Abstract
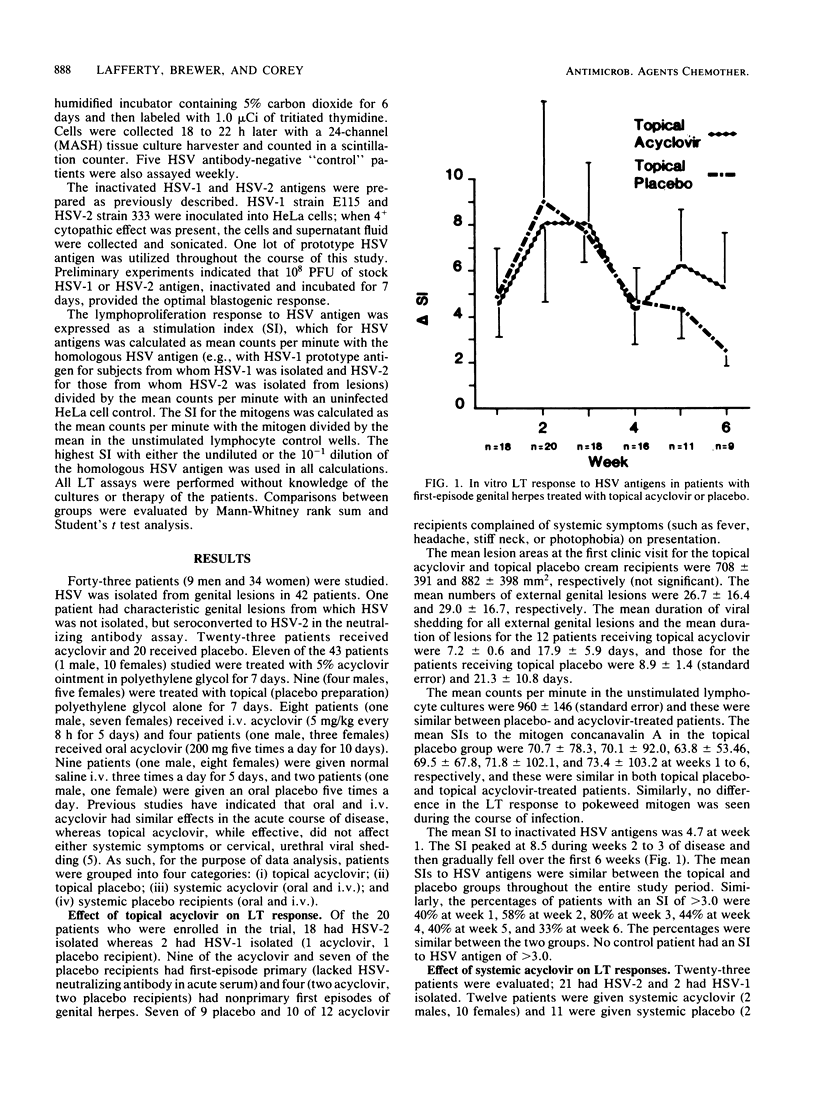
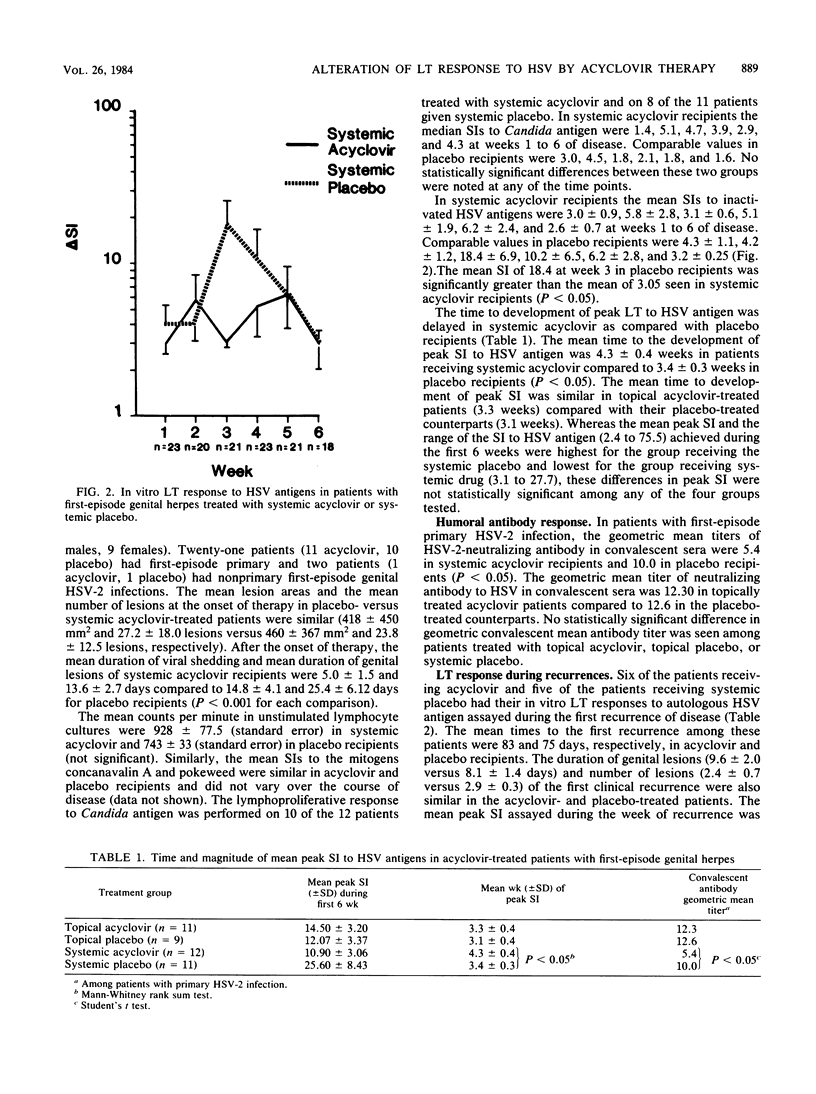
To evaluate the effect of acyclovir (ACV) therapy on the cellular immune response, we sequentially followed 43 patients with culture-proven first episodes of genital herpes simplex virus (HSV) infection. Twenty-three patients who were treated with ACV and 20 who received placebo had blood obtained weekly during the first 6 weeks after onset of lesions and had their in vitro lymphocyte transformation (LT) response to inactivated HSV antigens measured. The mean stimulation index to HSV antigens at week 3 among patients treated with systemic ACV was 3.5 +/- 0.64 compared to 18.4 +/- 6.89 in their placebo-treated counterparts (P less than 0.05). The mean time to the development of the peak LT response to HSV antigens was 4.3 weeks in systemic-treated versus 3.4 in placebo-treated patients (P less than 0.05). The time to the development of the peak in vitro LT response to HSV antigens and the height of that response were, however, similar between topical ACV- and topical placebo-treated patients. The geometric mean HSV-2-neutralizing titer in convalescent sera was 5.4 in recipients of systemic ACV compared to 10.0 in patients treated with systemic placebo (P less than 0.05). The LT response to HSV antigen was also measured at the first recurrence in 11 patients. No differences were found in the time to first recurrence, lesion duration, number of lesions, or mean stimulation index response to inactivated HSV antigens between the six patients treated with systemic ACV during their primary episode and the five given placebo during their primary episode. Systemic ACV therapy appears to diminish the peak in vitro LT response to inactivated HSV antigens as well as to delay the time to development of that peak response. However, the cell-mediated immune response to subsequent episodes appears similar.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. L., Corey L. Effect of acyclovir treatment of primary genital herpes on the antibody response to herpes simplex virus. J Clin Invest. 1984 Mar;73(3):681–688. doi: 10.1172/JCI111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston D. L., Cohen A., Spindler M. A. Herpesvirus hominis infection in patients with myeloproliferative and lymphoproliferative disorders. Br Med J. 1972 Nov 25;4(5838):462–465. doi: 10.1136/bmj.4.5838.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Dillon M., Lovett M., Acuna G., Taylor S., Cherry J. D., Johnson B. L., Wiesmeier E., Growdon W., Creagh-Kirk T. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- Corey L., Benedetti J. K., Critchlow C. W., Remington M. R., Winter C. A., Fahnlander A. L., Smith K., Salter D. L., Keeney R. E., Davis L. G. Double-blind controlled trial of topical acyclovir in genital herpes simplex virus infections. Am J Med. 1982 Jul 20;73(1A):326–334. doi: 10.1016/0002-9343(82)90117-6. [DOI] [PubMed] [Google Scholar]
- Corey L., Benedetti J., Critchlow C., Mertz G., Douglas J., Fife K., Fahnlander A., Remington M. L., Winter C., Dragavon J. Treatment of primary first-episode genital herpes simplex virus infections with acyclovir: results of topical, intravenous and oral therapy. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):79–88. doi: 10.1093/jac/12.suppl_b.79. [DOI] [PubMed] [Google Scholar]
- Corey L., Fife K. H., Benedetti J. K., Winter C. A., Fahnlander A., Connor J. D., Hintz M. A., Holmes K. K. Intravenous acyclovir for the treatment of primary genital herpes. Ann Intern Med. 1983 Jun;98(6):914–921. doi: 10.7326/0003-4819-98-6-914. [DOI] [PubMed] [Google Scholar]
- Corey L., Nahmias A. J., Guinan M. E., Benedetti J. K., Critchlow C. W., Holmes K. K. A trial of topical acyclovir in genital herpes simplex virus infections. N Engl J Med. 1982 Jun 3;306(22):1313–1319. doi: 10.1056/NEJM198206033062201. [DOI] [PubMed] [Google Scholar]
- Corey L., Reeves W. C., Holmes K. K. Cellular immune response in genital herpes simplex virus infection. N Engl J Med. 1978 Nov 2;299(18):986–991. doi: 10.1056/NEJM197811022991805. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Schwenteck M., Northoff H., Schöpf E. Defective in vitro lymphoproliferative responses to herpes simplex virus in patients with frequently recurring herpes infections during the disease-free interval. Clin Immunol Immunopathol. 1978 Nov;11(3):267–274. doi: 10.1016/0090-1229(78)90050-8. [DOI] [PubMed] [Google Scholar]
- Korsager B., Spencer E. S., Mordhorst C. H., Andersen H. K. Herpesvirus hominis infections in renal transplant recipients. Scand J Infect Dis. 1975;7(1):11–19. doi: 10.3109/inf.1975.7.issue-1.03. [DOI] [PubMed] [Google Scholar]
- Larsen H. S., Russell R. G., Rouse B. T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983 Jul;41(1):197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz G. J., Critchlow C. W., Benedetti J., Reichman R. C., Dolin R., Connor J., Redfield D. C., Savoia M. C., Richman D. D., Tyrrell D. L. Double-blind placebo-controlled trial of oral acyclovir in first-episode genital herpes simplex virus infection. JAMA. 1984 Sep 7;252(9):1147–1151. [PubMed] [Google Scholar]
- Montgomerie J. Z., Becroft D. M., Croxson M. C., Doak P. B., North J. D. Herpes-simplex-virus infection after renal transplantation. Lancet. 1969 Oct 25;2(7626):867–871. doi: 10.1016/s0140-6736(69)92327-7. [DOI] [PubMed] [Google Scholar]
- Muller S. A., Herrmann E. C., Jr, Winkelmann R. K. Herpes simplex infections in hematologic malignancies. Am J Med. 1972 Jan;52(1):102–114. doi: 10.1016/0002-9343(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Hirsch M. S., Kramer J. H., Murphy F. A. Effect of antithymocyte serum on herpesvirus hominis (type 1) infection in adult mice. Proc Soc Exp Biol Med. 1969 Nov;132(2):696–698. doi: 10.3181/00379727-132-34290. [DOI] [PubMed] [Google Scholar]
- Paty D. W., Cousin H. K., Stiller C. R., Boucher D. W., Furesz J., Warren K. G., Marchuk L., Dossetor J. B. HLA-D typing with an association of Dw2 and absent immune responses towards herpes simplex (type i) antigen in multiple sclerosis. Transplant Proc. 1977 Dec;9(4):1845–1848. [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Russell A. S. Cell-mediated immunity to herpes simplex virus in man. J Infect Dis. 1974 Feb;129(2):142–146. doi: 10.1093/infdis/129.2.142. [DOI] [PubMed] [Google Scholar]
- Sheridan J. F., Donnenberg A. D., Aurelian L., Elpern D. J. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J Immunol. 1982 Jul;129(1):326–331. [PubMed] [Google Scholar]
- Steele R. W., Marmer D. J., Keeney R. E. Comparative in vitro imunotoxicology of acyclovir and other antiviral agents. Infect Immun. 1980 Jun;28(3):957–962. doi: 10.1128/iai.28.3.957-962.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A. L., Smithwick E. M., Seligman S. J., Kim D. S. Fatal disseminated herpesvirus hominis type 2 infection in an adult with associated thymic dysplasia. Am J Med. 1974 Apr;56(4):545–553. doi: 10.1016/0002-9343(74)90487-2. [DOI] [PubMed] [Google Scholar]


