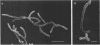Abstract
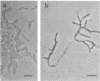
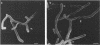
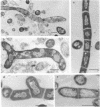
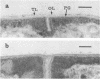
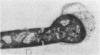
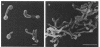
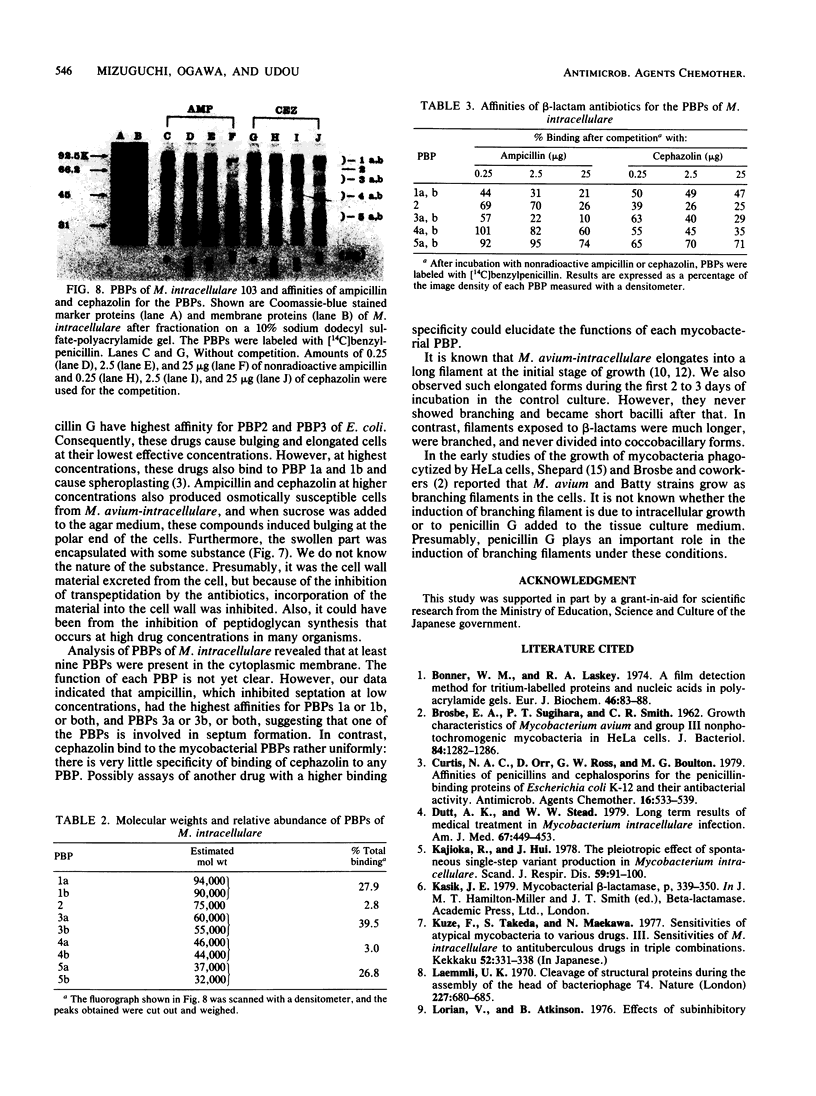
In vitro activity of seven beta-lactam antibiotics against strains of Mycobacterium avium-intracellulare was evaluated by the agar dilution method. The activity was influenced by the presence or absence of Tween 80 in Dubos medium, and cephazolin and cefotaxime were effective against most strains in the presence of Tween 80. beta-Lactam antibiotics at low concentrations induced long filamentous cells with branching. In contrast to the filaments induced by ampicillin, in which septation was rarely observed, filaments induced by cephazolin had many septa, suggesting that the mechanisms of filament induction were different from the drugs used. At high concentrations, ampicillin and cephazolin induced osmotically sensitive cells with bulging at polar end of the cells. Analysis of penicillin binding proteins (PBPs) of the organism showed that there were at least nine PBPs with molecular weights between 32,000 and 94,000 in the cytoplasmic membrane. Ampicillin showed the highest affinity for PBPs 1a or 1b, or both, and also PBPs 3a or 3b, or both. In contrast, there was very little specificity of binding of cephazolin for any of the PBPs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROSBE E. A., SUGIHARA P. T., SMITH C. R. Growth characteristics of Mycobacterium avium and group III nonphotochromogenic mycobacteria in HeLa cells. J Bacteriol. 1962 Dec;84:1282–1286. doi: 10.1128/jb.84.6.1282-1286.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A. K., Stead W. W. Long-term results of medical treatment in Mycobacterium intracellulare infection. Am J Med. 1979 Sep;67(3):449–453. doi: 10.1016/0002-9343(79)90792-7. [DOI] [PubMed] [Google Scholar]
- Kajioka R., Hui J. The pleiotropic effect of spontaneous single-step variant production in Mycobacterium intracellulare. Scand J Respir Dis. 1978 Apr;59(2):91–100. [PubMed] [Google Scholar]
- Kuze F., Takeda S., Maekawa N. [Sensitivities of atypical mycobacteria to various drugs. III. Sensitivities of M. intracellulare to antituberculous drugs in triple combinations (author's transl)]. Kekkaku. 1977 Jul;52(7):331–338. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorian V., Atkinson B. Effects of subinhibitory concentrations of antibiotics on cross walls of cocci. Antimicrob Agents Chemother. 1976 Jun;9(6):1043–1055. doi: 10.1128/aac.9.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C., Ashbaugh P. Factors that affect the cell cycle of Mycobacterium avium. Rev Infect Dis. 1981 Sep-Oct;3(5):914–925. doi: 10.1093/clinids/3.5.914. [DOI] [PubMed] [Google Scholar]
- Mizuguchi Y., Udou T., Yamada T. Mechanism of antibiotic resistance in Mycobacterium intracellulare. Microbiol Immunol. 1983;27(5):425–431. doi: 10.1111/j.1348-0421.1983.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Rastogi N., David H. L. Growth and cell division of Mycobacterium avium. J Gen Microbiol. 1981 Sep;126(1):77–84. doi: 10.1099/00221287-126-1-77. [DOI] [PubMed] [Google Scholar]
- Rosenzweig D. Y. Pulmonary mycobacterial infections due to Mycobacterium intracellulare-avium complex. Clinical features and course in 100 consecutive cases. Chest. 1979 Feb;75(2):115–119. doi: 10.1378/chest.75.2.115. [DOI] [PubMed] [Google Scholar]
- SHEPARD C. C. Behavior of the atypical mycobacteria in HeLa cells. Am Rev Tuberc. 1958 Jun;77(6):968–975. doi: 10.1164/artpd.1958.77.6.968. [DOI] [PubMed] [Google Scholar]
- Sakurai N. [In vitro susceptibility of atypical mycobacteria to Cephem and other antibiotics]. Kekkaku. 1983 Jun;58(6):355–362. [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Penicillin-binding proteins and the future of beta-lactam antibiotics. The Seventh Fleming Lecture. J Gen Microbiol. 1983 May;129(5):1247–1260. doi: 10.1099/00221287-129-5-1247. [DOI] [PubMed] [Google Scholar]
- Takade A., Takeya K., Taniguchi H., Mizuguchi Y. Electron microscopic observations of cell division in Mycobacterium vaccae V1. J Gen Microbiol. 1983 Jul;129(7):2315–2320. doi: 10.1099/00221287-129-7-2315. [DOI] [PubMed] [Google Scholar]
- Williamson R., Ward J. B. Benzylpenicillin-induced filament formation of Clostridium perfringens. J Gen Microbiol. 1982 Dec;128(12):3025–3035. doi: 10.1099/00221287-128-12-3025. [DOI] [PubMed] [Google Scholar]