Abstract
SRC-3/AIB1/ACTR/pCIP/RAC3/TRAM-1 is a primary transcriptional coactivator for the estrogen receptor. Here we report that deletion of the SRC-3 basic helix-loop-helix (bHLH) domain blocks its proteasome-dependent turnover. We further identified two residues (K17 and R18) in the SRC-3 bHLH domain that are essential for its stability. Moreover, we found that the bHLH domain contains a bipartite nuclear localization signal (NLS). SRC-3 NLS mutants block its translocation into the nucleus, and this correlates with its insensitivity to proteasome-dependent turnover. SRC-3 shows a time-dependent decay in the presence of cycloheximide which is not apparent for the cytoplasmic mutant. Fusion of a simian virus 40 T antigen NLS to the cytoplasmic localized SRC-3 mutant drives it back into the nucleus and restores its proteasomal sensitivity. In addition, the cytoplasmic mutants are inactive for transcriptional coactivation and cancer cell growth. Taken together, our data indicate that proteasome-dependent turnover of SRC-3 occurs in the nucleus and that two amino acid residues in the bHLH domain provide a signal for its nuclear localization and proteasome-dependent degradation as well as for regulation of SRC-3 transcriptional coactivator capacity.
Nuclear receptors comprise a large class of transcription factors that play central roles in development, reproduction, and homeostasis. Many nuclear receptor functions are regulated by specific steroid ligands. Ligand binding induces dissociation of corepressors and recruitment of coactivators to mediate the activation of gene transcription (15, 37, 39, 57). The most well-studied coactivators are members of the steroid receptor coactivator SRC/p160 family (39): SRC-1 (43), TIF2/GRIP1/SRC-2 (21, 59), and AIB1/pCIP/ACTR/RAC-3/TRAM-1/SRC-3 (2, 7, 18, 30, 54, 55).
SRC/p160 family proteins possess similar structures in their primary amino acid sequences (39). In the central region, there is an α-helical LXXLL motif, or NR box, which is implicated in their ligand-dependent recruitment by steroid receptors (20, 44, 60). At the C terminus, there is a HAT domain with acetyltransferase activity (7, 52). Between the NR box and HAT domain, there are CBP interaction and CARM1 interaction domains, both of which overlap with transferable activation domains (6, 7, 60). SRC proteins are most highly conserved in an N-terminal region that contains a basic helix-loop-helix (bHLH) domain and a PAS homology domain; those domains are present in members of the Per/Arnt/Sim family of transcription factors and mediate protein-protein interactions (15, 39). In contrast, the function of the bHLH domain in SRCs is largely unknown.
SRC family members have previously been reported to be overexpressed in various cancers (2, 17, 24, 33). SRC-3/AIB1 is genetically amplified in 5 to 10% of breast and gastric cancers but overexpressed in 40 to 60% of them (2, 33, 50, 61). It is an oncogene (56). Overexpression of SRC-3 stimulates the AKT signaling pathway and promotes cell growth (56, 73). When it is overexpressed in mice, SRC-3 induces spontaneous breast tumors (56). In contrast, SRC-3 deficiency suppresses v-Ha-ras-induced breast tumor initiation and progression in mice (27). Recently, it was demonstrated that phosphorylation plays a critical role in regulating SRC-3 activity for steroid and growth factor signaling and cell transformation (14, 65, 66).
Spatial and temporal regulation of essential regulatory molecules between different compartments is one of the key events in cellular signal transduction, gene activation, and protein dynamics during various biological processes. It has been demonstrated that estrogen induces phosphorylation of SRC-3 in an extranuclear complex (72), while the phosphorylated SRC-3 protein mediates gene activation in the nucleus (66). Multiple pathways are involved in cellular protein turnover, and the levels of important growth-promoting coactivators (and oncogenes) are likely tightly regulated in normal cells. Although the cellular levels of the SRC family of coactivators can be regulated at the transcription level under pathological conditions, posttranslational regulation appears to play the prominent role under differentiated physiological conditions (31, 35, 69). Nevertheless, the molecular mechanisms of regulating the nucleocytoplasmic shuttling and turnover of SRC-3 are obscure.
The ubiquitin/proteasome pathway plays a widely recognized role in promoting degradation of many regulatory proteins in eukaryotic cells (16). It is responsible for degradation of many nuclear receptors and coregulators (35, 36, 42). The underlying mechanisms for regulating transcription factors by the ubiquitin-proteasome system can occur at the level of regulation of their location, activity, or abundance through constitutive turnover or transcription-coupled destruction (10, 41). It is known that proteasomes are localized in both the nucleus and the cytosol, where intracellular proteins can be degraded by them (16). A number of transcription factors have previously been reported to be degraded in the nucleus (4, 9, 11, 13, 28, 46, 49, 63).
Recently, it has been reported that SRC-3 protein is continuously turned over by proteasomes while maintaining its essential role in steroid receptor signaling (31, 35, 36, 69). Regulation of SRC-3 protein stability by proteasomes has been reported to occur by both ubiquitin-dependent (35, 67) and ubiquitin-independent (31) pathways. However, the cellular compartment responsible for this proteasome-dependent turnover remains unclear.
Herein, we demonstrate the spatial regulation of SRC-3/AIB1 oncogene protein stability by proteasomes. Two amino acid residues (K17 and R18) within the N-terminal bHLH domain are identified to be essential for its proteasome-dependent turnover. These two residues also appear to be the signal residues for its nuclear localization (nuclear localization signals [NLS]). Mutation of these two residues in SRC-3 renders SRC-3 unable to be imported into the nucleus and degraded by proteasomes. Fusion of a simian virus 40 (SV40) T antigen NLS (TNLS) to this mutant rescues both its nuclear localization and its proteasome-dependent turnover, indicating a crucial role for the nuclear compartment in regulation of SRC-3 protein turnover and transcriptional regulatory capacity.
MATERIALS AND METHODS
Plasmids.
For most experiments, 293 cells were transiently transfected with expression vectors for the wild-type (SRC-3 wt) or mutant forms of SRC-3. FLAG-tagged SRC-3 wt was generated as described previously (66). A series of deletion mutants of SRC-3 were constructed by subcloning of Pfu-PCR-amplified SRC-3 fragments with XhoI or ApaI end sites into FLAG-SRC-3 wt's vector. Single point mutations of SRC-3 were generated according to Stratagene's site-directed mutagenesis protocol using point mutation primers. After Pfu-PCR amplification, DpnI digestion was used to remove the parental plasmid and the resultant plasmid constructs were sequenced. The K17A/R18A double mutant of SRC-3 was generated by two rounds of subcloning for each single point mutant. Mutation of the four basic residues to Ala in C-NLS (SRC-3-A4) was performed using K35A as a template to further mutate three additional sites. TNLS-SRC-3-K17A/R18A was generated by Pfu-PCR amplification using an N-terminal primer containing an XhoI site and flanking sequence encoding SV40 T antigen NLS (PPKKKRKV). pcDNA3.1-lacZ for β-galactosidase (β-Gal) was purchased from Invitrogen. Expression vector for hemagglutinin-ubiquitin (HA-Ub) was a generous gift from Dirk Bohmann (University of Rochester).
Cell culture and transfections.
Transient transfections were carried out in 293 or HeLa cells, which were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (36). For luciferase reporter assays, at least 24 h prior to transfection HeLa cells were plated in 24-well or 6-well plates in phenol red-free DMEM containing 5% charcoal dextran-stripped fetal bovine serum (Gemini Bio-Products, Woodland, CA). Transfections were carried out with TransIT-LT1 (Mirus, Madison, WI) according to the manufacturer's recommendations. One day after transfection, cells were treated with 10−8 M estradiol (E2), 10−9 M dihydrotestosterone (DHT), 10−8 M progesterone, or ethanol vehicle for corresponding to their luciferase reporters. Twenty-four hours after hormone treatment, cells were harvested for luciferase and Western analyses. Luciferase activities were normalized to SRC-3 wt and mutant protein levels.
Western analyses and antibodies.
Cells were lysed in an extraction buffer (66) on ice for 30 min with brief vortexing, followed by centrifugation for 15 min at 21,000 × g and 4°C. Supernatants were analyzed by 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to nitrocellulose membranes (Bio-Rad). In the case of FLAG-tagged SRC-3 wt and mutants, a horseradish peroxidase-conjugated anti-FLAG antibody (Sigma) with a 1:2,000 dilution was directly used for detection. Anti-β-Gal antibody (Roche) with a 1:2,000 dilution and anti-Hsp70 antibody (Transduction Laboratories, BD Bioscience) with a 1:5,000 dilution were used for detection of each protein. All other antibodies were from Santa Cruz Biotechnology. All blots were visualized by enhanced chemiluminescence (ECL; Amersham).
Cell fractionation.
Cell fractionation was carried out as described elsewhere (65), with several modifications. In brief, 293 tetracycline-inducible SRC-3 wt or its K17A/R18A mutant in stable cells were lysed in buffer A (10 mM HEPES, pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, 10 μg/ml phenylmethylsulfonyl fluoride, and protease inhibitor mix [Roche]). The homogenates were centrifuged for 10 min at 3,000 rpm to pellet nuclei. The supernatants were collected and used as cytosolic fractions. Pelleted nuclei were washed twice with buffer A. Nuclear fractions were extracted from nuclei with buffer C (20 mM HEPES, pH 7.9, 1.5 mM MgCl2, 600 mM NaCl, 0.5 mM dithiothreitol, 10 μg/ml phenylmethylsulfonyl fluoride, and protease inhibitor mix), followed by centrifugation for 15 min at 21,000 × g.
Proteasome-dependent protein stability analyses.
For proteasome-dependent protein stability assays, after transfection of expression plasmids, cells were treated with the proteasome inhibitor MG132 (5 μM) (Sigma) or DMSO vehicle overnight followed by Western analysis (36). Another highly specific proteasome inhibitor, epoxomicin (BostonBiochem) (500 ng/ml), also was used to examine proteasome-dependent protein stability in cells. For studies of the protein decay using treatment with cycloheximide (CHX), 2 days after cell transfection, cells were treated with CHX (0.5 mM) for indicated times, and SRC-3 wt or mutant protein levels were examined by Western blotting. For analysis of ubiquitination of SRC-3 wt and the cytoplasmic mutant, each above-described FLAG-tagged expression plasmid was transfected together with the HA-Ub plasmid into 293 cells, followed by immunoprecipitation (IP) with anti-FLAG antibody and Western blotting.
Stable cell lines and pulse-chase assays.
Stable 293 cell lines overexpressing SRC-3 wt and the K17A/R18A mutant were generated by an Flp-In T-REx system (Invitrogen). After induction by tetracycline for 2 days, cells were washed with DMEM without methionine (Met) and cysteine (Cys) (Invitrogen) and then incubated in this minimal medium for 1.5 h. Subsequently, the minimal medium was removed and the pulse-label medium (50 μCi [35S]Met/ml and 50 μCi [35S]Cys/ml [ICN] in DMEM without Met and Cys) was added for 1 h, followed by removal of the pulse-label medium and addition of the chase medium (DMEM with 1 mM unlabeled Met and Cys and 10% fetal calf serum) for indicated chase times. Cells were collected and lysed followed by immunoprecipitation with anti-FLAG antibodies, analyzed by SDS-PAGE, and quantified by use of a phosphorimager. T47D cell lines overexpressing SRC-3 wt or a cytoplasmic mutant were generated by use of a retrovirus method (66).
Cell proliferation assay.
293 tetracycline-inducible SRC-3 wt or its K17A/R18A mutant in stable cells was seeded into 96-well plates with 2,000 cells/well. One day after induction by tetracycline (1 μg/ml), viable cell numbers were determined by a CellTiter 96 AQueous One Solution cell proliferation assay (Promega). The optical density (490 nm) measurement was linear between cell numbers 1250 and 40000 in our calibration before the assay. 293/Flp recombination target host cells (Flp-In T-REx 293 cells) (Invitrogen) were also measured as a control. Breast cancer T47D cell proliferation assays also were performed by using the CellTiter 96 AQueous One Solution cell proliferation assay mentioned above.
Flow cytometry analysis was conducted at the Flow Cytometry Core Facility at Baylor College of Medicine. T47D cells overexpressing SRC-3 wt or a cytoplasmic mutant described above were grown as described previously (36), followed by use of a standard propidium iodide DNA staining procedure.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed as previously described (31). The pS2 gene promoter was analyzed by PCR after immunoprecipitation.
GFP fusion proteins.
pAcGFP1-C3 vector (Clontech, BD Biosciences) was used as a backbone for green fluorescent protein (GFP) fusion protein constructs. SRC-3 wt and the ΔN12, ΔN19, K17A/R18A, and TNLS-K17/R18A mutants were generated by subcloning each from the above-described SRC-3 wt and mutant constructs into pAcGFP1-C3 by use of XhoI- and ApaI-digested fragments. GFP-SRC-3-ΔC-NLS was generated by Pfu-PCR amplification to delete C-NLS, followed by ligation using a SalI site, which was flanked at two primer ends. GFP-N-NLS, GFP-C-NLS, and GFP-NLS were generated by Pfu-PCR amplification of N-NLS, C-NLS, and NLS fragments with XhoI and ApaI at two ends, followed by subcloning into pAcGFP1-C3. All GFP fusion proteins were expressed in 293 or HeLa cells by transient transfection of plasmid constructs. After 2 days, the GFP fusion proteins were visualized either directly under a fluorescence microscope or by prior fixation using 3.7% formaldehyde and nuclear staining by DAPI (4′,6′-diamidino-2-phenylindole) or Hoechst stain (2 μg/ml) (Sigma).
Immunofluorescence staining.
The basic protocol for the immunofluorescence staining experiment is described at http://microscopy.bcm.tmc.edu. Cells were transfected with plasmids for SRC-3 wt or mutants. After 2 days, cells were washed with phosphate-buffered saline (PBS) three times, fixed with 3.7% formaldehyde for 30 min on ice, and then washed again with PBS three times followed by permeabilization with 0.5% Triton X-100 in PBS at room temperature for 30 min. Cells then were washed with PBS three times and blocked in PBS-0.1% Tween 20 buffer with 5% milk for 30 min at room temperature. Subsequently, anti-FLAG-M2 antibody (Sigma) was incubated with cells at 4°C overnight, followed by washes and incubation with goat anti-mouse Alexa 555 or Alexa 488 (Molecular Probes) at room temperature for 30 min. Finally, the secondary antibody was removed, rinsed again, and applied to the coverslips prior to visualization by an Applied Precision deconvolution microscope (DeltaVision) (Applied Precision, Inc.).
RESULTS
The bHLH domain of SRC-3 contributes to SRC-3's proteasome-dependent stability.
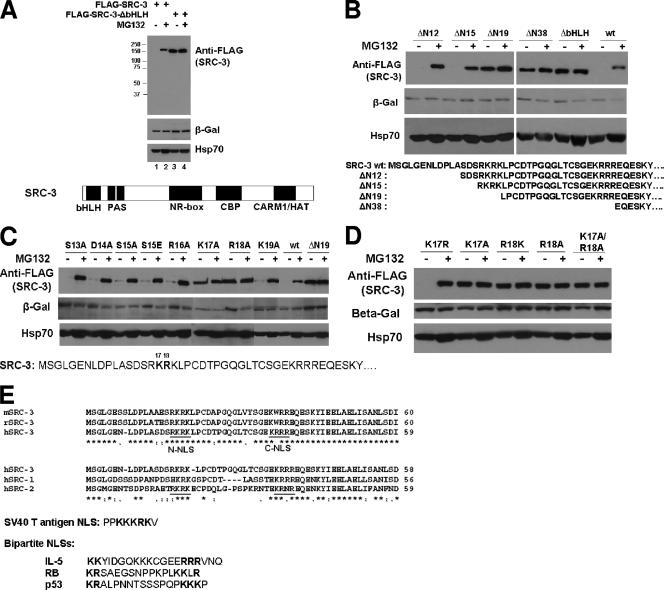
To study a functional role of the bHLH region of SRC-3, we generated an N-terminal mutant construct deleted in the bHLH domain (ΔbHLH). To our surprise, we observed a remarkably higher steady-state level of this SRC-3 mutant protein than of the SRC-3 wt protein when equal amounts of plasmid DNA were transfected into 293 cells (Fig. 1A, lanes 1 and 3). We previously reported that the SRC/p160 family proteins and the estrogen receptor (ER) are targets of the 26S proteasome, since disruption of proteasome function by its inhibitor MG132 results in increased steady-state levels of both ER and SRC/p160 proteins (36). Therefore, we examined whether the higher level of SRC-3 ΔbHLH protein was caused by a reduction in proteasome-dependent degradation of this mutant. Treatment of 293 cells with MG132 dramatically increased the wt SRC-3 protein level in comparison to cells treated with DMSO (Fig. 1A, lanes 1 and 2); in contrast, virtually no change was detected for the SRC-3 ΔbHLH mutant in the presence or absence of MG132 (Fig. 1A, lanes 3 and 4). Cotransfection of the LacZ gene plasmid into cells followed by Western detection using an anti-β-Gal antibody did not result in a similar response to MG132, indicating the observed changes were not due to differential transfection efficiency. In addition, Hsp70 was used in the same experiment to monitor cell numbers and MG132 treatment; it showed only a slight increase, consistent with previously reported observations (5, 29). Taken together, the results indicate that the SRC-3 ΔbHLH domain is essential for proteasome-dependent turnover of SRC-3.
FIG. 1.
Two residues of the SRC-3 bHLH domain essential for SRC-3's proteasome-dependent stability. (A) The bHLH domain of SRC-3 is essential for SRC-3's proteasome-dependent stability. 293 cells were transfected with 200 ng of the plasmids for wt FLAG-SRC-3 (FLAG-SRC-3) or a mutant with deletion of bHLH (FLAG-SRC-3-ΔbHLH) as well as 20 ng of the plasmid for β-Gal. After treatment with MG132 (+) or DMSO (−), cells were harvested for Western analysis using antibodies as indicated. Protein molecular mass markers (in kilodaltons) are shown at the left. The SRC-3 protein structure is indicated at the bottom. (B) Deletion mapping of the bHLH domain. The procedure was the same as that described for panel A, except a series of different SRC-3 deletion mutants was used as indicated. The bottom panel shows the N-terminal sequence of SRC-3 for the deletion mutants. (C) Point mutation analysis of the bHLH domain. The procedure was the same as that described for panel A, except a series of single amino acid point mutants of SRC-3 was used as indicated. The bottom panel shows the sequence position of each point mutant. (D) The procedure was the same as that described for panel A, except additional single point mutants and a double mutant, the K17A/R18A mutant, were used. (E) Sequence analysis. The top panel shows a sequence alignment of the mouse, rat, and human SRC-3 (mSRC-3, rSRC-3, and hSRC-3, respectively) N termini. Underlined putative NLS are labeled N-NLS and C-NLS. The middle panel shows a comparison between hSRC-1, hSRC-2, and hSRC-3, in addition to SV40 T antigen NLS. The bottom panel shows the bipartite NLS of interleukin-5 (IL-5), retinoblastoma (RB), and p53.
Two residues in the SRC-3 bHLH domain are essential for its stability.
To further map the region responsible for this proteasome-dependent stability, a number of truncation mutants in the SRC-3 bHLH domain were constructed (Fig. 1B, lower-panel sequences). The relative susceptibility of each SRC-3 mutant to proteasome-mediated degradation was assessed by comparing the difference in SRC-3 protein levels between cells that were treated with or without MG132. Two shorter deletions that remove the N-terminal 38 (ΔN38) or 19 (ΔN19) amino acid residues of SRC-3 resulted in the same effect, as shown by the deletion of the entire bHLH, which blocked the proteasome-dependent turnover (Fig. 1B). In contrast, a smaller deletion of only the N-terminal 12 (ΔN12) or 15 (ΔN15) residues showed no stabilization of SRC-3 protein (Fig. 1B), indicating that four basic N-terminal residues (N16 to N19) contain a determinant critical for proteasome-dependent degradation.
To precisely define the critical amino acids in the region between N16 and N19 that confer protein destabilization, additional SRC-3 mutants with single amino acid point substitutions were constructed. These expression constructs include mutation of Ser 13 to Ala (S13A), Asp 14 to Ala (D14A), Ser 15 to Ala (S15A), Ser 15 to Glu (S15E), Arg 16 to Ala (R16A), Lys 17 to Ala (K17A), Arg 18 to Ala (R18A), or Lys 19 to Ala (K19A) (Fig. 1C). In comparison to SRC-3 wt and ΔN19, which show a response and no response to MG132, respectively, responses of the K17A and R18A mutants appeared to be similar to that of ΔN19, while all other point mutants provided no stabilization effect (Fig. 1C). This result indicates that Lys 17 (K17) and Arg 18 (R18) are the two essential residues in the bHLH domain of SRC-3 that determine its susceptibility to proteasome-mediated degradation.
Lys 17 and Arg 18 are two basic charged residues. To test whether only basic charges in these two positions are necessary and sufficient for determining SRC-3 turnover, Lys 17 also was mutated to Arg (K17R) and Arg 18 to Lys (R18K). As shown in Fig. 1D, K17R behaved similarly to SRC-3 wt, indicating that a basic charged residue at the position of residue 17 is sufficient to render it sensitive to proteasome-dependent degradation. The R18K mutant, however, showed a similar lack of response to MG132, indicating that a simple basic charge at residue 18 is not sufficient to make it sensitive to proteasome degradation (Fig. 1D). A double mutant of K17A and R18A was constructed, and this mutant appeared to be unresponsive to MG132 as well and displayed a higher level of protein than the single K17A or R18A mutant. Transfection of a lower amount of vector expressing the K17A/R18A mutant (20%) resulted in a level of protein similar to that observed for the single K17A or R18 mutant (Fig. 1D).
Since the two basic K17 and R18 residues are required for normal proteasome-dependent turnover of SRC-3, we analyzed the primary amino acid sequence of SRC-3 surrounding this region in different species. The N-terminal 60 residues of human SRC-3 (hSRC-3), which consist of a majority of the bHLH domain (ending at the 84th residue), was compared with mouse SRC-3 and rat SRC-3 sequences (Fig. 1E, top panel). This region is highly conserved between human, mouse, and rat. Interestingly, there are two putative NLS in this region (Fig. 1E, underlined sequences, N-NLS and C-NLS); the first N-NLS encompasses K17 and R18. The putative NLS motifs also are conserved among the SRC/p160 family, including SRC-1, -2, and -3 (Fig. 1E, middle panel). The amino acid spacer between the two putative NLS in the SRC-3 family (1) is composed of 15 residues, which is longer than a typical bipartite NLS spacer of 10 to 12 residues, such as those of the well-known bipartite NLS in interleukin-5 (23), retinoblastoma (70), and p53 (32) (Fig. 1E, bottom panel).
Correlations exist between SRC-3 stability and its subcellular localization.
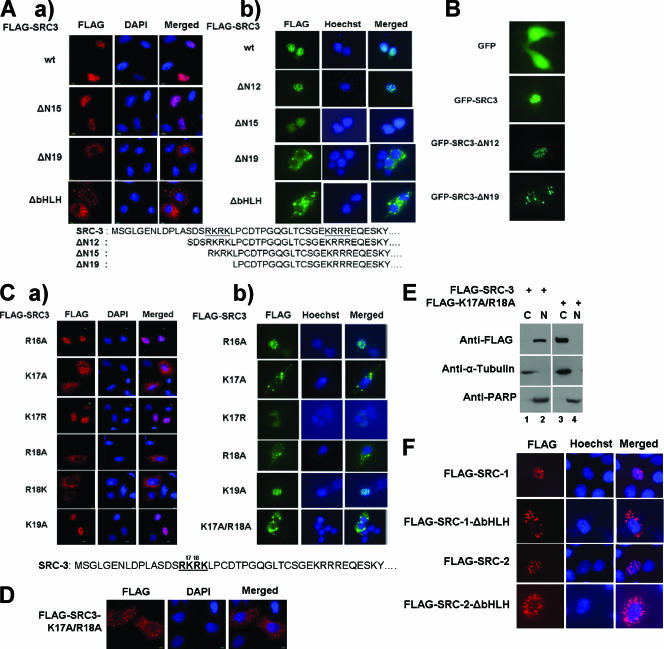
To determine whether the putative SRC-3 NLS are functional in cells, a number of SRC-3 N-terminal deletion mutants were tested by immunofluorescence staining. Plasmid DNA encoding FLAG-tagged SRC-3 wt and mutants was transfected into HeLa cells cultured in a complete-serum medium, followed by immunofluorescence staining with anti-FLAG antibodies to detect SRC-3 wt or mutants. SRC-3 wt and deletion of N15 (ΔN15) appeared in the nucleus, whereas the deletions of N19 (ΔN19) and bHLH (ΔbHLH) were present in the cytoplasm (Fig. 2A, panel a). As expected, an SRC-3 mutant with a deletion of N12 had the same subcellular localization as an SRC-3 mutant with a deletion of N15 (data not shown). The same results were also seen in 293 cells (Fig. 2A, panel b). The results indicate that some or all amino acids within the four basic residues from R16 to K19 are required for SRC-3 nuclear localization.
FIG. 2.
Subcellular localization of SRC-3 N-terminal mutants. (A) Immunofluorescence staining of SRC-3 wt and deletion mutants. Plasmids for FLAG-tagged SRC-3 wt or mutants, as indicated in the legends for Fig. 1A and B, were transfected into (a) HeLa or (b) 293 cells, which were maintained in a complete-serum medium. Anti-FLAG antibodies were used for detection. DAPI and Hoechst staining showing the nucleus are labeled. (B) GFP fusions with SRC-3 wt or mutants as indicated above were expressed in HeLa cells cultured in a full-serum medium. Cells were fixed and visualized under a microscope. (C) The procedure was the same as that described for panel A, except single amino acid point mutants of SRC-3 were used in (a) HeLa or (b) 293 cells. Each designation indicates the amino acid residue position and the letter for the amino acid, e.g., R16A indicates Arg 16 mutated to Ala. (D) The procedure was the same as that described for panel A, except a double point mutant of SRC-3 (K17A/R18A mutant) was used. (E) Cell fractionation of 293 cells stably expressing wt FLAG-SRC-3 (FLAG-SRC3) or its K17A/R18A mutant (FLAG-K17A/R18A). Cytoplasmic (C) or nuclear (N) fractions of cell lysates were subjected to Western blot analysis using antibodies as indicated. PARP, poly(ADP-ribose) polymerase. (F) Subcellular localizations of the wt and bHLH deletion mutants of SRC-1 and SRC-2. The FLAG-tagged wt and mutants of each constructs, as indicated, were transfected into HeLa cells, followed by immunofluorescence staining using anti-FLAG antibodies.
To substantiate our results, GFP fusions with SRC-3 wt or mutants were expressed in HeLa cells cultured in a full-serum medium. GFP alone appeared in both the nucleus and the cytosol, and GFP-SRC-3 wt possessed a nuclear localization. GFP fusion with deletion of N12 of SRC-3 also appeared to be in the nucleus; in contrast, GFP fusion with deletion of N19 of SRC-3 resulted in cytoplasmic localization (Fig. 2B). In addition, all of these GFP fusion proteins were expressed in 293 cells and were localized in the same manner as in HeLa cells (data not shown). Therefore, the correlation between SRC-3 stability and subcellular localization was demonstrated by both immunofluorescence staining and GFP fusion methods.
Next we asked whether there was a further correlation between SRC-3 stability and its subcellular localization among single point mutations in the SRC-3 N-NLS. According to the stability studies described above, the R16A, K17R, and K19A mutants are unstable mutants with strong responses to MG132, while the K17A, R18A, and R18K mutants are stable mutants with no responses to MG132 (Fig. 1C and D). All of these single mutants were examined for their subcellular localizations by immunofluorescence staining. As expected, the unstable point mutants (R16A, K17R, and K19A mutants) were in the nucleus, whereas the stable point mutants (K17A, R18A, and R18K mutants) were in the cytoplasm in HeLa cells (Fig. 2C, panel a). Similar results were observed in 293 cells (Fig. 2C, panel b). The double point mutant (K17A/R18A mutant) showed a strong cytoplasmic localization (Fig. 2C and D).
Moreover, we performed a cell fractionation experiment using cells expressing either SRC-3 wt or its K17A/R18A mutant. SRC-3 wt protein appeared in the nuclear fraction, while the K17A/R18A mutant was present in the cytoplasmic fraction (Fig. 2E). α-Tubulin and poly(ADP-ribose) polymerase were used as the cytoplasmic and nuclear protein markers, respectively, in the same experiment (Fig. 2E). Again, these data support the conclusion that K17/R18 is required for the nuclear localization of SRC-3.
Since the primary sequences of the bHLH region containing the NLS are conserved among the SRC family (Fig. 1E), we examined subcellular localization of the wild type and ΔbHLH mutants of SRC-1 and SRC-2 by using immunofluorescence staining. As expected, both wt SRC-1 and wt SRC-2 appeared in the nucleus whereas the ΔbHLH mutants were in the cytoplasm (Fig. 2F). Therefore, the bHLH sequence is important for the nuclear localization of the SRC (p160) family.
Proteasome-dependent turnover of SRC-3 occurs in the nucleus.
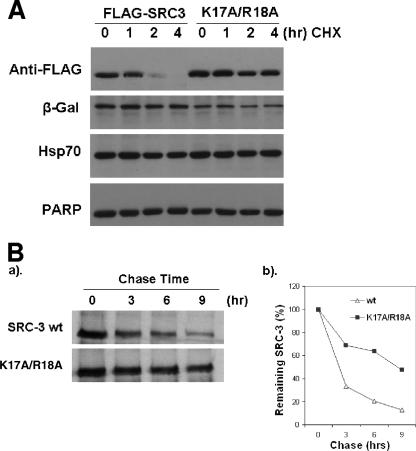
To further assess whether the higher protein levels of the SRC-3 cytoplasmic mutants were the consequence of their increased protein stabilities, FLAG-SRC-3 wt and its double point mutant (K17A/R18A mutant) were expressed in 293 cells, and CHX was added to block new SRC-3 protein synthesis, followed by monitoring the decay of SRC-3 wt and double mutant proteins after 1, 2, and 4 h. SRC-3 wt (FLAG-SRC-3) decreased after 2 h, but the double mutant (K17A/R18A mutant) was almost unchanged (Fig. 3A), indicating that the cytoplasmic SRC-3 mutant is much more stable than the wild type. Pulse-chase assays then were performed to measure degradation rates of SRC-3 wt and its K17A/R18A mutant. As expected, SRC-3 wt levels decreased so that at 3 h there was about 40% remaining; in contrast, the K17A/R18A mutant showed a slower degradation rate and at 3 h there was about 70% remaining (Fig. 3B). This result was consistent with the CHX experiment, substantiating that the cytoplasmic mutants of SRC-3 are indeed more stable.
FIG. 3.
Two residues, K17 and R18, of SRC-3 essential for its protein turnover rate. (A) FLAG-SRC-3 wt (FLAG-SRC3) or its double mutant (K17A/R18A mutant), along with β-Gal, was expressed in 293 cells. After treatment with CHX for the indicated times, cells were harvested and analyzed by Western blotting using antibodies as indicated. PARP, poly(ADP-ribose) polymerase. (B) Pulse-chase analysis of SRC-3 wt and its K17A/R18A mutant. Inducible 293 cells for overexpressing SRC-3 wt or the K17A/R18A mutant were induced for 2 days, followed by 35S labeling and chasing for times indicated. 35S-labeled SRC-3 protein was analyzed by IP with anti-FLAG antibodies, followed by (a) SDS-PAGE and (b) phosphorimager quantitation.
SRC-3 contains a bipartite NLS.
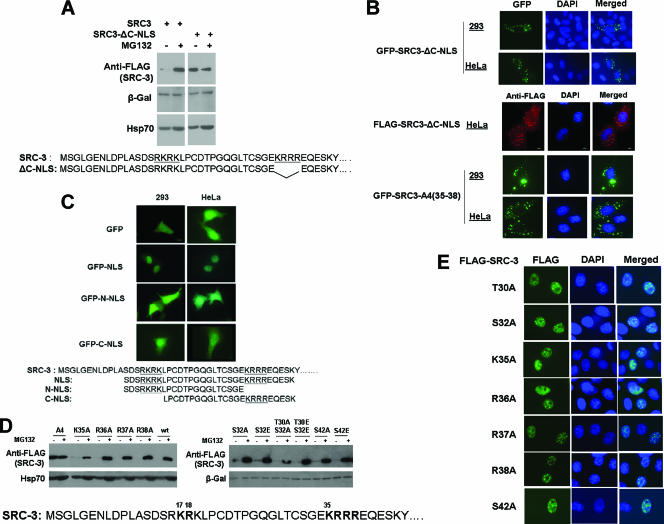
Since sequence analysis (Fig. 1E) of SRC-3 revealed two putative NLS, we asked whether the C-NLS in SRC-3 functions in a way similar to N-NLS. A mutant containing a deletion of the four basic residues in the C-NLS of SRC-3 (SRC3-ΔC-NLS) was expressed in 293 cells, followed by treatment with MG132. As shown in Fig. 4A, SRC3-ΔC-NLS protein was at a higher level than SRC-3 wt in the absence of MG132 and did not respond to MG132 (Fig. 4A). This result was the same as that for deletion of SRC-3 N19, which encompasses N-NLS (Fig. 1B), indicating that both NLS in SRC-3 are required for its proteasome-dependent turnover.
FIG. 4.
A bipartite NLS in SRC-3 and its functions. (A) 293 cells were transfected with either FLAG-tagged SRC-3 wt (SRC3) or its deletion of C-NLS (SRC3-ΔC-NLS), as indicated at the bottom. After treatment with MG132 (+) or DMSO (−), cell extracts were analyzed by Western blotting. (B) Subcellular localization of SRC-3-ΔC-NLS. GFP fusion with SRC-3-ΔC-NLS was analyzed in 293 or HeLa cells (upper images). FLAG-SRC-3-ΔC-NLS was analyzed in HeLa cells by immunofluorescence staining (middle images). GFP-SRC-3 with four basic residues from K35 to R38 mutated to Ala [GFP-SRC-3-A4(35-38)] was also analyzed in 293 and HeLa cells (bottom images). (C) A bipartite NLS in SRC-3. Short peptides containing SRC-3's bipartite NLS (NLS), only N-NLS (N-NLS), or only C-NLS (C-NLS) were fused to GFP and expressed in 293 or HeLa cells, followed by GFP detection. The peptide sequences of the constructs are indicated at the bottom. (D) Proteasome-dependent stability of SRC-3 point mutants in C-NLS and nearby amino acids. Each of the point mutants of FLAG-SRC-3, as indicated, was expressed in 293 cells, followed by treatment with MG132 and Western analysis. A4 indicates the mutant with all basic residues from K35 to R38 replaced with Ala. (E) Immunofluorescence staining of the point mutants at SRC-3 C-NLS.
Next, we examined whether the second NLS is functional in cells. Immunofluorescence staining and fluorescent imaging of SRC-3-ΔC-NLS and GFP-SRC-3-ΔC-NLS, respectively, were analyzed in both 293 and HeLa cells (Fig. 4B). SRC-3-ΔC-NLS was localized in the cytoplasm in these experiments (Fig. 4B). In addition, the four basic residues in C-NLS were mutated to Ala in the GFP fusion protein [GFP-SRC-3-A4(35-38)], and this mutant appeared in the cytoplasm as well, substantiating that C-NLS is required for SRC-3 nuclear localization (Fig. 4B).
One of the criteria for an NLS sequence is that when the NLS is fused to another cytoplasmic protein, it is capable of driving the fusion protein into the nuclear compartment. To test whether N-NLS and C-NLS are capable of this as well, a short peptide containing the N-NLS and C-NLS of SRC-3 was fused to GFP (GFP-NLS), while GFP by itself appears in both the nucleus and the cytosol (Fig. 4C). The fusion protein, GFP-NLS, was detected only in the nucleus, while GFP-N-NLS and GFP-C-NLS, which lack C-NLS and N-NLS sequences, respectively, were detected in both the nucleus and the cytosol (Fig. 4C). This confirmed that SRC-3 contains a bona fide bipartite NLS.
In addition, we further studied point mutations in C-NLS and potential critical residues surrounding C-NLS in terms of proteasome-dependent turnover and subcellular localization. Unlike the point mutants in N-NLS (Fig. 1C and D), each of the single point mutants in C-NLS was unstable and responsive to MG132 similarly to SRC-3 wt, but mutations of all four basic residues (A4) resulted in stabilization of this mutant (Fig. 4D). As expected, similarly to SRC-3 wt, all of these single point mutants in C-NLS remained in the nucleus (Fig. 4E).
SV40 T antigen NLS rescues the cytoplasmic localized mutant of SRC-3 as well as its proteasome-dependent turnover.
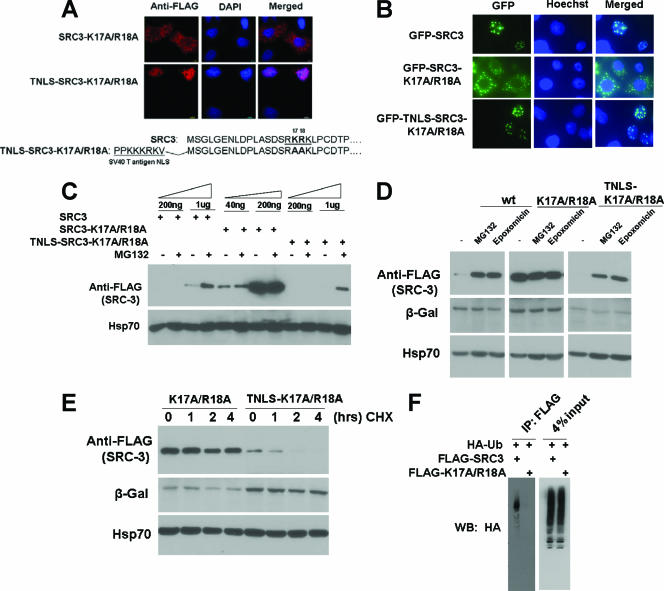
If nuclear or cytoplasmic localization is one of the prime determinants of SRC-3's proteasome-dependent stability, we reasoned that if an SRC-3 cytoplasmic mutant could be driven back into the nucleus using a heterologous NLS, it should be able to recover its capacity for turnover. To test this, a well-known SV40 TNLS was fused to the N terminus of the SRC-3 K17A/R18A mutant (TNLS-SRC-3-K17A/R18A), and the new fusion protein was expressed in cells. Immunofluorescence staining revealed that TNLS-SRC-3-K17A/R18A was localized in the nucleus, in contrast to SRC3-K17A/R18A, which was localized in the cytosol (Fig. 5A). This result was confirmed additionally by the GFP detection method. In 293 live cells cultured in a complete-serum medium, GFP-SRC-3 wt appeared in the nucleus, GFP-SRC-3-K17A/R18A was present in the cytoplasm, and GFP-TNLS-SRC-3-K17A/R18A again was back in the nucleus (data not shown). In HeLa cells, the same observation was obtained by fixation of the GFP fusion proteins in cells (Fig. 5B).
FIG. 5.
Rescue of SRC-3 cytoplasmic mutant by SV40 TNLS and recovery of its proteasome-dependent turnover. (A) SV40 TNLS fusion with FLAG-SRC-3-K17A/R18A was analyzed in HeLa cells by immunofluorescence staining. At bottom, sequences of the SRC-3 cytoplasmic mutant and TNLS are shown. (B) GFP fusions with SRC-3 wt, the K17A/R18A mutant, or TNLS-SRC-3-K17A/R18A were detected in HeLa cells. (C) 293 cells were transfected with increasing amounts (as indicated at the top) of plasmids for FLAG-tagged SRC-3 wt (SRC3), SRC-3-K17A/R18A, or TNLS-SRC-3-K17A/R18A. After treatment of MG132, cell extracts were analyzed by Western blotting. (D) Epoxomicin, a highly specific proteasome inhibitor, was used to treat cells expressing FLAG-tagged SRC-3 wt, the K17A/R18A mutant, or the TNLS-K17A/R18A mutant, followed by Western analysis. (E) CHX was used to treat cells expressing FLAG-tagged SRC-3-K17A/R18A (K17A/R18A) or TNLS-SRC-3-K17A/R18A (TNLS-K17A/R18A) mutants for the indicated times, followed by Western analysis. (F) FLAG-tagged SRC-3 wt or its K17A/R18A mutant was transfected along with HA-Ub into 293 cells, followed by IP with FLAG antibody and Western blotting (WB) with HA antibody.
We next asked whether TNLS-SRC-3-K17A/R18A recovers its proteasome-dependent turnover. Since levels of protein stability can be different for different SRC-3 mutant constructs, transient transfections were carried out with increasing amounts of plasmids to express FLAG-tagged SRC-3 wt (SRC-3), the FLAG-tagged SRC-3-K17A/R18A double mutant (SRC-3-K17A/R18A), or FLAG-tagged TNLS-SRC-3-K17A/R18A (TNLS-SRC-3-K17A/R18A) in 293 cells. After treatment with MG132, Western analysis was carried out using an anti-FLAG antibody. As shown before, SRC-3 wt showed responsiveness to MG132, but the double mutant did not. In contrast, TNLS-SRC-3-K17A/R18A recovered its wild-type responsiveness to MG132, compared to the double mutant (Fig. 5C). These results substantiate that proteasome-dependent turnover of SRC-3 occurs in the nucleus. Furthermore, epoxomicin, a highly specific irreversible proteasome inhibitor, was used to test proteasome-dependent turnover of SRC-3. Both SRC-3 wt and the TNLS fusion mutant showed similar responses to both MG132 and epoxomicin, whereas the SRC-3 K17A/R18A mutant was not responsive to either MG132 or epoxomicin (Fig. 5D). In addition, TNLS-SRC-3-K17A/R18A protein decay was examined in a CHX treatment experiment. As shown above, SRC-3-K17A/R18A was stable after treatment with CHX for 4 h, but when it was fused to TNLS, this mutant decayed dramatically after 2 h (Fig. 5E). Furthermore, we examined the ubiquitination status of SRC-3 wt and its K17A/R18A cytoplasmic mutant by transfecting each expression plasmid and HA-Ub. After immunoprecipitation of SRC-3 proteins, we observed a strong signal from SRC-3 wt but very little signal was observed with the cytoplasmic mutant (Fig. 5F).
SRC-3 cytoplasmic mutants are inactive in transcription and cell growth.
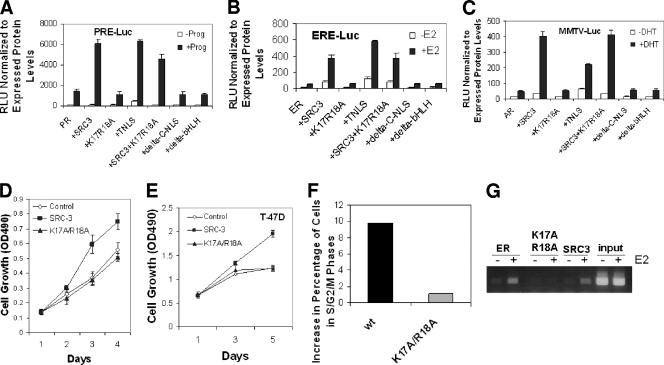
To assess the effects of the SRC-3 cytoplasmic localized mutants on steroid receptor-mediated transcription, progesterone receptor (PR), ER, and androgen receptor (AR) were tested with progesterone-responsive element luciferase reporter (PRE-luc), estrogen-responsive element luciferase reporter (ERE-luc), and mouse mammary tumor virus luciferase reporter (MMTV-luc), respectively. Transfection of plasmids for each receptor and its respective reporter was performed in HeLa cells, in combinations with SRC-3 wt, the SRC-3-K17A/R18A mutant, TNLS-SRC-3-K17A/R18A, SRC-3 wt and the K17A/R18A mutant together, the deletion mutant of C-NLS, or the deletion mutant of bHLH, in the presence or absence of each hormone, progesterone, E2, or DHT, respectively. The luciferase activities revealed that SRC-3 wt coactivated receptors in a hormone-dependent manner. In contrast, the K17A/R18A, ΔC-NLS, and bHLH mutants were not able to activate the reporters under any conditions. However, the TNLS-K17A/R18A mutant regained its coactivation activity (Fig. 6A, B, and C). Similar results were obtained using the single point mutants (data not shown). We also have not observed a “dominant negative” effect of the cytoplasmic SRC-3 mutant. This is likely due to the fact that stable coactivator complex formation occurs only in the nucleus at promoters following signal-dependent activation.
FIG. 6.
Effects of SRC-3 mutants on receptor-dependent luciferase reporters and cancer cell growth. (A) Luciferase assays using PRE-luc. HeLa cells were transfected with PR and PRE-luc either alone or together with SRC-3 wt (+SRC3), its K17A/R18A mutant (+K17R18A), the TNLS fusion with this mutant (+TNLS), combined SRC-3 and K17R18A (+SRC3+K17R18A), deletion of C-NLS (+delta-C-NLS), or deletion of bHLH (+delta-bHLH), followed by treatment with (+Prog) or without (−Prog) progesterone and analyses of relative luciferase activities (RLU) normalized to expressed protein levels. (B) The procedure was the same as that described for panel A, except that ER, ERE-luc, and hormone estrogen (E2) were used. (C) The procedure was the same as that described for panel A, except that AR, MMTV-luc, and hormone DHT were used. (D) Effect of SRC-3 wt and its K17A/R18A mutant on 293 cell growth. Equal numbers of 293 host cells (Control) and tetracycline-inducible 293 cells expressing SRC-3 wt (SRC-3) or its K17A/R18A mutant (K17A/R18A) were seeded into 96-well plates. One day after induction with tetracycline, cell proliferation was determined as described in Materials and Methods. OD490, optical density at 490 nm. (E) T47D breast cancer cells overexpressing SRC-3 wt or its cytoplasmic mutant (see Materials and Methods) were examined for cell growth using the method described for panel D. (F) Cell cycle analysis of cells described for panel E. Increases in percentage of cells in S/G2/M phases were analyzed for SRC-3 wt or its mutant (see Materials and Methods). (G) ChIP analysis of cells described for panel E. Recruitments of ER, SRC-3 wt, or its cytoplasmic mutant on the pS2 promoter were examined.
Finally, we examined the effect of SRC-3 wt and its K17A/R18A cytoplasmic mutant on cell growth. Using the tetracycline-inducible stable cell lines expressing SRC-3 wt or its K17A/R18A mutant, we observed a significant increase in cell growth by SRC-3 wt, but not by the K17A/R18A mutant, after 2 days of induction (Fig. 6D). A similar result was obtained when using the breast cancer cell line T47D (Fig. 6E). In addition, the cell cycle was examined in T47D cells and showed an increase of cells in S/G2/M phases for the wild type but virtually no change for the mutant (Fig. 6F). We also monitored recruitment of ER, SRC-3 wt, and its mutant to an ER target gene. As expected, ER and SRC-3 wt were recruited onto the promoter but the cytoplasmic mutant was not (Fig. 6G). These results clearly indicate that cytoplasmic localized mutants of SRC-3 are not biologically active in inducing cell growth or inducing target gene expression.
DISCUSSION
It has been reported previously that posttranscriptional mechanisms play an important role in regulation of the steady-state levels of SRC-3 protein (31, 35, 36, 69). Treatment with MG132, a proteasome inhibitor, resulted in elevation of the level of the SRC/p160 family proteins, indicating that a proteasome-dependent degradation of SRC protein occurs in cells (35, 36). In this study, we observed the same effect of MG132 on SRC-3 by using multiple cell lines. Experiments with another highly specific proteasome inhibitor, epoxomicin, a natural product inhibitor with higher proteasome specificity than MG132, also supported that SRC-3 protein turnover is indeed proteasome dependent. It is known that the ubiquitin and proteasome pathways in mammalian cells are usually coupled to regulate target protein degradation (16, 58). It was reported that degradation of SRC-3 protein is impaired in the temperature-sensitive UBA-defective ts85 cell line, indicating that the ubiquitin-activating enzyme (UBA) is necessary for normal SRC-3 degradation (35). Moreover, ubiquitination of other coactivators as well as SRC-3 has been reported previously (42, 67). Therefore, it is likely that the proteasome-mediated turnover of SRC-3, at least in part, follows the ubiquitination of SRC-3. In addition, however, it has been reported recently that ubiquitin-independent degradation of SRC-3 by proteasomes also occurs and contributes to maintenance of appropriate levels of this oncogene (31).
Using SRC-3 mutants, we discovered that some were much more stable in cells and did not respond to MG132 treatment. We eventually identified two amino acid residues, K17 and R18, that are essential for responsiveness to MG132. These two residues are in the bHLH domain and are highly conserved in the SRC family, suggesting that similar determinants in SRC-1 and SRC-2 proteins may exist. Moreover, we identified the essential bipartite NLS sequences in the bHLH domain of SRC-3. There was a precise correlation between SRC-3 stability and its subcellular localization: it is stable in the cytoplasm and unstable in the nucleus. Using an SV40 T antigen NLS fusion protein, we further demonstrated that SRC-3 turnover by proteasomes occurs in the nucleus. In addition, using GFP fusion to SRC-3 NLS peptides in cells treated with MG132, we did not detect GFP-SRC-3-NLS responsiveness to MG132, while GFP-SRC-3 behaved in the same way as FLAG-SRC-3 by Western analysis (data not shown). This result suggests that the proteasome-dependent turnover factors and the nuclear localization factors for SRC-3 are not identical. We also examined the subcellular localization of GFP-SRC-3 in the presence or absence of MG132. Under the both conditions, SRC-3 protein remained in the nucleus (data not shown). Although the SRC-3 cytoplasmic mutant showed no response to MG132, our result does not preclude some proteasome-independent degradation in the cytoplasm. Indeed, our pulse-chase experiment showed that there was a much slower degradation of the SRC-3 K17A/R18A cytoplasmic mutant than of SRC-3 wt (Fig. 3B).
It has been reported that proteasomes are localized in both the nucleus and the cytosol in eukaryotic cells, while in Schizosaccharomyces pombe the majority of proteasomes are nuclear and/or may accumulate at the inner nuclear envelope and the endoplasmic reticulum (12, 64). Many of the enzymatic components of the ubiquitin system, such as E1, have nuclear localization signals (19, 38) and have been identified in the nucleus (51). It has been reported previously that intracellular proteins can be degraded either in the nucleus and/or in the cytoplasm (16). Some examples of nuclear degraded transcription factors are dioxin receptor (49), MyoD (13), Gcn4p (46), POU4F3 (63), and Msn2 (11). Although we have demonstrated that proteasome-dependent turnover of SRC-3 occurs in the nucleus, it is unclear precisely where its turnover occurs in the nuclear subcompartments. For example, it is known that proteasome functions are required for efficient continued steroid receptor regulation of transcription (36, 45). A key question is whether this proteasome-dependent turnover is tightly coupled to the promoter-bound complexes or whether it is a downstream nuclear event that follows activation of transcription. The destruction of SRC-3 in the nucleus may serve as a part of a use-and-destroy strategy to limit the overall action of coactivators in gene expression. Additional SRC-3 molecules residing in an inactive state would thus be preserved for later activation and transcriptional use.
Given that there are proteasomes in the cytoplasm (16, 58), it remains largely unknown why SRC-3 is not degraded effectively in that compartment. It is accepted that proteasomes are heterogeneous in cells. Also, an additional interacting factor(s) required for SRC-3 to be accessible to and be degraded by the ubiquitin/proteasome pathway may not be present in the cytoplasm; alternatively, a negative inhibitory factor(s) could bind to SRC-3 in the cytoplasm and block proteasome-dependent degradation. Using transient transfection of exogenous HA-Ub, we observed a significant reduction in the amount of ubiquitinated SRC-3 for the cytoplasmic mutant relative to that for its wild type (Fig. 5F), suggesting that diminished cytoplasmic SRC-3 ubiquitination may account in part for stabilization of the SRC-3 cytoplasmic mutants. Finally, ubiquitin-independent proteasomal degradation of SRC-3 also is a contributor to its nuclear turnover (31).
Available data on subcellular localization of the SRC family proteins are contradictory (1, 8, 25, 47, 53, 62, 65, 74). These variations may be attributed to different cell culture conditions (i.e., serum or serum-free), cell differentiation state, and different detection methods. In this study and under full-serum culture conditions, SRC-3 wt is localized in the nucleus, which is the consensus result among all other reports.
Degradation of a number of transcription factors has been shown to be physiologically regulated. Protein transport into the nucleus also is regulated in numerous cases. Phosphorylation is a well-known modification that can either enhance or inhibit target protein stability as well as protein import or export via regulating the function of NLS (22). A well-documented example is the protein kinase A phosphorylation of c-rel, an oncoprotein transcription factor, thereby enhancing c-rel NLS function (40). SRC-3 protein is a target for phosphorylation in the cytoplasm by various kinases in complexes containing estrogen receptor (66, 72). To date, we have not been able to determine the role that phosphorylation plays in the regulation of the bipartite NLS of SRC-3.
As might be expected, we demonstrated that SRC-3 cytoplasmic point mutants lose their coactivation capacity for ER-, AR-, and PR-mediated transcription. Rescue of the K17A/R18A cytoplasmic mutant using the SV40 T antigen NLS allows recovery of coactivation potential (Fig. 6). Since the receptor interaction and transactivation domains of SRC-3 remain intact when the bipartite NLS is mutated, it is likely that the NLS mutants of SRC-3 would still function under cell-free conditions. By use of cell-free chromatin transcription assays, an N-terminally deleted form of SRC-1, which lacks the bHLH domain, exhibited a coactivation of PR-dependent transcription comparable to that of wild-type SRC-1 (34).
The larger N-terminal bHLH-PAS domain has previously been reported to be essential for CoCoA interaction and coactivation of NR (26), as well as dimerization between SRC proteins and differential regulation of target genes (71). Therefore, the N-terminal region of SRC-3 appears to have multiple functions. Nevertheless, other studies produced mixed results as to whether the bHLH-PAS domain was necessary for SRC coactivator function (3, 7, 48). Certain discrepancies may occur due to use of transient transfection and luciferase reporter assays as the experimental design does not account for expressed protein levels. As we showed above (Fig. 5C), the cytoplasmic mutant of SRC-3 is stable and expressed at levels dramatically increased relative to wt levels when transfecting comparable amounts of plasmid DNA.
SRC-3/AIB1 is an oncogene (56). Recent findings demonstrate that regulation of SRC-3 by proteasomes is an important aspect of steroid receptor signaling. In this report, we identified a specific intrinsic determinant in the bHLH domain that is essential for subcellular localization and proteasome-dependent stability of SRC-3 in the nucleus. This signal represents another piece of the puzzle by which the function of this potent oncogene is subject to temporal and spatial regulation and cellular trafficking.
ADDENDUM
While this paper was under review, Yeung et al. (68) published a paper on SRC-3's bipartite NLS with a conclusion similar to that reported here.
Acknowledgments
This work was supported by NIH grants (NICHD; NURSA/NIDDK) to B.W.O. C.L. was a recipient of a postdoctoral fellowship from the U.S. Department of Defense Breast Cancer Research Program. M.-J.T. is supported by the Baylor Prostate SPORE.
We thank Fuzhong F. Zheng for providing some plasmids and constructive discussions, David M. Lonard for critical reading of the manuscript, and Jianming Xu for providing access to lab equipment. We are also grateful to Dirk Bohmann (University of Rochester) for kindly providing the expression vector for HA-Ub.
Footnotes
Published ahead of print on 11 December 2006.
REFERENCES
- 1.Amazit, L., Y. Alj, R. K. Tyagi, A. Chauchereau, H. Loosfelt, C. Pichon, J. Pantel, E. Foulon-Guinchard, P. Leclerc, E. Milgrom, and A. Guiochon-Mantel. 2003. Subcellular localization and mechanisms of nucleocytoplasmic trafficking of steroid receptor coactivator-1. J. Biol. Chem. 278:32195-32203. [DOI] [PubMed] [Google Scholar]
- 2.Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965-968. [DOI] [PubMed] [Google Scholar]
- 3.Belandia, B., and M. G. Parker. 2000. Functional interaction between the p160 coactivator proteins and the transcriptional enhancer factor family of transcription factors. J. Biol. Chem. 275:30801-30805. [DOI] [PubMed] [Google Scholar]
- 4.Blondel, M., M. Galan, Y. Chi, C. Lafourcade, C. Longaretti, R. J. Deshaies, and M. Peter. 2000. Nuclear-specific degradation of Far1 is controlled by localization of the F-box protein Cdc4. EMBO J. 19:6085-6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, K. T., A. L. Goldberg, and S. K. Nigam. 1997. Proteasome inhibition leads to a heat-shock response induction of endoplasmic reticulum chaperones and thermotolerance. J. Biol. Chem. 272:9086-9092. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D., H. Ma, H. Hong, S. S. Koh, S. M. Huang, B. T. Schurter, D. W. Aswad, and M. R. Stallcup. 1999. Regulation of transcription by a protein methyltransferase. Science 284:2174-2177. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., R. J. Lin, R. L. Schiltz, D. Chakravarti, A. Nash, L. Nagy, M. L. Privalsky, Y. Nakatani, and R. M. Evans. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569-580. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S. L., S. C. Wang, B. Hosking, and G. E. Muscat. 2001. Subcellular localization of the steroid receptor coactivators (SRCs) and MEF2 in muscle and rhabdomyosarcoma cells. Mol. Endocrinol. 15:783-796. [DOI] [PubMed] [Google Scholar]
- 9.Chuang, L.-C., and P. R. Yew. 2001. Regulation of nuclear transport and degradation of the Xenopus cyclin-dependent kinase inhibitor p27Xic1. J. Biol. Chem. 276:1610-1617. [DOI] [PubMed] [Google Scholar]
- 10.Conaway, R. C., C. S. Brower, and J. W. Conaway. 2002. Emerging roles of ubiquitin in transcription regulation. Science 296:1254-1258. [DOI] [PubMed] [Google Scholar]
- 11.Durchschlag, E., W. Reiter, G. Ammerer, and C. Schuller. 2004. Nuclear localization destabilizes the stress-regulated transcription factor Msn2. J. Biol. Chem. 279:55425-55432. [DOI] [PubMed] [Google Scholar]
- 12.Enenkel, C., A. Lehmann, and P. M. Kloetzel. 1998. Subcellular distribution of proteasomes implicates a major location of protein degradation in the nuclear envelope-ER network in yeast. EMBO J. 17:6144-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floyd, Z. E., J. S. Trausch-Azar, E. Reinstein, A. Ciechanover, and A. L. Schwartz. 2001. The nuclear ubiquitin-proteasome system degrades MyoD. J. Biol. Chem. 276:22468-22475. [DOI] [PubMed] [Google Scholar]
- 14.Font de Mora, J., and M. Brown. 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol. Cell. Biol. 20:5041-5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 16.Glickman, M. H., and A. Ciechanover. 2002. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol. Rev. 82:373-428. [DOI] [PubMed] [Google Scholar]
- 17.Gregory, C. W., B. He, R. T. Johnson, O. H. Ford, J. L. Mohler, F. S. French, and E. M. Wilson. 2001. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 61:4315-4319. [PubMed] [Google Scholar]
- 18.Halachmi, S., E. Marden, G. Martin, H. MacKay, C. Abbondanza, and M. Brown. 1994. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science 264:1455-1458. [DOI] [PubMed] [Google Scholar]
- 19.Handley, P. M., M. Mueckler, N. R. Siegel, A. Ciechanover, and A. L. Schwartz. 1991. Molecular cloning, sequence, and tissue distribution of the human ubiquitin-activating enzyme, E1. Proc. Natl. Acad. Sci. USA 88:258-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 21.Hong, H., K. Kohli, A. Trivedi, D. L. Johnson, and M. R. Stallcup. 1996. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl. Acad. Sci. USA 93:4948-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jans, D. A., and S. Hubner. 1996. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol. Rev. 76:651-685. [DOI] [PubMed] [Google Scholar]
- 23.Jans, D. A., L. J. Briggs, S. E. Gustin, P. Jans, S. Ford, and I. G. Young. 1997. A functional bipartite nuclear localisation signal in the cytokine interleukin-5. FEBS Lett. 406:315-320. [DOI] [PubMed] [Google Scholar]
- 24.Kershah, S. M., M. M. Desouki, K. L. Koterba, and B. G. Rowan. 2004. Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol. Oncol. 92:304-313. [DOI] [PubMed] [Google Scholar]
- 25.Kim, H. J., J. Y. Yi, H. S. Sung, D. D. Moore, B. H. Jhun, Y. C. Lee, and J. W. Lee. 1999. Activating signal cointegrator 1, a novel transcription coactivator of nuclear receptors, and its cytosolic localization under conditions of serum deprivation. Mol. Cell. Biol. 19:6323-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim, J. H., H. Li, and M. R. Stallcup. 2003. CoCoA, a nuclear receptor coactivator which acts through an N-terminal activation domain of p160 coactivators. Mol. Cell 12:1537-1549. [DOI] [PubMed] [Google Scholar]
- 27.Kuang, S. Q., L. Liao, H. Zhang, A. V. Lee, B. W. O'Malley, and J. Xu. 2004. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 64:1875-1885. [DOI] [PubMed] [Google Scholar]
- 28.Lanning, C. C., J. L. Daddona, R. Ruiz-Velasco, S. H. Shafer, and C. L. Williams. 2004. The Rac1 C-terminal polybasic region regulates the nuclear localization and protein degradation of Rac1. J. Biol. Chem. 279:44197-44210. [DOI] [PubMed] [Google Scholar]
- 29.Lee, D. H., and A. L. Goldberg. 1998. Proteasome inhibitors cause induction of heat shock proteins and trehalose, which together confer thermotolerance in Saccharomyces cerevisiae. Mol. Cell. Biol. 18:30-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, H., P. J. Gomes, and J. D. Chen. 1997. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. USA 94:8479-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, X., D. M. Lonard, S. Y. Jung, A. Malovannaya, Q. Feng, J. Qin, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 2006. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell 124:381-392. [DOI] [PubMed] [Google Scholar]
- 32.Liang, S.-H., and M. F. Clarke. 1999. A bipartite nuclear localization signal is required for p53 nuclear import regulated by a carboxyl-terminal domain. J. Biol. Chem. 274:32699-32703. [DOI] [PubMed] [Google Scholar]
- 33.List, H. J., R. Reiter, B. Singh, A. Wellstein, and A. T. Riegel. 2001. Expression of the nuclear coactivator AIB1 in normal and malignant breast tissue. Breast Cancer Res. Treat. 68:21-28. [DOI] [PubMed] [Google Scholar]
- 34.Liu, Z., J. Wong, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 2001. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc. Natl. Acad. Sci. USA 98:12426-12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lonard, D. M., S. Y. Tsai, and B. W. O'Malley. 2004. Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol. Cell. Biol. 24:14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lonard, D. M., Z. Nawaz, C. L. Smith, and B. W. O'Malley. 2000. The 26S proteasome is required for estrogen receptor-alpha and coactivator turnover and for efficient estrogen receptor-alpha transactivation. Mol. Cell 5:939-948. [DOI] [PubMed] [Google Scholar]
- 37.Mangelsdorf, D. J., C. Thummel, M. Beato, P. Herrlich, G. Schutz, K. Umesono, B. Blumberg, P. Kastner, M. Mark, P. Chambon, and R. M. Evans. 1995. The nuclear receptor superfamily: the second decade. Cell 83:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrath, J. P., S. Jentsch, and A. Varshavsky. 1991. UBAl: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J. 10:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 40.Mosialos, G., P. Hamer, A. J. Capobianco, R. A. Laursen, and T. D. Gilmore. 1991. A protein kinase-A recognition sequence is structurally linked to transformation by p59v-rel and cytoplasmic retention of p68c-rel. Mol. Cell. Biol. 11:5867-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muratani, M., and W. P. Tansey. 2003. How the ubiquitin-proteasome system controls transcription. Nat. Rev. Mol. Cell Biol. 4:192-201. [DOI] [PubMed] [Google Scholar]
- 42.Nawaz, Z., and B. W. O'Malley. 2004. Urban renewal in the nucleus: is protein turnover by proteasomes absolutely required for nuclear receptor-regulated transcription? Mol. Endocrinol. 18:493-499. [DOI] [PubMed] [Google Scholar]
- 43.Onate, S. A., S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354-1357. [DOI] [PubMed] [Google Scholar]
- 44.Onate, S. A., V. Boonyaratanakornkit, T. E. Spencer, S. Y. Tsai, M.-J. Tsai, D. P. Edwards, and B. W. O'Malley. 1998. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J. Biol. Chem. 273:12101-12108. [DOI] [PubMed] [Google Scholar]
- 45.Perissi, V., A. Aggarwal, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2004. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 116:511-526. [DOI] [PubMed] [Google Scholar]
- 46.Pries, R., K. Bomeke, S. Irniger, O. Grundmann, and G. H. Braus. 2002. Amino acid-dependent Gcn4p stability regulation occurs exclusively in the yeast nucleus. Eukaryot. Cell 1:663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qutob, M. S., R. N. Bhattacharjee, E. Pollari, S. P. Yee, and J. Torchia. 2002. Microtubule-dependent subcellular redistribution of the transcriptional coactivator p/CIP. Mol. Cell. Biol. 22:6611-6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiter, R., A. Wellstein, and A. T. Riegel. 2001. An isoform of the coactivator AIB1 that increases hormone and growth factor sensitivity is overexpressed in breast cancer. J. Biol. Chem. 276:39736-39741. [DOI] [PubMed] [Google Scholar]
- 49.Roberts, B. J., and M. L. Whitelaw. 1999. Degradation of the basic helix-loop-helix/Per-ARNT-Sim homology domain dioxin receptor via the ubiquitin/proteasome pathway. J. Biol. Chem. 274:36351-36356. [DOI] [PubMed] [Google Scholar]
- 50.Sakakura, C., A. Hagiwara, R. Yasuoka, Y. Fujita, M. Nakanishi, K. Masuda, A. Kimura, Y. Nakamura, J. Inazawa, T. Abe, and H. Yamagishi. 2000. Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers. Int. J. Cancer 89:217-223. [PubMed] [Google Scholar]
- 51.Schwartz, A. L., J. S. Trausch, A. Ciechanover, J. W. Slot, and H. Geuze. 1992. Immunoelectron microscopic localization of the ubiquitin-activating enzyme E1 in HepG2 cells. Proc. Natl. Acad. Sci. USA 89:5542-5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer, T. E., G. Jenster, M. M. Burcin, C. D. Allis, J. Zhou, C. A. Mizzen, N. J. McKenna, S. A. Onate, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1997. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194-198. [DOI] [PubMed] [Google Scholar]
- 53.Stenoien, D. L., M. G. Mancini, K. Patel, E. A. Allegretto, C. L. Smith, and M. A. Mancini. 2000. Subnuclear trafficking of estrogen receptor-alpha and steroid receptor coactivator-1. Mol. Endocrinol. 14:518-534. [DOI] [PubMed] [Google Scholar]
- 54.Takeshita, A., G. R. Cardona, N. Koibuchi, C. S. Suen, and W. W. Chin. 1997. TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J. Biol. Chem. 272:27629-27634. [DOI] [PubMed] [Google Scholar]
- 55.Torchia, J., D. W. Rose, J. Inostroza, Y. Kamei, S. Westin, C. K. Glass, and M. G. Rosenfeld. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677-684. [DOI] [PubMed] [Google Scholar]
- 56.Torres-Arzayus, M. I., J. Font de Mora, J. Yuan, F. Vazquez, R. Bronson, M. Rue, W. R. Sellers, and M. Brown. 2004. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6:263-274. [DOI] [PubMed] [Google Scholar]
- 57.Tsai, M.-J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 58.Varshavsky, A. 2005. Regulated protein degradation. Trends Biochem. Sci. 30:283-286. [DOI] [PubMed] [Google Scholar]
- 59.Voegel, J. J., M. J. Heine, C. Zechel, P. Chambon, and H. Gronemeyer. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 15:3667-3675. [PMC free article] [PubMed] [Google Scholar]
- 60.Voegel, J. J., M. J. Heine, M. Tini, V. Vivat, P. Chambon, and H. Gronemeyer. 1998. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 17:507-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, Y., M. C. Wu, J. S. Sham, W. Zhang, W. Q. Wu, and X. Y. Guan. 2002. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 95:2346-2352. [DOI] [PubMed] [Google Scholar]
- 62.Wang, Z., D. W. Rose, O. Hermanson, F. Liu, T. Herman, W. Wu, D. Szeto, A. Gleiberman, A. Krones, K. Pratt, R. Rosenfeld, C. K. Glass, and M. G. Rosenfeld. 2000. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 97:13549-13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss, S., I. Gottfried, I. Mayrose, S. L. Khare, M. Xiang, S. J. Dawson, and K. B. Avraham. 2003. The DFNA15 deafness mutation affects POU4F3 protein stability, localization, and transcriptional activity. Mol. Cell. Biol. 23:7957-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson, C. R., M. Wallace, M. Morphew, P. Perry, R. Allshire, J. P. Javerzat, J. R. McIntosh, and C. Gordon. 1998. Localization of the 26S proteasome during mitosis and meiosis in fission yeast. EMBO J. 17:6465-6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu, R. C., J. Qin, Y. Hashimoto, J. Wong, J. Xu, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 2002. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol. Cell. Biol. 22:3549-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, R. C., J. Qin, P. Yi, J. Wong, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 2004. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic responses to multiple cellular signaling pathways. Mol. Cell 15:937-949. [DOI] [PubMed] [Google Scholar]
- 67.Yan, F., X. Gao, D. M. Lonard, and Z. Nawaz. 2003. Specific ubiquitin-conjugating enzymes promote degradation of specific nuclear receptor coactivators. Mol. Endocrinol. 17:1315-1331. [DOI] [PubMed] [Google Scholar]
- 68.Yeung, P. L., A. Zhang, and J. D. Chen. 2006. Nuclear localization of coactivator RAC3 is mediated by a bipartite NLS and importin alpha3. Biochem. Biophys. Res. Commun. 348:13-24. [DOI] [PubMed] [Google Scholar]
- 69.Yi, P., R. C. Wu, J. Sandquist, J. Wong, S. Y. Tsai, M.-J. Tsai, A. R. Means, and B. W. O'Malley. 2005. Peptidyl-prolyl isomerase 1 (Pin1) serves as a coactivator of steroid receptor by regulating the activity of phosphorylated steroid receptor coactivator 3 (SRC-3/AIB1). Mol. Cell. Biol. 25:9687-9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zacksenhaus, E., R. Bremner, R. A. Phillips, and B. L. Gallie. 1993. A bipartite nuclear localization signal in the retinoblastoma gene product and its importance for biological activity. Mol. Cell. Biol. 13:4588-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, H., X. Yi, X. Sun, N. Yin, B. Shi, H. Wu, D. Wang, G. Wu, and Y. Shang. 2004. Differential gene regulation by the SRC family of coactivators. Genes Dev. 18:1753-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng, F. F., R. C. Wu, C. L. Smith, and B. W. O'Malley. 2005. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol. Cell. Biol. 25:8273-8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou, G., Y. Hashimoto, I. Kwak, S. Y. Tsai, and M.-J. Tsai. 2003. Role of the steroid receptor coactivator SRC-3 in cell growth. Mol. Cell. Biol. 23:7742-7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zilliacus, J., E. Holter, H. Wakui, H. Tazawa, E. Treuter, and J. A. Gustafsson. 2001. Regulation of glucocorticoid receptor activity by 14-3-3-dependent intracellular relocalization of the corepressor RIP140. Mol. Endocrinol. 15:501-511. [DOI] [PubMed] [Google Scholar]