Abstract
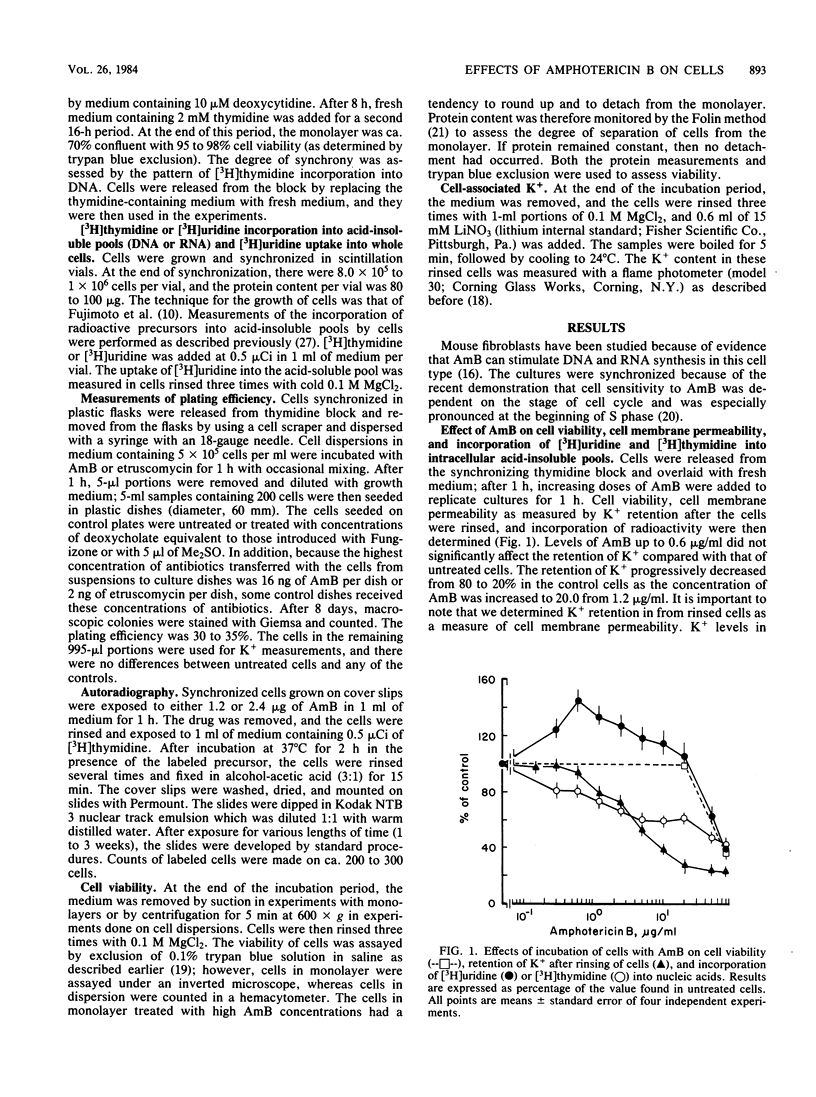
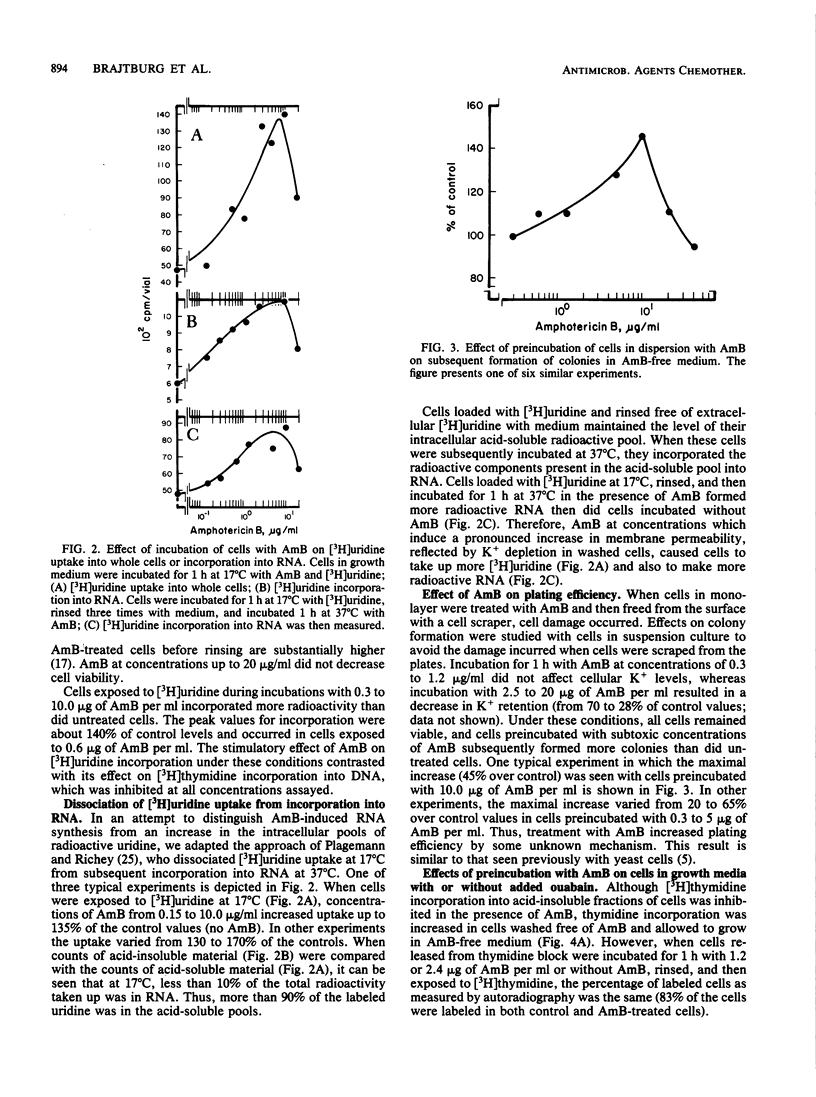
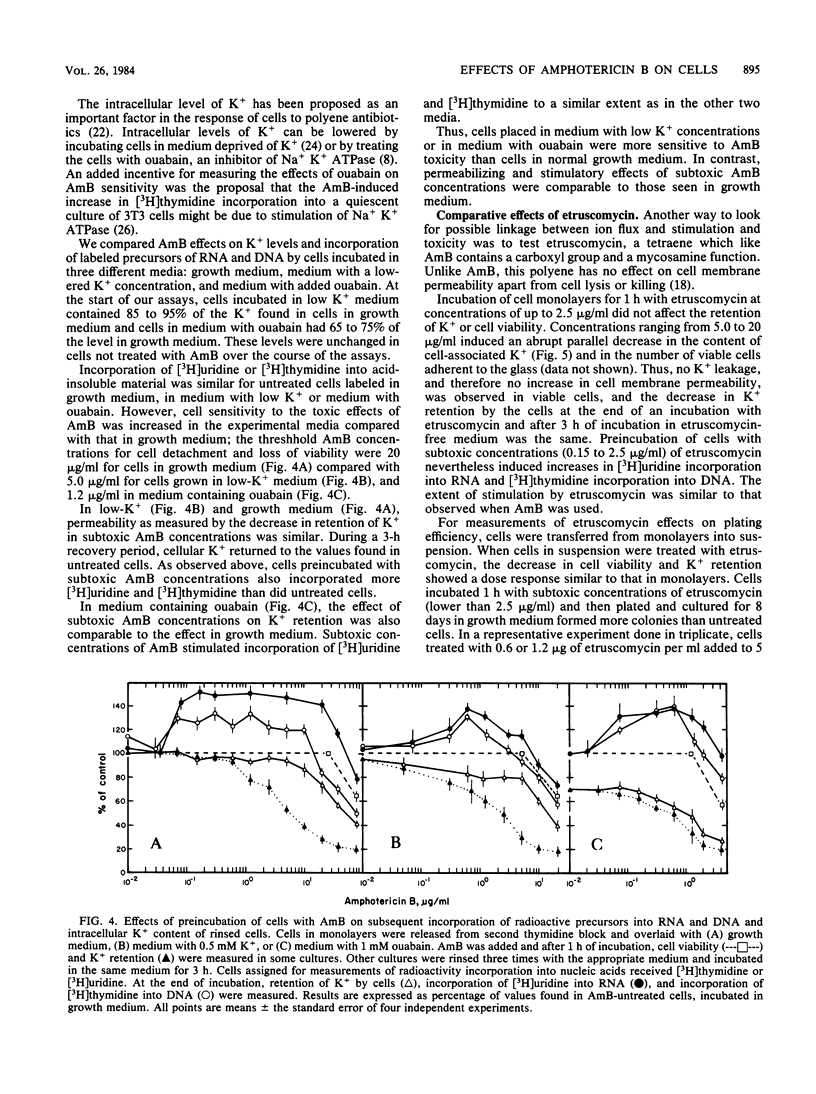
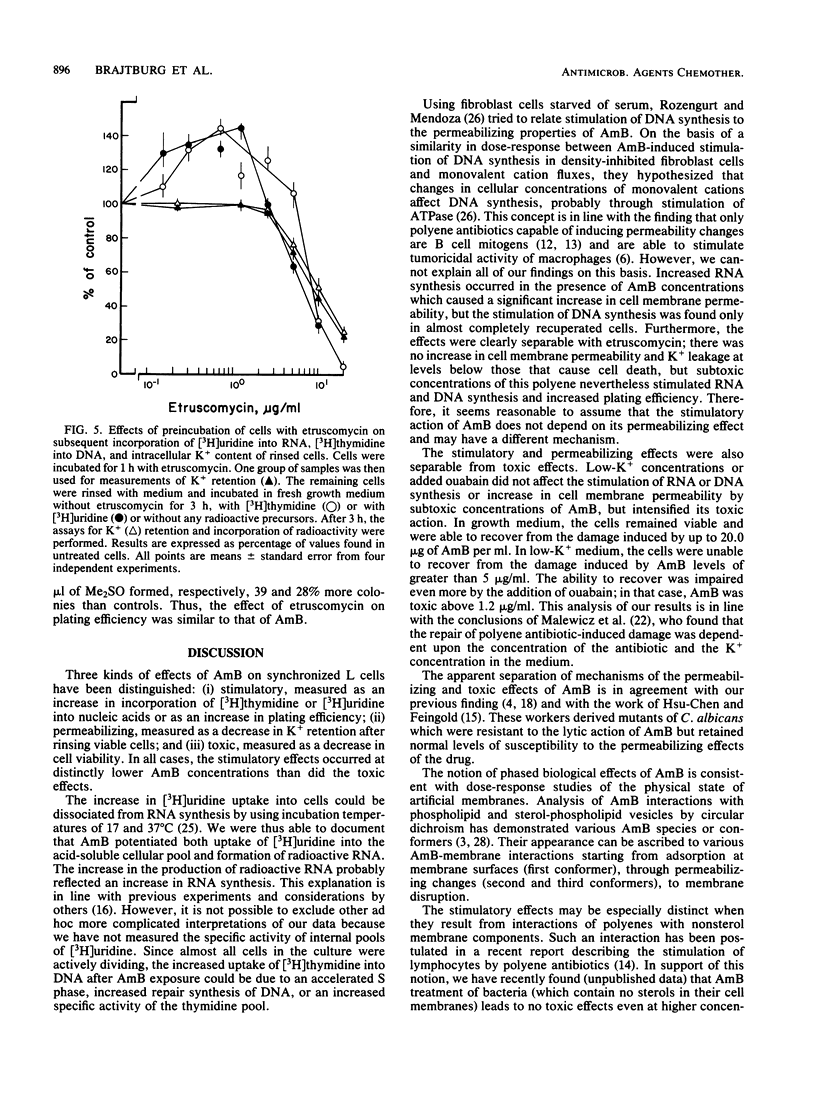
High concentrations of amphotericin B (AmB) killed mouse L cells, but low concentrations increased plating efficiency and stimulated the incorporation of labeled precursors into DNA and RNA. Thus, there were two disparate effects of AmB on L cells, stimulatory and toxic, and they occurred in distinct dose-related stages. AmB also affected the permeability of L cells. In dose-response studies, increases in cell membrane permeability, measured as the loss of K+ ions, occurred along with the stimulation of [3H]uridine incorporation into RNA. In contrast, stimulation of [3H]thymidine incorporation into DNA was only observed in cells recuperating from AmB-induced permeability changes. When the K+ concentration in the medium was lowered to 0.5 from 4.5 mM, or when 1 mM ouabain was added to the cultures, cell killing was potentiated, but the stimulatory and permeabilizing effects of subtoxic concentrations of AmB were unaffected. Furthermore, etruscomycin, a polyene antibiotic without any permeabilizing effects, nevertheless induced an enhancement of plating efficiency and of incorporation of [3H]uridine into RNA and [3H]thymidine into DNA. Our results suggest that the dose-related stimulatory, permeabilizing, and toxic effects of AmB most probably have distinct mechanisms of action and may be independent of one another.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. I., Hidaka K., Komiyama S., Kuwano M. Control of permeation of bleomycin A2 by polyene antibiotics in cultured Chinese hamster cells. Cancer Res. 1979 Dec;39(12):5150–5154. [PubMed] [Google Scholar]
- Akiyama S., Tabuki T., Kaneko M., Komiyama S., Kuwano M. Classification of polyene antibiotics according to their synergistic effect in combination with bleomycin A2 or fusidic acid. Antimicrob Agents Chemother. 1980 Aug;18(2):226–230. doi: 10.1128/aac.18.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J., Seigneuret M., Boudet G. Interaction between phospholipid bilayer membranes and the polyene antibiotic amphotericin B: lipid state and cholesterol content dependence. Biochim Biophys Acta. 1980 Jun 20;599(1):280–293. doi: 10.1016/0005-2736(80)90074-7. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Elberg S., Medoff G., Kobayashi G. S. Increase in colony-forming units of Candida albicans after treatment with polyene antibiotics. Antimicrob Agents Chemother. 1981 Jan;19(1):199–200. doi: 10.1128/aac.19.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Medoff G., Kobayashi G. S., Elberg S., Finegold C. Permeabilizing and hemolytic action of large and small polyene antibiotics on human erythrocytes. Antimicrob Agents Chemother. 1980 Oct;18(4):586–592. doi: 10.1128/aac.18.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Hibbs J. B., Jr Modulation of macrophage tumoricidal capability by polyene antibiotics: support for membrane lipid as a regulatory determinant of macrophage function. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4349–4353. doi: 10.1073/pnas.75.9.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti M., Amati P. Influence of amphotericin B on leucine uptake in 3T3 cells. Biochim Biophys Acta. 1983 Jul 13;732(1):251–255. doi: 10.1016/0005-2736(83)90209-2. [DOI] [PubMed] [Google Scholar]
- Frantz C. N., Nathan D. G., Scher C. D. Intracellular univalent cations and the regulation of the BALB/c-3T3 cell cycle. J Cell Biol. 1981 Jan;88(1):51–56. doi: 10.1083/jcb.88.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto W. Y., Starman B. J., Rowe D. W. The effect of amphotericin B-deoxycholate on proliferation and protein synthesis in human skin fibroblast cultures. In Vitro. 1978 Dec;14(12):1003–1009. doi: 10.1007/BF02616214. [DOI] [PubMed] [Google Scholar]
- Fujimoto W. Y., Teague J., Williams R. H. Fibroblast monolayer cultures in scintillation counting vials: metabolic and growth experiments using radioisotopes and a microfluoremetric DNA assay. In Vitro. 1977 Apr;13(4):237–244. doi: 10.1007/BF02615081. [DOI] [PubMed] [Google Scholar]
- Glassy M. C., Furlong C. E. Neutral amino acid transport during the cell cycle of cultured human lymphocytes. J Cell Physiol. 1981 Apr;107(1):69–74. doi: 10.1002/jcp.1041070109. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Smith C. I., Dresdner G. Is cholesterol the receptor for polyene antibiotic-induced B-lymphocyte activation. Cell Immunol. 1980 Nov;56(1):193–204. doi: 10.1016/0008-8749(80)90094-5. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Smith C. I. In vitro activating properties of polyene antibiotics for murine lymphocytes. Acta Pathol Microbiol Scand C. 1977 Aug;85C(4):277–283. doi: 10.1111/j.1699-0463.1977.tb03642.x. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Smith E. Mitogenic properties of polyene antibiotics for murine B cells. Scand J Immunol. 1976;5(1-2):37–43. doi: 10.1111/j.1365-3083.1976.tb02990.x. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. Two types of resistance to polyene antibiotics in Candida albicans. Nature. 1974 Oct 18;251(5476):656–659. doi: 10.1038/251656a0. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Andoh T. Stimulation by amphotericin B of uridine transport, RNA synthesis and DNA synthesis in density-inhibited fibroblasts. Exp Cell Res. 1978 Aug;115(1):37–46. doi: 10.1016/0014-4827(78)90399-3. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Boggs S., Schlessinger D., Pandey R. C., Rinehart K. L., Jr Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob Agents Chemother. 1979 May;15(5):716–722. doi: 10.1128/aac.15.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Kobayashi G. S., Schlessinger D. Sensitivity to amphotericin B and the cholesterol: phospholipid molar ratios of 3T3, L, BHK and HeLa cells. Biochem Pharmacol. 1977 Apr 15;26(8):705–710. doi: 10.1016/0006-2952(77)90212-x. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Schlessinger D., Kobayashi G. S. Amphotericin B and filipin effects on L and HeLa cells: dose response. Antimicrob Agents Chemother. 1977 May;11(5):803–808. doi: 10.1128/aac.11.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano M., Akiyama S. I., Takaki R., Okano H., Nishimoto T. Inhibition of DNA synthesis and cell cycle in Chinese hamster cells by sterol-binding polyene amphotericin B. Biochim Biophys Acta. 1981 Feb 26;652(2):266–273. doi: 10.1016/0005-2787(81)90116-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malewicz B., Jenkin H. M., Borowski E. Repair of membrane alterations induced in baby hamster kidney cells by polyene macrolide antibiotics. Antimicrob Agents Chemother. 1981 Feb;19(2):238–247. doi: 10.1128/aac.19.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff G., Brajtburg J., Kobayashi G. S., Bolard J. Antifungal agents useful in therapy of systemic fungal infections. Annu Rev Pharmacol Toxicol. 1983;23:303–330. doi: 10.1146/annurev.pa.23.040183.001511. [DOI] [PubMed] [Google Scholar]
- Moscatelli D., Sanui H., Rubin A. H. Effects of depletion of K+, Na+, or Ca2+ on DNA synthesis and cell cation content in chick embryo fibroblasts. J Cell Physiol. 1979 Oct;101(1):117–128. doi: 10.1002/jcp.1041010114. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P. Transport of nucleosides, nucleic acid bases, choline and glucose by animal cells in culture. Biochim Biophys Acta. 1974 Dec 16;344(3-4):263–305. doi: 10.1016/0304-4157(74)90010-0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann N Y Acad Sci. 1980;339:175–190. doi: 10.1111/j.1749-6632.1980.tb15977.x. [DOI] [PubMed] [Google Scholar]
- Schmidt R. A., Glomset J. A., Wight T. N., Habenicht A. J., Ross R. A study of the Influence of mevalonic acid and its metabolites on the morphology of swiss 3T3 cells. J Cell Biol. 1982 Oct;95(1):144–153. doi: 10.1083/jcb.95.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertut-Croquin A., Bolard J., Chabbert M., Gary-Bobo C. Differences in the interaction of the polyene antibiotic amphotericin B with cholesterol- or ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study. Biochemistry. 1983 Jun 7;22(12):2939–2944. doi: 10.1021/bi00281a024. [DOI] [PubMed] [Google Scholar]


