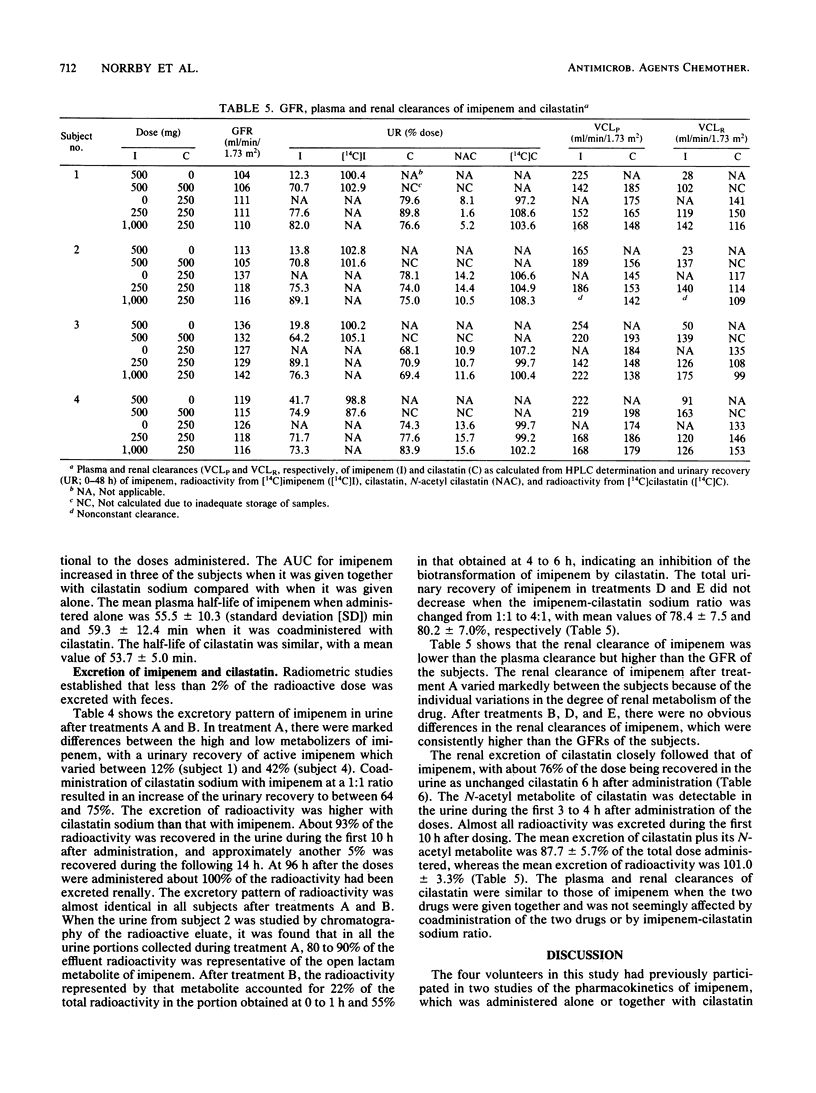
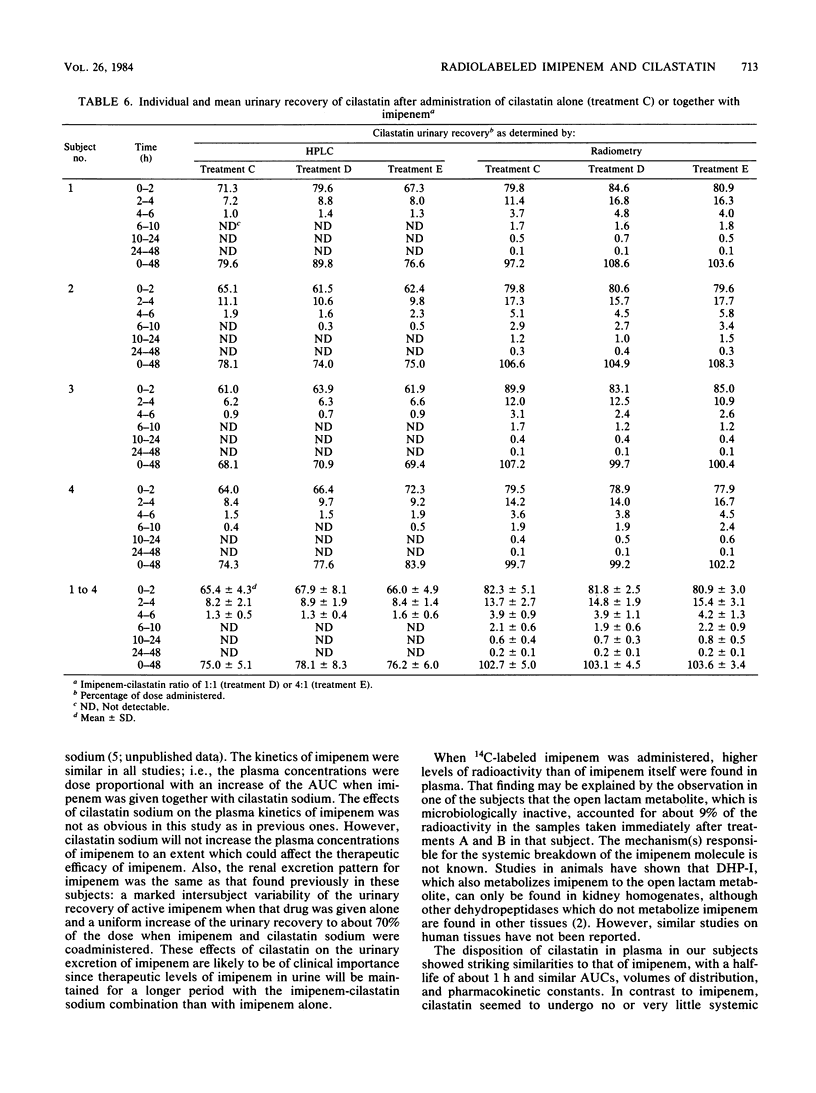
In the first of two successive studies, four healthy male subjects received 500 mg of 14C-labeled imipenem alone and together with 500 mg of unlabeled cilastatin sodium. In the second study, the same subjects were given 250 mg of 14C-labeled cilastatin sodium alone and together with 250 and 1,000 mg of cold imipenem. Concentrations of imipenem and cilastatin in plasma, urine, and feces were assayed by high-pressure liquid chromatography and radiometry. Plasma concentrations of imipenem assayed radiometrically were higher than those measured by high-pressure liquid chromatography. In one subject studied at the end of drug administration, the open lactam metabolite of imipenem represented 9% of the radioactivity. Plasma levels of cilastatin determined by high-pressure liquid chromatography and radiometry were virtually identical. Urinary recovery of imipenem varied between 12 and 42% of the dose when that drug was given alone but increased to between 64 and 75% when administered with cilastatin sodium at a 1:1 ratio. Almost all radioactivity of imipenem was recovered in the urine within 96 h after drug administration. The open lactam metabolite, resulting from the metabolism of imipenem in the kidneys by a dipeptidase, dehydropeptidase-I, represented 80 to 90% of the effluent radioactivity when imipenem was given alone and about 20% when cilastatin sodium was coadministered. Renal excretion of cilastatin followed closely that of imipenem. Almost all of the administered radioactivity was recovered in 24 h, and about 75% of the dose was recovered as unchanged cilastatin within 6 h. The N-acetyl metabolite of cilastatin was found to represent about 12% of the total radioactivity.
Full text
PDF
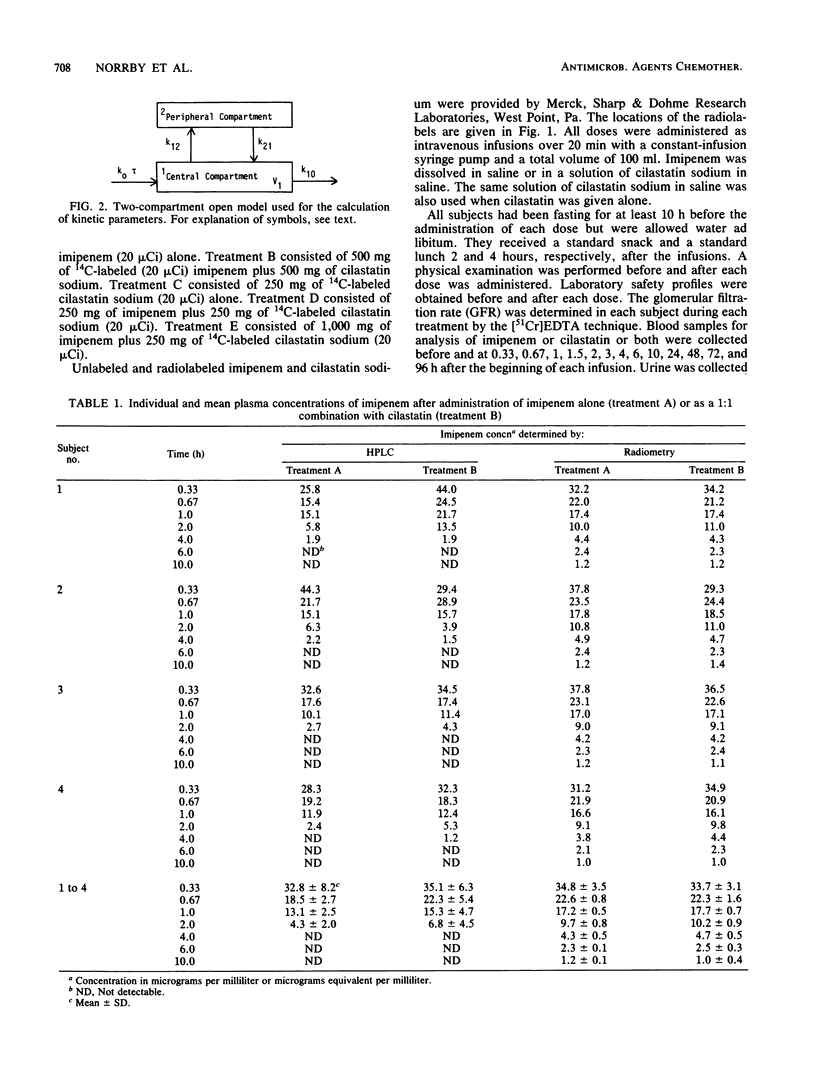
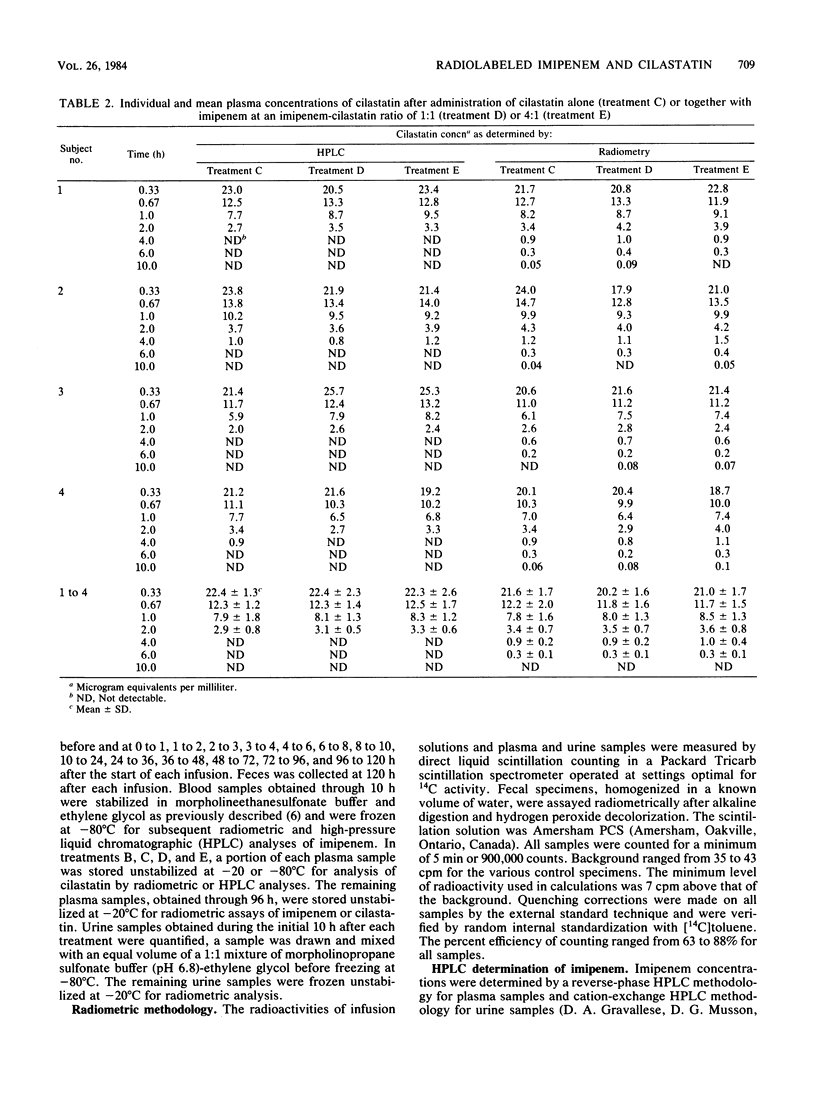
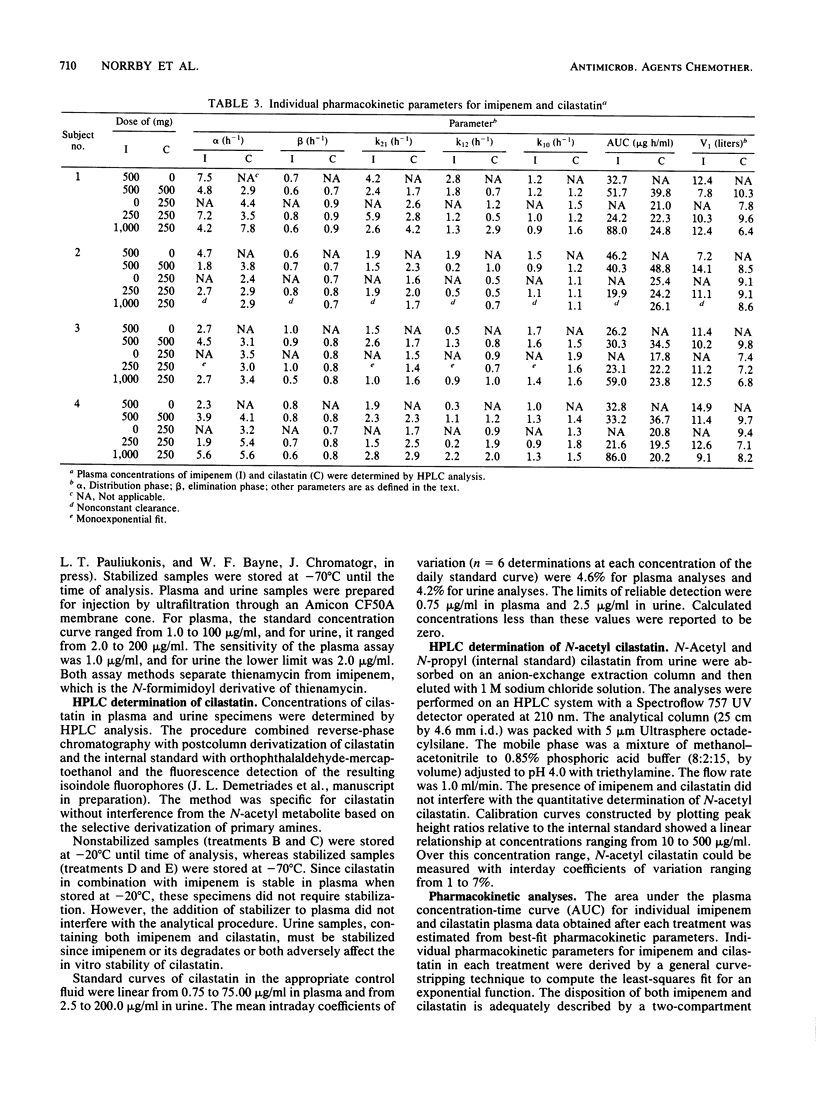
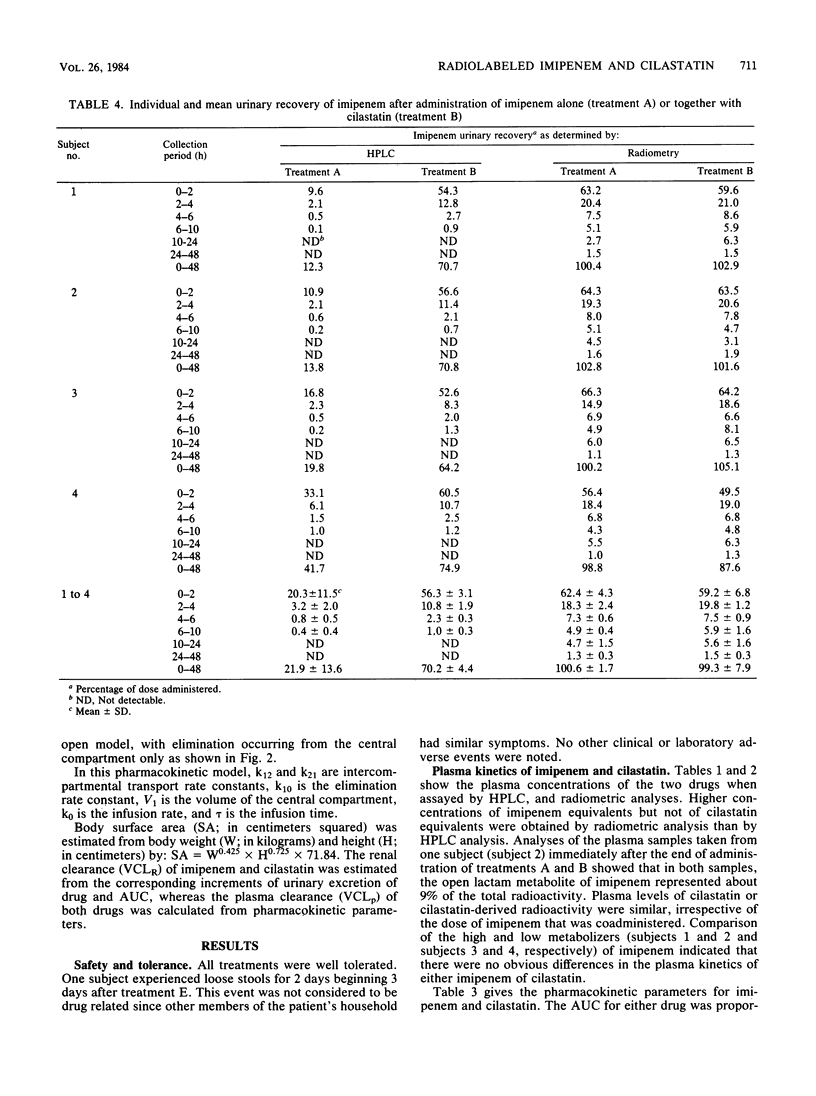

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calandra G. B., Ricci F. M., Wang C., Brown K. R. Safety and tolerance comparison of imipenem-cilastatin to cephalothin and cefazolin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):125–131. doi: 10.1093/jac/12.suppl_d.125. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kropp H., Sundelof J. G., Birnbaum J. Thienamycin: development of imipenen-cilastatin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):1–35. doi: 10.1093/jac/12.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Hajdu R., Kahan F. M. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob Agents Chemother. 1982 Jul;22(1):62–70. doi: 10.1128/aac.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp H., Sundelof J. G., Kahan J. S., Kahan F. M., Birnbaum J. MK0787 (N-formimidoyl thienamycin): evaluation of in vitro and in vivo activities. Antimicrob Agents Chemother. 1980 Jun;17(6):993–1000. doi: 10.1128/aac.17.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Björnegård B., Burman L. A., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Kahan J. S., Kropp H. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983 Feb;23(2):300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Meisinger M. A., Rogers J. D. Pharmacokinetics and tolerance of N-formimidoyl thienamycin (MK0787) in humans. Antimicrob Agents Chemother. 1983 Feb;23(2):293–299. doi: 10.1128/aac.23.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L., Verhaegen J. In vitro activity of N-formimidoyl thienamycin in comparison with cefotaxime, moxalactam, and ceftazidime. Antimicrob Agents Chemother. 1981 Mar;19(3):402–406. doi: 10.1128/aac.19.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]


