Abstract
Myocardin, a serum response factor (SRF)-dependent cofactor, is a potent activator of smooth muscle gene activity but a poor activator of cardiogenic genes in pluripotent 10T1/2 fibroblasts. Posttranslational modification of GATA4, another myocardin cofactor, by sumoylation strongly activated cardiogenic gene activity. Here, we found that myocardin's activity was strongly enhanced by SUMO-1 via modification of a lysine residue primarily located at position 445 and that the conversion of this residue to arginine (K445R) impaired myocardin transactivation. PIAS1 was involved in governing myocardin activity via its E3 ligase activity that stimulated myocardin sumoylation on an atypical sumoylation site(s) and by its physical association with myocardin. Myocardin initiated the expression of cardiac muscle-specified genes, such as those encoding cardiac α-actin and α-myosin heavy chain, in an SRF-dependent manner in 10T1/2 fibroblasts, but only in the presence of coexpressed SUMO-1/PIAS1. Thus, SUMO modification acted as a molecular switch to promote myocardin's role in cardiogenic gene expression.
SUMOs (small ubiquitin-like modifiers) are implicated in numerous physiological and pathological processes through altering the functions of its target proteins. SUMO covalent linkage is usually through the lysine residue(s) in the consensus sequence ψKXE (ψ stands for a bulky hydrophobic amino acid, and X represents any residue) (37, 47). Some targets are involved with gene regulation, such as the coactivator P300 (14), the corepressor CtBP (32), and other transcription factors (38). SUMOs govern transcription target activity by altering DNA binding activity, subcellular localization, and/or protein stability. In contrast to what is seen for invertebrates, which have only one SUMO gene, at least four members of the SUMO gene family have been identified in vertebrates (7, 13, 16, 25). SUMO-1 has about 50% homology to SUMO-2, -3, and -4. These SUMO members exert preferential target activities. For instance, SUMO modulation of RanGAP1 was favored by SUMO-1 but poorly modified by SUMO-2/-3 (49), while SUMO-2/-3 became highly active upon oxidative stress but SUMO-1 did not (49); thus, distinct roles for SUMO members under physiological and pathological states are exhibited.
SUMO conjugation is part of an enzymatic cascade basically involving two enzymes, heterodimer E1-activating enzyme (SAE1/2) and E2-conjugating enzyme (Ubc9). Unlike ubiquitination, some sumoylation assays revealed that in the presence of E1 and E2, the E3 ligase was dispensable to accomplish SUMO conjugation. However, SUMO E3 ligases, such as PIAS1, contributed to the efficiency and specificity of SUMO conjugation (29, 57) and were attributed to the RING domain, which is similar to the corresponding structure in E3 ligases involved in the ubiquitination. The following three distinct classes of E3 ligase have been revealed: (i) the PIAS family (19), whose members, in addition to having their E3 ligase activity, may influence targets independently of sumoylation, as shown by PIAS1 and PIASy (11, 15); (ii) RanBP2, which is involved in nuclear transport (44); and (iii) polycomb protein 2, which is involved in gene repression (20).
Myocardin and its other related proteins, MRTF-A and MRTF-B (56), belong to a SAP superfamily (SAF-A/B, Acinus, and PIAS) harboring the chromatin-remodeling SAP domain (1). Myocardin expressed in embryonic heart and vascular smooth muscle cells was identified as a serum response factor (SRF) coactivator that triggers an SRF-dependent smooth muscle differentiation program (12, 55). Other cofactors, such as GATA4 and p300, also enhanced myocardin activity (4, 43). Inactivation of myocardin revealed a severe disruption of vascular smooth muscle development (28), demonstrating its indispensability in vascular smooth muscle cell differentiation. Myocardin has been shown to be important for normal heart development in Xenopus embryos (53, 55). On occasion, the forced expression of myocardin was able to induce the expression of some cardiac muscle-specified genes in cell lines such as human mesenchymal stem cells, foreskin fibroblasts, and L6 myoblasts (8, 54, 59). However, myocardin was not sufficient to activate cardiogenic genes in pluripotent 10T1/2 fibroblast cells (28, 58).
Recently, we reported that SUMO modification of GATA4 activated several cardiac muscle-restricted genes in 10T1/2 fibroblasts (57). In addition, SRF, a chief coaccessory factor of myocardin and GATA4, was shown to be a SUMO target (36). Since myocardin, SRF, and GATA factors are cointeractive and enriched in the heart, we asked if myocardin might also be a SUMO target. In fact, bioinformatics revealed a potential SUMO modification consensus sequence in myocardin. We then asked whether myocardin could be sumoylated and if so what the consequence for myocardin's activity would be. Here, we provide evidence that myocardin is a target for sumoylation which can be facilitated by the E3 ligase PIAS1 not only in 10T1/2 cells but also in other noncardiogenic cell types. In addition, SUMO-conjugated myocardin switched on cardiogenic gene activity in pluripotent 10T1/2 fibroblasts in an SRF-dependent fashion.
MATERIALS AND METHODS
Plasmid constructs.
The construction of cardiac α-actin promoter-driven luciferase reporters and promoter mutants was described previously (50). The construction of SUMO-1 and its defective C-terminal deletion mutant SUMO-1ΔGG was previously detailed (57). The wild-type myocardin expression vector and its insert was amplified by PCR and then ligated into the pcDNA4A-V5/(His)6 vector at the EcoRV and HindIII cleavage sites. A myocardin mutant was generated by conversion of amino acid (aa) 445 lysine to an arginine by a two-step PCR mutagenesis protocol, with oligonucleotide primers overlapping the lysine 445 mutation and terminal cDNA sequences, as described previously (57). The myocardin cDNA was inserted into pcDNA4A-V5/(His)6 and verified by sequencing DNA inserts. Glutathione S-transferase (GST)-fused myocardin was produced by the insertion of the myocardin cDNA into SmaI and NotI sites of pGEX4T-2. Plasmids for wild-type Ubc9 and its dominant negative (d/n) form, C93R/L97A, were obtained from K. Tashiro (52) and cloned into the expression vector pcDNA3.1. All other mammalian expression plasmids for deletion mutants of PIAS1 and myocardin were previously described (29, 55).
Cell culture, transfection, and trans-differentiation assays.
Transient transfections with plasmid-based expression vectors into tissue-cultured CV1 and/or HeLa cells and reporter gene assays were previously described (5, 57). Promoter activity was expressed as the ratio of luciferase reporter activity induced by coexpression of a specific factor(s) to that of the controls, represented by the expressions of empty vector. Data are expressed as the means ± standard deviations of the means from at least two independent assays, with each assay carried out in duplicate. Pluripotent 10T1/2 fibroblasts were maintained at low density (<30%) in 10% fetal bovine serum plus Dulbecco's modified Eagle's medium prior to transfection, and conditions for transfections in 10T1/2 cells followed by reverse transcription-PCR (RT-PCR) were detailed previously (57). The sequences of all specific primers used in RT-PCR assays are available upon request.
Sumoylation assays and Ni-NTA pulldown assays.
Sumoylation assays in vivo were detailed previously (57). Nickel affinity chromatography was performed according to the protocol described previously (31). Briefly, the vector encoding wild-type V5-His6-tagged myocardin was transfected into HeLa cells in the absence or presence of the SUMO-1 expression vector. After 48 h, HeLa cells were then lysed and sonicated in 6 M guanidine buffer, and six-His-tagged myocardin was immobilized on Ni-nitrilotriacetic acid (NTA) beads (QIAGEN), eluted by elution buffer (200 mM imidazole, 5% sodium dodecyl sulfate, 0.15 M Tris [pH 6.7], 30% glycerol, 0.72 M β-mercaptoethanol). Western blots were used to analyze the eluted proteins by using anti-V5 antibody to reveal the presence of myocardin. Anti-SUMO-1 antibody was used to detect SUMO-1-conjugated myocardin. All proteins were visualized by use of the SuperSignal West Pico chemiluminescent substrate from Pierce (Rockford, IL).
Coimmunoprecipitation and GST pulldown assays.
Plasmid-based expression vectors encoding V5-tagged myocardin and hemagglutinin (HA)-tagged PIAS1 were cotransfected into CV1 cells. Lysates were collected 48 h posttransfection, and HA antibody was used to precipitate HA-PIAS1. The resulting mixture was washed, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE), transferred to a polyvinylidene difluoride membrane, first revealed by V5 antibody to visualize V5 epitoped myocardin, and then striped and reprobed with HA antibody to visualize HA epitoped PIAS1. The GST pulldown assay was detailed previously (5).
Immunobiochemistry.
Immunostaining of transfected fibroblasts was performed as described previously (57). Briefly, transfected 10T1/2 cells were fixed and then probed with MF20 anti-sarcomeric α-myosin heavy chain (α-MHC) antibody from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA) and anti-V5 antibody from Bethyl Laboratories (Montgomery, TX) followed by green- and red-labeled secondary antibodies from Molecular Probes (Eugene, OR), respectively. DAPI (4′,6′-diamidino-2-phenylindole) was used to stain nuclei.
RESULTS
Myocardin activity regulated via SUMO-1 modification.
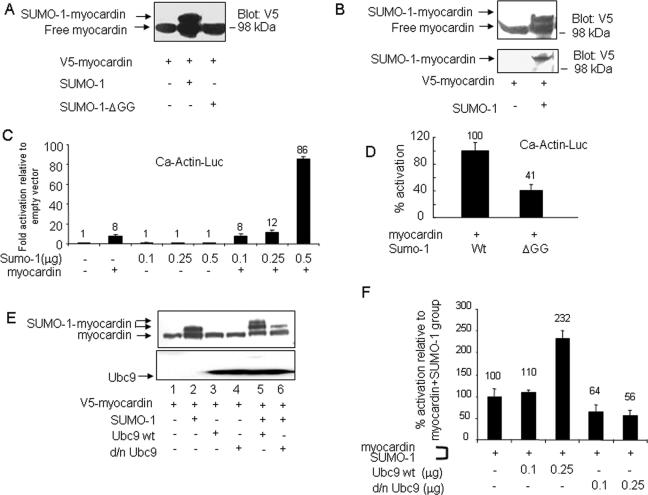
Bioinformatics revealed a myocardin SUMO modification consensus sequence that was validated by in vivo sumoylation assays. Transfections of plasmid-based expression vectors into cultured HeLa cells revealed by the combination of PAGE and immunoblots of cell lysates a well-defined but slower-migrating myocardin species, which was detected by anti-V5 antibody in the presence of Flag-SUMO-1 but was absent from samples of expressed conjugation-defective Flag-SUMO-1ΔGG (Fig. 1A). To determine if the slower-migrating species was a SUMO-1 conjugate, cellularly expressed V5-His6-myocardin was retrieved by nickel affinity chromatography, as shown by anti-V5 blotting (Fig. 1B, upper panel) and also by use of anti-SUMO-1 antibody (Fig. 1B, lower panel). SUMO-1 antibody identified a single band that reacted with both anti-V5-His6-myocardin and anti-Flag-SUMO-1. Similarly, myocardin sumoylation by SUMO-1 in vivo was observed for CV1 and 10T1/2 cells (data not shown). Thus, SUMO-1 was conjugated to V5-His6-myocardin. Similarly, myocardin was SUMO modified in vitro in the presence of SAE1/2, Ubc9, and SUMO-1 (data not shown). Taken together, these data demonstrated that myocardin is a de novo SUMO-1 substrate.
FIG. 1.
Myocardin transcriptional activity regulated via SUMO-1 conjugation. (A) Myocardin was modified by SUMO-1 in vivo. Soluble extracts taken from HeLa cells previously transfected with expression vectors were evaluated by PAGE and protein blotting with V5 antibody. (B) Expressed V5-His6-tagged myocardin alone or in the presence of SUMO-1 was isolated by nickel affinity chromatography. Both free and SUMO-1-modified myocardin forms were detected by V5 antibody (upper panel), and the latter form was also detected by SUMO-1 antibody (lower panel). Data shown are the representative from three similar assays. (C and D) SUMO-1 potentiated myocardin activity. Luciferase reporter gene activities were analyzed for CV1 cells transfected with indicated expression vector plasmids (panel C, myocardin [0.25 μg]; panel D, SUMO-1 [0.5 μg] and SUMO-1ΔGG [0.5 μg]). Promoter activity was expressed either as activation (n-fold) (with values shown on each group within the panel) as detailed in Materials and Methods (C) or as the percent activation (with values shown on each group within the panel) with luciferase activity relative to that with myocardin and wild-type (wt) SUMO-1, which was taken as 100-percent activation (D). These data were obtained from three (C) or two (D) independent assays, each carried out in duplicate. (E) Dominant negative Ubc9 (C93R/L97A) impeded myocardin sumoylation. The upper panel showed a protein blot carried out on extracts taken from HeLa cells transfected with indicated combinations of expression vectors and then probed with V5 antibody; the lower panel showed that the same blot shown in the upper panel was stripped and reprobed with Ubc9 antibody. (F) Transcription activities were determined on CV1 cells transfected with a Ca-Actin-Luc reporter construct together with myocardin in the presence or absence of increasing amounts of wild-type or d/n Ubc9 (0.1 or 0.25 μg, respectively). Data obtained from two independent determinations with each carried out in duplicate are shown as percent activation relative to that for the group of myocardin and SUMO-1, which was taken as 100-percent activation. The numbers shown above each bar inside the panel indicate the percent activation for each group.
Transfection assays done with CV1 cells in the presence of myocardin and the coaddition of SUMO-1 showed robust cardiac α-actin promoter activation (Fig. 1C). Similar observations were made for transfected HeLa and 10T1/2 cells (data not shown). Substitution of SUMO-1 by SUMO-1ΔGG reached only 41% of the full promoter activation that was achieved with wild-type SUMO-1 (Fig. 1D), thus implicating SUMO-1 conjugation in the regulation of myocardin activity. To further explore the involvement of the sumoylation pathway in the modulation of myocardin activity, we evaluated the role of Ubc9, the only known E2 SUMO ligase, and its d/n form (C93R/L97A), which cannot transfer SUMO-1 (52). Expressed wild-type Ubc9 enhanced myocardin sumoylation, as shown by the appearance of an additional slower-migrating band, while d/n Ubc9 reduced sumoylation, as shown in Fig. 1E (upper panel, compare lanes 5 and 6 with lane 2). The potentiation or reduction of myocardin sumoylation led by Ubc9 or by C93R/L97A correlated well with myocardin transcription activity, as reflected by promoter reporter assays. In comparison to the robust promoter activity stimulated by the cotransfections with myocardin and SUMO-1 expression vectors, the addition of wild-type Ubc9 further increased promoter activity by ∼2-fold, whereas d/n Ubc9 decreased activity by 40 to 50%, as shown in Fig. 1F. Collectively, data from transfected CV1 and a variety of other cell types indicated that myocardin activity was modulated by the SUMO conjugation pathway.
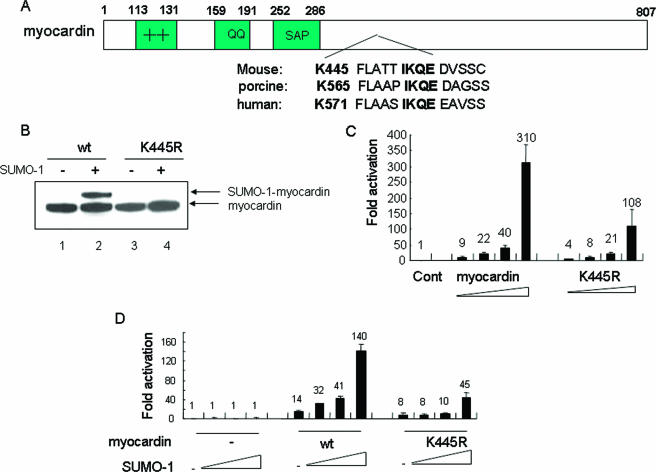
Lysine 445, the principal sumoylation site.
Consensus sumoylation sites usually contain a target lysine within the sequence ψKXE (47). Informatics directed our attention to lysine 445 present within the sequence IKQE of myocardin, which is conserved across species (Fig. 2A). The site-directed mutant in which lysine 445 was converted to arginine (the K445R mutant) blocked SUMO conjugation of myocardin (Fig. 2B) and blocked approximately 70% of myocardin's transcription activity in the presence of SUMO-1 (Fig. 2C and D). Therefore, lysine 445 was the primary sumoylation site in myocardin and contributed to myocardin transcription activity.
FIG. 2.
Lysine 445, the primary myocardin SUMO-1 target site in myocardin. (A) Schematic diagram of the conserved canonical sumoylation sequence in myocardin. (B) The K445R mutant blocked sumoylation. Lysates taken from HeLa cells transfected with wild-type (wt) myocardin or its K445R mutant in the presence or absence of SUMO-1 as indicated were stained with V5 antibody. Data are representative of three independent assays. (C and D) The K445R mutant displayed impaired activity. Ca-Actin-Luc activity was assayed for CV1 cells transfected with dosed myocardin or its mutant K445R (C) or with increasing amounts of SUMO-1 alone or in the presence of either myocardin or the K445R mutant (D). Data obtained from three separate experiments carried out in duplicate are shown as activation levels (n-fold) relative to that for the empty vector group, which was taken as 1. Cont, control.
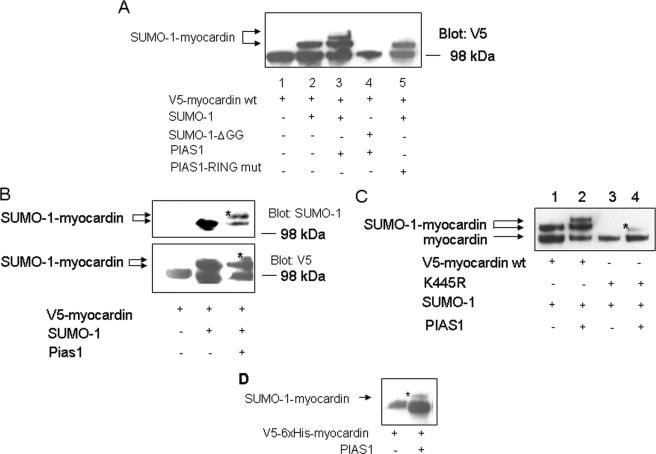
PIAS1 stimulated myocardin sumoylation on a nonconsensus sumoylation site(s) via its RING domain.
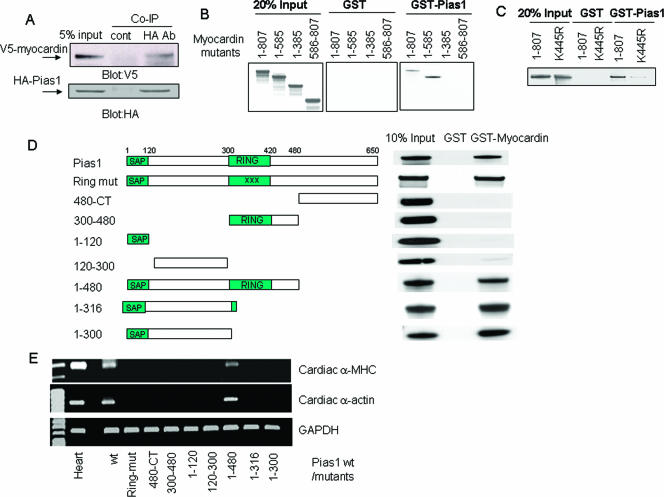
PIAS1 is an E3 ligase involved in the SUMO conjugation pathway (24, 57), and PIAS1 also enhanced sumoylation of myocardin. As shown in Fig. 3A, one slow migratory band on a PAGE gel was observed in the presence of SUMO-1 only (lane 2); however, the presence of PIAS1 further introduced an additional slow migratory band (lane 3), which was not detected in the presence of either SUMO-1ΔGG (lane 4) or the PIAS1 RING mutant (lane 5). Nickel-NTA pulldown followed by protein blotting confirmed that the additional protein species induced by PIAS1 was SUMO-1-conjugated myocardin, since it was detected by both anti-V5 and anti-SUMO-1 antibodies (Fig. 3B). Although the K445R mutation blocked myocardin sumoylation in the presence of SUMO-1, it was unable to totally abolish myocardin sumoylation that was enhanced by PIAS1 (Fig. 3C, compare lanes 3 and 4). Interestingly, PIAS1 alone was able to promote myocardin sumoylation (Fig. 3D). Thus, PIAS1 stimulated myocardin sumoylation via its RING domain and also promoted sumoylation at a nonconsensus SUMO conjugation site(s).
FIG. 3.
PIAS1 stimulated myocardin sumoylation on a nonconsensus sumoylation site(s) via its RING domain. (A) Enhancement of myocardin sumoylation by PIAS1 via its RING domain was observed by Western blotting with lysates of HeLa cells transfected with indicated combinations of specified factors (PIAS1 or its RING mutant [mut]; 50 ng). Data are representative of two independent assays. (B) Ni-NTA pulldown followed by a protein blotting assay confirmed that PIAS1 potentiated myocardin sumoylation via transfected SUMO-1. (Upper panel) The blot was revealed by SUMO-1 antibody; (lower panel) the blot was revealed by V5 antibody. The asterisks inside the panels refer to the enhanced SUMO-1-V5-myocardin by PIAS1. (C) The K445R mutant was sumoylated in the presence of PIAS1. The asterisk inside the panel indicates the SUMO-1-conjugated K445R mutant. The results shown in panels B and C are representative of three independent assays. (D) Ni-NTA pulldown followed by a Western blotting assay confirmed that PIAS1 potentiated endogenous SUMO conjugation to myocardin. Analogous results were obtained from two independent assays. The asterisk inside the panel denotes the SUMO-conjugated myocardin. wt, wild type.
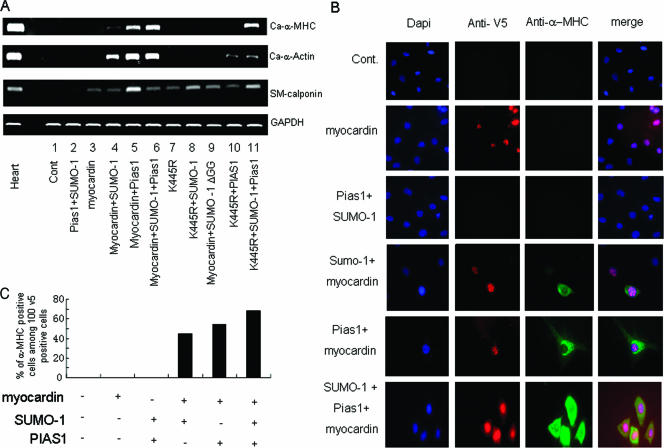
Myocardin triggered endogenous cardiac muscle-specified gene expression in fibroblast 10T1/2 cells in the presence of SUMO-1/PIAS1.
Myocardin expression vectors transfected into 10T1/2 fibroblasts were unable to induce the expression of cardiac muscle-specific genes. However, myocardin induced endogenous cardiac α-actin and cardiac α-MHC gene activity when cotransfected with SUMO-1 and/or PIAS1 (Fig. 4A, lanes 4, 5, and 6). In contrast, neither the expression vectors driving the K445R myocardin mutant together with SUMO-1 nor myocardin together with SUMO-1ΔGG was capable of triggering cardiac gene activation (Fig. 4A, lanes 8 and 9). Interestingly, the K445R mutant together with PIAS1 in the presence or absence of SUMO-1 was still able to induce cardiac α-actin expression, although at a reduced level (Fig. 4A, lanes 10 and 11), but the K445R mutant only along with PIAS1 and SUMO-1 modulated cardiac α-MHC gene activity, suggesting an intricate mechanism for regulating myocardin's ability to stimulate cardiac muscle-specified genes and revealing critical roles of both lysine 445 and a yet-to-be-identified atypical sumoylation site(s). Also, it is noteworthy that the K445R mutant was equivalent to the wild-type myocardin with regard to the induction of smooth muscle differentiation (smooth muscle calponin expression) in 10T1/2 cells (Fig. 4A, compare lanes 3 and 7). Furthermore, sarcomeric α-MHC gene expression, induced by myocardin via SUMO-1/PIAS1 in 10T1/2 fibroblasts, was confirmed by MF20 immunostaining, which specifically detected sarcomeric α-MHC (Fig. 4B). Thus, sumoylation acted as a molecular switch to convert myocardin into a potent transactivator of cardiac gene expression.
FIG. 4.
Myocardin induced cardiac muscle-specific gene expressions in fibroblast 10T1/2 cells in the presence of SUMO-1/PIAS1. (A) RT-PCR was performed on total RNA from 10T1/2 cells transfected with specified expression vectors alone or in various combinations as indicated. GAPDH expression level was measured as a control (Cont). Comparable data were obtained from two separate experiments. Ca, cardiac. (B) Sarcomeric α-MHC was detected in 10T1/2 cells transfected with various specified factors as indicated using MF20 in immunofluorescence staining assays. V5 antibody was used to detect myocardin-positive cells. The data shown represent three independent assays. (C) The percentage scores of 100 cells among the V5-positive are shown.
PIAS1 coassociated with myocardin.
Coimmunoprecipitation assays demonstrated physical association between myocardin and PIAS1 (Fig. 5A). 35S-labeled myocardin was bound by GST-PIAS1, as was the myocardin mutant with a deletion of aa 1 to 585. Fragmented myocardin (aa 1 to 385 and aa 586 to 807) abolished the association with GST-PIAS1, while the K445R mutant displayed reduced binding with GST-PIAS1 in comparison to wild-type myocardin (Fig. 5B and C, respectively). Thus, myocardin residues from aa 385 to 586 contributed to the physical association with PIAS1. To map the domain(s) of PIAS1 interacting with myocardin, PIAS1 serial deletions and RING domain mutants were 35S labeled, in vitro translated, and then used in GST pulldown assays with GST alone or with GST-myocardin (Fig. 5C). The PIAS1 RING mutant was bound by GST-myocardin, whereas the fragment containing only the RING domain (aa 300 to 480), the C terminus, the SAP domain (aa 1 to 120) or the fragment consisting of aa 120 to 300 failed to associate with myocardin. However, the N-terminal fragments of PIAS1 (aa 1 to 480, aa 1 to 316, and aa 1 to 300) were bound by myocardin (Fig. 5D). Thus, the association-mapping data correlated well with the myocardin-dependent induction of both cardiac α-actin and α-MHC expression in that the PIAS1 mutant with a deletion of aa 1 to 480 possessed both E3 ligase activity and the interactive region which bound myocardin. Other PIAS1 deletion mutants failed to empower myocardin to trigger cardiogenic gene expression (Fig. 5E). Collectively, these data demonstrated that both the RING domain and the N terminus of PIAS1 were indispensable for PIAS1 to switch on cardiac gene activity through myocardin.
FIG. 5.
Protein regions responsible for the physical interaction between PIAS1 and myocardin. (A) Total extracts from CV1 cells transfected with V5-tagged myocardin and HA epitoped PIAS1 were coimmunoprecipitated (Co-IP) with a control (cont) antibody (Ab) and HA antibody, respectively. Protein Western blots were revealed by staining with V5 antibody (upper panel) or HA antibody (lower panel). (B and C) Amino acid residues 385 to 586 contributed to the physical interaction with PIAS1. In vitro-translated 35S-labeled serial deletion or point mutants of myocardin were incubated with either GST or GST-fused PIAS1 and precipitated by GST beads. (D) The N-terminal region of PIAS1 physically interacted with myocardin. In vitro-translated 35S-labeled serial deletion mutants of PIAS1 were incubated with either GST or GST-fused myocardin and precipitated by GST beads. (E) Both the RING domain and the protein-interacting region in PIAS1 were required by myocardin to activate cardiogenic gene expression. The expression of selected cardiac muscle-specific genes were determined by RT-PCR on total RNAs from 10T1/2 fibroblast cells transfected with myocardin along with the indicated one serial deletion mutant of PIAS1. All data shown represent at least two independent assays. wt, wild type; mut, mutant; CT, C terminus.
Myocardin transcription activity enhanced by SUMO-1/PIAS1 was SRF dependent.
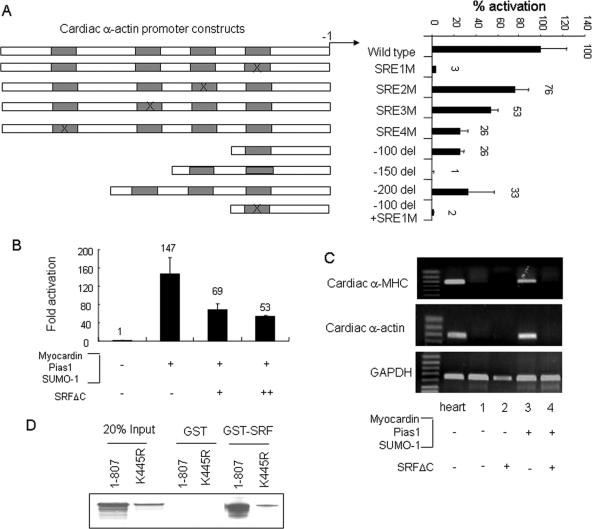
Did myocardin function via serum response elements (SREs) in cardiac muscle-specific gene promoters in the presence of SUMO-1/PIAS1? Mutation of SRE1 decreased cardiac α-actin promoter activation induced by cotransfected myocardin, SUMO-1, and PIAS1 by 90%, and the other mutated SREs (SRE2M, SRE3M, and SRE4M) also inhibited cardiac α-actin promoter activity, but to different degrees (Fig. 6A). A dominant negative SRFΔC protein (6) depressed promoter activity by myocardin, SUMO-1, and PIAS1 (Fig. 6B) and abrogated the induction of both cardiac α-MHC and cardiac α-actin in 10T1/2 cells (Fig. 6C, compare lanes 3 and 4). In addition, myocardin interacted with SRF via its N terminus (55), and a GST pulldown assay confirmed that the K445 myocardin mutant still retained physical association with SRF (Fig. 6D). Thus, SUMO-1 modification strongly potentiated the activity of myocardin but did not alter its SRF-dependent properties.
FIG. 6.
Myocardin transactivation enhanced by SUMO-1/PIAS1 was SRF dependent. (A) Reporter gene analysis was conducted with CV1 cells transfected with intact or point deletion (del) mutants of Ca-Actin-Luc reporter constructs, as indicated, in the presence of myocardin, SUMO-1, and PIAS1 expression vectors. Data obtained from two separate assays show percent activation for each corresponding group relative to that of the wild-type Ca-Actin-Luc group, which was taken as 100% activation. The X symbol indicates a site-mutated SRE. (B) SRF with deletion of the transactivation domain, SRFΔC, reduced promoter activity elicited by the presence of myocardin, PIAS1, and SUMO-1. (C) SRFΔC abrogated endogenous cardiac gene induction caused by coexpression of myocardin, PIAS1, and SUMO-1 in 10T1/2 cells. (D) K445R preserved physical association with SRF. GST pulldown assays were performed using in vitro-translated 35S-labeled wild-type myocardin or the K445R mutant incubated with either GST or GST-fused SRF.
Myocardin was differentially regulated by SUMO-1, -2, and -3.
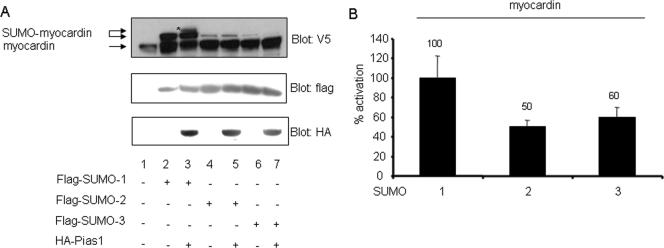
The SUMO gene family is composed of four members (13, 16), and some targets may be differentially favored by these SUMO isotypes (49). We asked if these SUMO isotypes are equivalent and whether PIAS1 showed a preference for any of these species in myocardin sumoylation. Sumoylation assays were conducted with HeLa cells transfected with expression vectors of V5-tagged myocardin together with Flag epitoped SUMO-1, -2, or -3, respectively, in the presence or absence of HA-PIAS1 (Fig. 7A). Even though all three of these SUMO members were expressed to about the same extent (middle panel), only SUMO-1 potently sumoylated myocardin (compare lane 2 with lanes 4 and 6). Also, PIAS1 preferred SUMO-1 in driving myocardin sumoylation (compare lane 3 with lanes 5 and 7). Consistent with these results, myocardin drove cardiac α-actin promoter activity in the presence of SUMO-2/-3 by only ∼50% of that in the presence of SUMO-1 (Fig. 7B). Thus, SUMO isotypes differentially modulated myocardin activity, and the E3 ligase, PIAS1, favored SUMO-1 over SUMO-2 or -3.
FIG. 7.
Sumoylation of myocardin was differentially regulated by the small SUMO multigene family members. (A) Free myocardin and forms of myocardin sumoylated by Flag-tagged SUMO-1, -2, and -3 were detected by anti-V5 Western blotting (upper panel). Flag antibody and HA antibody were used to evaluate the equivalent expression levels of SUMO proteins (middle panel) or PIAS1 (lower panel), respectively, in various groups. Western blotting was conducted with extracts from HeLa cells transfected with V5-tagged myocardin alone or in combination with one of the Flag-tagged SUMO-encoding vectors in the presence or absence of HA epitoped PIAS1 as indicated. The asterisk denotes the SUMO-conjugated myocardin induced by PIAS1 (upper panel). (B) Reporter gene activity was analyzed for CV1 cells transfected with myocardin together with either SUMO-1, SUMO-2, or SUMO-3. Data obtained from two independent experiments are shown as percent activation relative to that for myocardin and SUMO-1, which is taken as 100-percent activation.
DISCUSSION
Sumoylated myocardin, a potent activator of cardiac muscle-specified genes.
Myocardin is a powerful regulator of smooth muscle-specified genes in concert with SRF, but it was not sufficient to activate the endogenous cardiogenic program in pluripotent 10T1/2 fibroblast cells (58). Two forms of myocardin have been identified so far: a full-length myocardin (937 aa) and an N-terminally truncated one (807 aa) (55, 56). Moreover, functional studies have revealed that there was no significant difference between these two isoforms in terms of activating target genes (56). Here, we demonstrate that myocardin induced the expression of cardiac muscle-specific genes (encoding cardiac α-actin and cardiac α-MHC) in these pluripotent fibroblasts via SUMO modification. We noticed that SUMO-1ΔGG still exhibited some positive effect on myocardin activity in transactivation assays, which was consistent with the findings that SUMO-1 may perform its role independent of its conjugation competency (27). However, the positive effect of SUMO-1ΔGG on myocardin was not sufficient to initiate a cardiac differentiation program, indicating the pivotal role of SUMO linkage in the induction of this cardiac muscle-specific gene. SUMO-1 up-regulated myocardin activity primarily through modifying lysine 445 in the absence of E3 ligase. It appeared that K445 is the primary regulatory site that directed cardiogenic gene activity, which did not significantly alter smooth muscle cell-specified gene induction (Fig. 4A). The mutation of K445 did not affect myocardin nuclear localization or its physical interaction with SRF (Fig. 6D and data not shown), consistent with the report that the nuclear occupancy of MRTF-A, as well as its association with SRF, was not influenced by its sumoylation site mutation (40). However, in contrast to our findings, MRTF-A transcriptional activity was suppressed by SUMO modification. The possible explanations for these different observations are as follows: (i) although these two proteins share some close homology, they may not be equivalent and able to substitute for each other; (ii) they exhibit different specificities in tissue distributions (56), which might suggest different biological roles; and/or (iii) the functional consequence of sumoylation on its targets may be context dependent (17). We also noted that the downstream reporters employed for SUMO-regulated MRTF-A and myocardin were also different, which may also contribute to the dissimilar functions. The fact that cardiac gene induction by myocardin/SUMO-1/PIAS1 was hindered by a dominant negative form of SRF also suggested that SUMO-1 conjugation did not change the functional dependence of myocardin upon SRF.
Myocardin was targeted well by SUMO-1 and to a lesser extent by SUMO-2/-3 (Fig. 7). Correspondingly, SUMO-2/-3 was less effective than SUMO-1 in stimulating cardiac α-actin promoter activity. SUMO-1 also appeared to have a preferred role in directing myocardin cardiogenic activity. Saitoh and Hinchey (49) reported that the free pool of unconjugated SUMO-2/-3 was much greater than that of SUMO-1 under normal physiological conditions but that this changed dramatically under conditions of stress, which may relate to the preferential usage of these isotypes under altered physiological states. For example, the expression of SUMO-1 was also shown to be increased in response to hypoxia in the heart (51), a condition which activates HIF1-dependent pathways (9). It remains to be determined how the myocardin activity is modulated by other SUMO isotypes under pathological conditions.
PIAS1, a potent myocardin coactivator of cardiogenic genes.
PIAS family proteins, represented by five different members, i.e., PIAS1, PIAS3, PIASxα, PIASxβ, and PIASy, were shown to inhibit STATs via various mechanisms (2, 10, 33, 34). Recently, PIAS proteins were shown to be implicated in the modulation of the transcriptional activities of a multitude of transcription factors via protein-protein interaction and/or SUMO E3 ligase activity that facilitates SUMO attachment to the substrates (24, 35). PIAS1 may stimulate sumoylation reaction as follows: (i) by enhancing the sumoylation on the existing major sumoylation sites in target proteins; (ii) by promoting the formation of a SUMO chain on the existing major sumoylation site; and/or (iii) by introducing a novel sumoylation site that is rarely detected in the absence of E3 ligase. PIAS1 enhanced GATA4 sumoylation on the typical SUMO attachment site (57), but as shown here, PIAS1-directed myocardin sumoylation led us to consider the presence of a nonconsensus sumoylation site(s) on myocardin. Like lysine 445, the atypical sumoylation site(s) also appeared to contribute to the regulatory role of myocardin in cardiogenic gene induction, since PIAS1 assisted wild-type myocardin and even the K445R myocardin mutant in triggering a portion of the cardiogenic differentiation program (Fig. 4A). The RING domain of PIAS1 appeared to be indispensable in this induction (Fig. 5E), thus indicating the critical role of E3 ligase activity. However, given the fact that PIAS1 is a cofactor for a variety of proteins and may even recruit a specific factor(s) to execute some special actions, such as directing smooth muscle cell differentiation in fibroblasts (22, 26, 34, 45), we cannot completely rule out the potential involvement of a PIAS1-recruited factor(s) in PIAS1-assisted cardiac differentiation in fibroblasts. Unexpectedly, Ubc9, the only E2 in the sumoylation pathway, also stimulated myocardin sumoylation, which was not abrogated by K445 mutation (Fig. 1F and data not shown). In addition, sumoylation E3 ligases exhibit substrate specificity. For instance, PIAS1 served as an E3 ligase upon p53 sumoylation (21), and RanBP2, another E3 ligase, was involved in the SUMO modification of SP100 and HDAC4 (23, 44). Our observation that PIAS1 acted as the E3 ligase for SUMO-1 but not for SUMO-2/-3 in the modification of myocardin indicated that E3 ligase was specific not only for substrates but also for the various SUMO isoforms.
Our data demonstrated that the physical association of PIAS1 with myocardin plays a vital role in governing myocardin cardiogenic activity. We speculated that mutual-factor interactions shared between PIAS1 and myocardin are among the earliest steps required for conjugating SUMO to myocardin. Our mapping association study revealed the importance of amino acid residues 385 to 586 of myocardin in the physical interaction with PIAS1. Indeed, conversion of lysine residue 445 to arginine weakened this association; however, the residual coassociation with PIAS1 was sufficient for the K445R myocardin mutant to trigger cardiac-α actin expression in fibroblasts, albeit at a reduced level. Interestingly, K445R/PIAS1 was unable to induce cardiac α-MHC expression unless SUMO-1 was present, again indicating the importance of lysine 445 and/or a nonconsensus sumoylation site(s) in directing cardiac α-MHC gene activity.
Potential involvement of sumoylation in cardiogenesis.
Reversible SUMO conjugation and deconjugation regulate the activities of a large pool of proteins controlling cellular proliferation and differentiation. For example, mice with knockdowns of sumoylation pathway components, such as Ubc9, died at the early embryonic postimplantation stage (18, 39). More recently, Ubc9 was shown to be required for myotube formation in C2C12 cells and pharyngeal muscle development in Caenorhabditis elegans (46, 48), thus implicating the sumoylation pathway in muscle development. We demonstrated that SUMO modification of GATA4 elicited cardiac muscle-specific gene expression (57), and myocardin sumoylation by SUMO-1/PIAS1 showed induced cardiogenic gene expression. Given the facts that transcription factors such as myocardin, SRF, and GATA4 are all SUMO targeted and physically interact with each other (3, 43, 55) and that all of them are crucial to heart development (30, 41, 42), these noteworthy findings point to the possibility that the sumoylation pathway may contribute significantly to heart development via the modification of heart-enriched transcription factors as well as cofactors.
Acknowledgments
The laboratories of Robert J. Schwartz, XinHua Feng, and Eric N. Olson were supported by grants from the National Institutes of Health and the Foundation Leducq Transatlantic Networks of Excellence for Cardiovascular Research (to Robert J. Schwartz).
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Aravind, L., and E. V. Koonin. 2000. SAP—a putative DNA-binding motif involved in chromosomal organization. Trends Biochem. Sci. 25:112-114. [DOI] [PubMed] [Google Scholar]
- 2.Arora, T., B. Liu, H. He, J. Kim, T. L. Murphy, K. M. Murphy, R. L. Modlin, and K. Shuai. 2003. PIASx is a transcriptional co-repressor of signal transducer and activator of transcription 4. J. Biol. Chem. 278:21327-21330. [DOI] [PubMed] [Google Scholar]
- 3.Belaguli, N. S., J. L. Sepulveda, V. Nigam, F. Charron, M. Nemer, and R. J. Schwartz. 2000. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol. Cell. Biol. 20:7550-7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao, D., Z. Wang, C. L. Zhang, J. Oh, W. Xing, S. Li, J. A. Richardson, D. Z. Wang, and E. N. Olson. 2005. Modulation of smooth muscle gene expression by association of histone acetyltransferases and deacetylases with myocardin. Mol. Cell. Biol. 25:364-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, D. F., N. S. Belaguli, D. Iyer, W. B. Roberts, S. P. Wu, X. R. Dong, J. G. Marx, M. S. Moore, M. C. Beckerle, M. W. Majesky, and R. J. Schwartz. 2003. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev. Cell 4:107-118. [DOI] [PubMed] [Google Scholar]
- 6.Chang, J., L. Wei, T. Otani, K. A. Youker, M. L. Entman, and R. J. Schwartz. 2003. Inhibitory cardiac transcription factor, SRF-N, is generated by caspase 3 cleavage in human heart failure and attenuated by ventricular unloading. Circulation 108:407-413. [DOI] [PubMed] [Google Scholar]
- 7.Chen, A., H. Mannen, and S. S. Li. 1998. Characterization of mouse ubiquitin-like SMT3A and SMT3B cDNAs and gene/pseudogenes. Biochem. Mol. Biol. Int. 46:1161-1174. [DOI] [PubMed] [Google Scholar]
- 8.Chen, J., C. M. Kitchen, J. W. Streb, and J. M. Miano. 2002. Myocardin: a component of a molecular switch for smooth muscle differentiation. J. Mol. Cell. Cardiol. 34:1345-1356. [DOI] [PubMed] [Google Scholar]
- 9.Chi, N. C., and J. S. Karliner. 2004. Molecular determinants of responses to myocardial ischemia/reperfusion injury: focus on hypoxia-inducible and heat shock factors. Cardiovasc. Res. 61:437-447. [DOI] [PubMed] [Google Scholar]
- 10.Chung, C. D., J. Liao, B. Liu, X. Rao, P. Jay, P. Berta, and K. Shuai. 1997. Specific inhibition of Stat3 signal transduction by PIAS3. Science 278:1803-1805. [DOI] [PubMed] [Google Scholar]
- 11.Collavin, L., M. Gostissa, F. Avolio, P. Secco, A. Ronchi, C. Santoro, and G. Del Sal. 2004. Modification of the erythroid transcription factor GATA-1 by SUMO-1. Proc. Natl. Acad. Sci. USA 101:8870-8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du, K. L., H. S. Ip, J. Li, M. Chen, F. Dandre, W. Yu, M. M. Lu, G. K. Owens, and M. S. Parmacek. 2003. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol. 23:2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill, G. 2004. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 18:2046-2059. [DOI] [PubMed] [Google Scholar]
- 14.Girdwood, D., D. Bumpass, O. A. Vaughan, A. Thain, L. A. Anderson, A. W. Snowden, E. Garcia-Wilson, N. D. Perkins, and R. T. Hay. 2003. P300 transcriptional repression is mediated by SUMO modification. Mol. Cell 11:1043-1054. [DOI] [PubMed] [Google Scholar]
- 15.Gross, M., R. Yang, I. Top, C. Gasper, and K. Shuai. 2004. PIASy-mediated repression of the androgen receptor is independent of sumoylation. Oncogene 23:3059-3066. [DOI] [PubMed] [Google Scholar]
- 16.Guo, D., M. Li, Y. Zhang, P. Yang, S. Eckenrode, D. Hopkins, W. Zheng, S. Purohit, R. H. Podolsky, A. Muir, J. Wang, Z. Dong, T. Brusko, M. Atkinson, P. Pozzilli, A. Zeidler, L. J. Raffel, C. O. Jacob, Y. Park, M. Serrano-Rios, M. T. Larrad, Z. Zhang, H. J. Garchon, J. F. Bach, J. I. Rotter, J. X. She, and C. Y. Wang. 2004. A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat. Genet. 36:837-841. [DOI] [PubMed] [Google Scholar]
- 17.Hay, R. T. 2005. SUMO: a history of modification. Mol. Cell 18:1-12. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi, T., M. Seki, D. Maeda, W. Wang, Y. Kawabe, T. Seki, H. Saitoh, T. Fukagawa, H. Yagi, and T. Enomoto. 2002. Ubc9 is essential for viability of higher eukaryotic cells. Exp. Cell Res. 280:212-221. [DOI] [PubMed] [Google Scholar]
- 19.Jackson, P. K. 2001. A new RING for SUMO: wrestling transcriptional responses into nuclear bodies with PIAS family E3 SUMO ligases. Genes Dev. 15:3053-3058. [DOI] [PubMed] [Google Scholar]
- 20.Kagey, M. H., T. A. Melhuish, and D. Wotton. 2003. The polycomb protein Pc2 is a SUMO E3. Cell 113:127-137. [DOI] [PubMed] [Google Scholar]
- 21.Kahyo, T., T. Nishida, and H. Yasuda. 2001. Involvement of PIAS1 in the sumoylation of tumor suppressor p53. Mol. Cell 8:713-718. [DOI] [PubMed] [Google Scholar]
- 22.Kawai-Kowase, K., M. S. Kumar, M. H. Hoofnagle, T. Yoshida, and G. K. Owens. 2005. PIAS1 activates the expression of smooth muscle cell differentiation marker genes by interacting with serum response factor and class I basic helix-loop-helix proteins. Mol. Cell. Biol. 25:8009-8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsh, O., J. S. Seeler, A. Pichler, A. Gast, S. Muller, E. Miska, M. Mathieu, A. Harel-Bellan, T. Kouzarides, F. Melchior, and A. Dejean. 2002. The SUMO E3 ligase RanBP2 promotes modification of the HDAC4 deacetylase. EMBO J. 21:2682-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotaja, N., U. Karvonen, O. A. Janne, and J. J. Palvimo. 2002. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 22:5222-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapenta, V., P. Chiurazzi, P. van der Spek, A. Pizzuti, F. Hanaoka, and C. Brahe. 1997. SMT3A, a human homologue of the S. cerevisiae SMT3 gene, maps to chromosome 21qter and defines a novel gene family. Genomics 40:362-366. [DOI] [PubMed] [Google Scholar]
- 26.Lee, H., J. C. Quinn, K. V. Prasanth, V. A. Swiss, K. D. Economides, M. M. Camacho, D. L. Spector, and C. Abate-Shen. 2006. PIAS1 confers DNA-binding specificity on the Msx1 homeoprotein. Genes Dev. 20:784-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, Y. S., M. S. Jang, J. S. Lee, E. J. Choi, and E. Kim. 2005. SUMO-1 represses apoptosis signal-regulating kinase 1 activation through physical interaction and not through covalent modification. EMBO Rep. 6:949-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, S., D. Z. Wang, Z. Wang, J. A. Richardson, and E. N. Olson. 2003. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc. Natl. Acad. Sci. USA 100:9366-9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang, M., F. Melchior, X. H. Feng, and X. Lin. 2004. Regulation of Smad4 sumoylation and transforming growth factor-beta signaling by protein inhibitor of activated STAT1. J. Biol. Chem. 279:22857-22865. [DOI] [PubMed] [Google Scholar]
- 30.Liang, Q., L. J. De Windt, S. A. Witt, T. R. Kimball, B. E. Markham, and J. D. Molkentin. 2001. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J. Biol. Chem. 276:30245-30253. [DOI] [PubMed] [Google Scholar]
- 31.Lin, X., M. Liang, Y. Y. Liang, F. C. Brunicardi, F. Melchior, and X. H. Feng. 2003. Activation of transforming growth factor-beta signaling by SUMO-1 modification of tumor suppressor Smad4/DPC4. J. Biol. Chem. 278:18714-18719. [DOI] [PubMed] [Google Scholar]
- 32.Lin, X., B. Sun, M. Liang, Y. Y. Liang, A. Gast, J. Hildebrand, F. C. Brunicardi, F. Melchior, and X. H. Feng. 2003. Opposed regulation of corepressor CtBP by SUMOylation and PDZ binding. Mol. Cell 11:1389-1396. [DOI] [PubMed] [Google Scholar]
- 33.Liu, B., M. Gross, J. ten Hoeve, and K. Shuai. 2001. A transcriptional corepressor of Stat1 with an essential LXXLL signature motif. Proc. Natl. Acad. Sci. USA 98:3203-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, B., J. Liao, X. Rao, S. A. Kushner, C. D. Chung, D. D. Chang, and K. Shuai. 1998. Inhibition of Stat1-mediated gene activation by PIAS1. Proc. Natl. Acad. Sci. USA 95:10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long, J., I. Matsuura, D. He, G. Wang, K. Shuai, and F. Liu. 2003. Repression of Smad transcriptional activity by PIASy, an inhibitor of activated STAT. Proc. Natl. Acad. Sci. USA 100:9791-9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuzaki, K., T. Minami, M. Tojo, Y. Honda, Y. Uchimura, H. Saitoh, H. Yasuda, S. Nagahiro, H. Saya, and M. Nakao. 2003. Serum response factor is modulated by the SUMO-1 conjugation system. Biochem. Biophys. Res. Commun. 306:32-38. [DOI] [PubMed] [Google Scholar]
- 37.Melchior, F. 2000. SUMO-nonclassical ubiquitin. Annu. Rev. Cell Dev. Biol. 16:591-626. [DOI] [PubMed] [Google Scholar]
- 38.Muller, S., C. Hoege, G. Pyrowolakis, and S. Jentsch. 2001. SUMO, ubiquitin's mysterious cousin. Nat. Rev. Mol. Cell Biol. 2:202-210. [DOI] [PubMed] [Google Scholar]
- 39.Nacerddine, K., F. Lehembre, M. Bhaumik, J. Artus, M. Cohen-Tannoudji, C. Babinet, P. P. Pandolfi, and A. Dejean. 2005. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev. Cell 9:769-779. [DOI] [PubMed] [Google Scholar]
- 40.Nakagawa, K., and N. Kuzumaki. 2005. Transcriptional activity of megakaryoblastic leukemia 1 (MKL1) is repressed by SUMO modification. Genes Cells 10:835-850. [DOI] [PubMed] [Google Scholar]
- 41.Narita, N., M. Bielinska, and D. B. Wilson. 1997. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev. Biol. 189:270-274. [DOI] [PubMed] [Google Scholar]
- 42.Niu, Z., W. Yu, S. X. Zhang, M. Barron, N. S. Belaguli, M. D. Schneider, M. Parmacek, A. Nordheim, and R. J. Schwartz. 2005. Conditional mutagenesis of the murine serum response factor gene blocks cardiogenesis and the transcription of downstream gene targets. J. Biol. Chem. 280:32531-32538. [DOI] [PubMed] [Google Scholar]
- 43.Oh, J., Z. Wang, D. Z. Wang, C. L. Lien, W. Xing, and E. N. Olson. 2004. Target gene-specific modulation of myocardin activity by GATA transcription factors. Mol. Cell. Biol. 24:8519-8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pichler, A., A. Gast, J. S. Seeler, A. Dejean, and F. Melchior. 2002. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell 108:109-120. [DOI] [PubMed] [Google Scholar]
- 45.Prigge, J. R., and E. E. Schmidt. 2006. Interaction of protein inhibitor of activated STAT (PIAS) proteins with the TATA-binding protein, TBP. J. Biol. Chem. 281:12260-12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riquelme, C., K. K. Barthel, X. F. Qin, and X. Liu. 2006. Ubc9 expression is essential for myotube formation in C2C12. Exp. Cell Res. 312:2132-2141. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez, M. S., C. Dargemont, and R. T. Hay. 2001. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J. Biol. Chem. 276:12654-12659. [DOI] [PubMed] [Google Scholar]
- 48.Roy Chowdhuri, S., T. Crum, A. Woollard, S. Aslam, and P. G. Okkema. 2006. The T-box factor TBX-2 and the SUMO conjugating enzyme UBC-9 are required for ABa-derived pharyngeal muscle in C. elegans. Dev. Biol. 295:664-677. [DOI] [PubMed] [Google Scholar]
- 49.Saitoh, H., and J. Hinchey. 2000. Functional heterogeneity of small ubiquitin-related protein modifiers SUMO-1 versus SUMO-2/3. J. Biol. Chem. 275:6252-6258. [DOI] [PubMed] [Google Scholar]
- 50.Sepulveda, J. L., S. Vlahopoulos, D. Iyer, N. Belaguli, and R. J. Schwartz. 2002. Combinatorial expression of GATA4, Nkx2-5, and serum response factor directs early cardiac gene activity. J. Biol. Chem. 277:25775-25782. [DOI] [PubMed] [Google Scholar]
- 51.Shao, R., F. P. Zhang, F. Tian, P. Anders Friberg, X. Wang, H. Sjoland, and H. Billig. 2004. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 569:293-300. [DOI] [PubMed] [Google Scholar]
- 52.Tashiro, K., M. P. Pando, Y. Kanegae, P. M. Wamsley, S. Inoue, and I. M. Verma. 1997. Direct involvement of the ubiquitin-conjugating enzyme Ubc9/Hus5 in the degradation of IkappaBalpha. Proc. Natl. Acad. Sci. USA 94:7862-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueyama, T., H. Kasahara, T. Ishiwata, Q. Nie, and S. Izumo. 2003. Myocardin expression is regulated by Nkx2.5, and its function is required for cardiomyogenesis. Mol. Cell. Biol. 23:9222-9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Tuyn, J., S. Knaan-Shanzer, M. J. van de Watering, M. de Graaf, A. van der Laarse, M. J. Schalij, E. E. van der Wall, A. A. de Vries, and D. E. Atsma. 2005. Activation of cardiac and smooth muscle-specific genes in primary human cells after forced expression of human myocardin. Cardiovasc. Res. 67:245-255. [DOI] [PubMed] [Google Scholar]
- 55.Wang, D., P. S. Chang, Z. Wang, L. Sutherland, J. A. Richardson, E. Small, P. A. Krieg, and E. N. Olson. 2001. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 105:851-862. [DOI] [PubMed] [Google Scholar]
- 56.Wang, D. Z., S. Li, D. Hockemeyer, L. Sutherland, Z. Wang, G. Schratt, J. A. Richardson, A. Nordheim, and E. N. Olson. 2002. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc. Natl. Acad. Sci. USA 99:14855-14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, J., X. H. Feng, and R. J. Schwartz. 2004. SUMO-1 modification activated GATA4-dependent cardiogenic gene activity. J. Biol. Chem. 279:49091-49098. [DOI] [PubMed] [Google Scholar]
- 58.Wang, Z., D. Z. Wang, G. C. Pipes, and E. N. Olson. 2003. Myocardin is a master regulator of smooth muscle gene expression. Proc. Natl. Acad. Sci. USA 100:7129-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshida, T., K. Kawai-Kowase, and G. K. Owens. 2004. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler. Thromb. Vasc. Biol. 24:1596-1601. [DOI] [PubMed] [Google Scholar]