Abstract
The neural cell-adhesion molecule L1 is involved in intercellular recognition and neuronal migration in the CNS. Recently, we have shown that mutations in the gene encoding L1 are responsible for three related disorders; X-linked hydrocephalus, MASA (mental retardation, aphasia, shuffling gait, and adducted thumbs) syndrome, and spastic paraplegia type I (SPG1). These three disorders represent a clinical spectrum that varies not only between families but sometimes also within families. To date, 14 independent L1 mutations have been reported and shown to be disease causing. Here we report nine novel L1 mutations in X-linked hydrocephalus and MASA-syndrome families, including the first examples of mutations affecting the fibronectin type III domains of the molecule. They are discussed in relation both to phenotypes and to the insights that they provide into L1 function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel F., Holm J., Conscience J. F., Schachner M. Several extracellular domains of the neural cell adhesion molecule L1 are involved in neurite outgrowth and cell body adhesion. J Neurosci. 1993 Nov;13(11):4764–4775. doi: 10.1523/JNEUROSCI.13-11-04764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchine J. W., Lewis R. C., Jr The MASA syndrome: a new heritable mental retardation syndrome. Clin Genet. 1974;5(4):298–306. doi: 10.1111/j.1399-0004.1974.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Bieber A. J., Snow P. M., Hortsch M., Patel N. H., Jacobs J. R., Traquina Z. R., Schilling J., Goodman C. S. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989 Nov 3;59(3):447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Burgoon M. P., Grumet M., Mauro V., Edelman G. M., Cunningham B. A. Structure of the chicken neuron-glia cell adhesion molecule, Ng-CAM: origin of the polypeptides and relation to the Ig superfamily. J Cell Biol. 1991 Mar;112(5):1017–1029. doi: 10.1083/jcb.112.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucke P., Vits L., Van Camp G., Serville F., Lyonnet S., Kenwrick S., Rosenthal A., Wehnert M., Munnich A., Willems P. J. Identification of a 5' splice site mutation in intron 4 of the L1CAM gene in an X-linked hydrocephalus family. Hum Mol Genet. 1994 Apr;3(4):671–673. doi: 10.1093/hmg/3.4.671. [DOI] [PubMed] [Google Scholar]
- Davis J. Q., Bennett V. Ankyrin binding activity shared by the neurofascin/L1/NrCAM family of nervous system cell adhesion molecules. J Biol Chem. 1994 Nov 4;269(44):27163–27166. [PubMed] [Google Scholar]
- EDWARDS J. H., NORMAN R. M., ROBERTS J. M. Sex-linked hydrocephalus. Report of a family with 15 affected members. Arch Dis Child. 1961 Oct;36:481–485. doi: 10.1136/adc.36.189.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen E., Schrander-Stumpel C., Vits L., Coucke P., Van Camp G., Willems P. J. X-linked hydrocephalus and MASA syndrome present in one family are due to a single missense mutation in exon 28 of the L1CAM gene. Hum Mol Genet. 1994 Dec;3(12):2255–2256. doi: 10.1093/hmg/3.12.2255. [DOI] [PubMed] [Google Scholar]
- Friedlander D. R., Milev P., Karthikeyan L., Margolis R. K., Margolis R. U., Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994 May;125(3):669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryns J. P., Spaepen A., Cassiman J. J., van den Berghe H. X linked complicated spastic paraplegia, MASA syndrome, and X linked hydrocephalus owing to congenital stenosis of the aqueduct of Sylvius: variable expression of the same mutation at Xq28. J Med Genet. 1991 Jun;28(6):429–431. doi: 10.1136/jmg.28.6.429-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareis F. J., Mason J. D. X-linked mental retardation associated with bilateral clasp thumb anomaly. Am J Med Genet. 1984 Jan;17(1):333–338. doi: 10.1002/ajmg.1320170126. [DOI] [PubMed] [Google Scholar]
- Grumet M. Cell adhesion molecules and their subgroups in the nervous system. Curr Opin Neurobiol. 1991 Oct;1(3):370–376. doi: 10.1016/0959-4388(91)90055-c. [DOI] [PubMed] [Google Scholar]
- Halliday J., Chow C. W., Wallace D., Danks D. M. X linked hydrocephalus: a survey of a 20 year period in Victoria, Australia. J Med Genet. 1986 Feb;23(1):23–31. doi: 10.1136/jmg.23.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
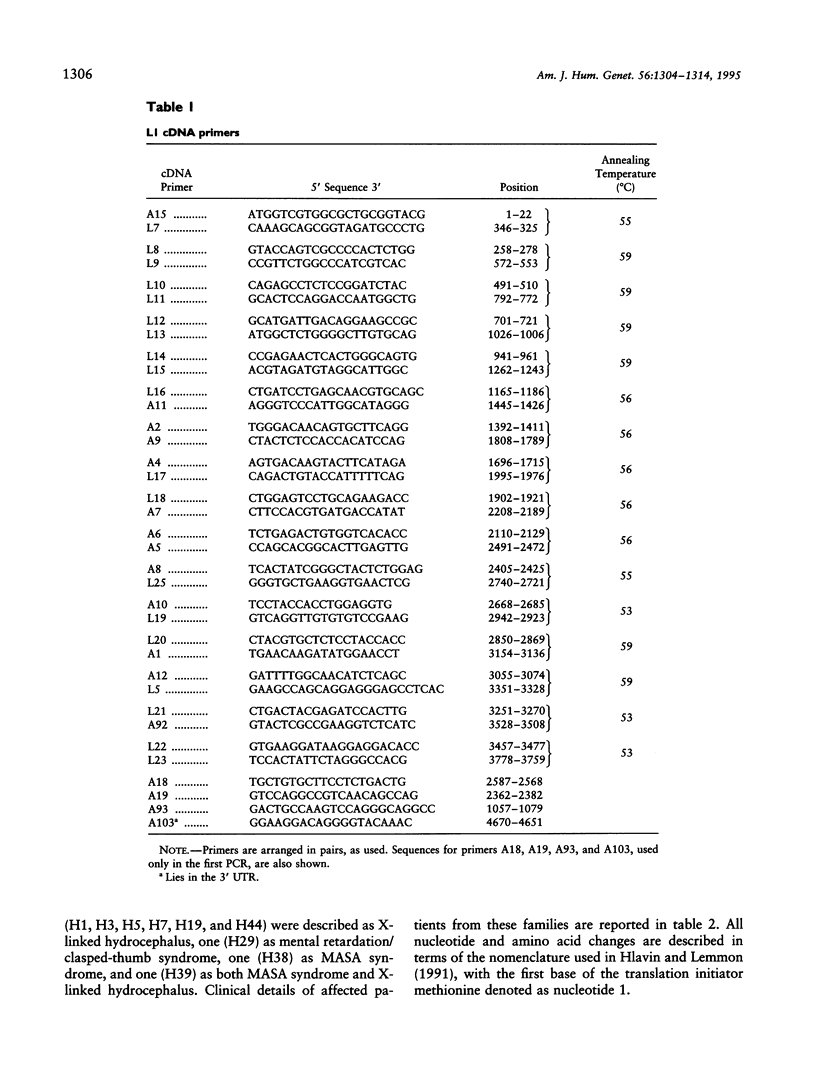
- Hlavin M. L., Lemmon V. Molecular structure and functional testing of human L1CAM: an interspecies comparison. Genomics. 1991 Oct;11(2):416–423. doi: 10.1016/0888-7543(91)90150-d. [DOI] [PubMed] [Google Scholar]
- Jouet M., Feldman E., Yates J., Donnai D., Paterson J., Siggers D., Kenwrick S. Refining the genetic location of the gene for X linked hydrocephalus within Xq28. J Med Genet. 1993 Mar;30(3):214–217. doi: 10.1136/jmg.30.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouet M., Kenwrick S. Gene analysis of L1 neural cell adhesion molecule in prenatal diagnosis of hydrocephalus. Lancet. 1995 Jan 21;345(8943):161–162. doi: 10.1016/s0140-6736(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Jouet M., Rosenthal A., Armstrong G., MacFarlane J., Stevenson R., Paterson J., Metzenberg A., Ionasescu V., Temple K., Kenwrick S. X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat Genet. 1994 Jul;7(3):402–407. doi: 10.1038/ng0794-402. [DOI] [PubMed] [Google Scholar]
- Jouet M., Rosenthal A., MacFarlane J., Kenwrick S., Donnai D. A missense mutation confirms the L1 defect in X-linked hydrocephalus (HSAS) Nat Genet. 1993 Aug;4(4):331–331. doi: 10.1038/ng0893-331. [DOI] [PubMed] [Google Scholar]
- Kenwrick S., Ionasescu V., Ionasescu G., Searby C., King A., Dubowitz M., Davies K. E. Linkage studies of X-linked recessive spastic paraplegia using DNA probes. Hum Genet. 1986 Jul;73(3):264–266. doi: 10.1007/BF00401241. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Kuhn T. B., Stoeckli E. T., Condrau M. A., Rathjen F. G., Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4). J Cell Biol. 1991 Nov;115(4):1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppuswamy M. N., Hoffmann J. W., Kasper C. K., Spitzer S. G., Groce S. L., Bajaj S. P. Single nucleotide primer extension to detect genetic diseases: experimental application to hemophilia B (factor IX) and cystic fibrosis genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1143–1147. doi: 10.1073/pnas.88.4.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon V., Farr K. L., Lagenaur C. L1-mediated axon outgrowth occurs via a homophilic binding mechanism. Neuron. 1989 Jun;2(6):1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Miura M., Kobayashi M., Asou H., Uyemura K. Molecular cloning of cDNA encoding the rat neural cell adhesion molecule L1. Two L1 isoforms in the cytoplasmic region are produced by differential splicing. FEBS Lett. 1991 Sep 2;289(1):91–95. doi: 10.1016/0014-5793(91)80915-p. [DOI] [PubMed] [Google Scholar]
- Moos M., Tacke R., Scherer H., Teplow D., Früh K., Schachner M. Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988 Aug 25;334(6184):701–703. doi: 10.1038/334701a0. [DOI] [PubMed] [Google Scholar]
- Reed R. The organization of 3' splice-site sequences in mammalian introns. Genes Dev. 1989 Dec;3(12B):2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Charnock-Jones D. S. New protocols for DNA sequencing with dye terminators. DNA Seq. 1992;3(1):61–64. doi: 10.3109/10425179209039697. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Jouet M., Kenwrick S. Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat Genet. 1992 Oct;2(2):107–112. doi: 10.1038/ng1092-107. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Neural cell-to-cell adhesion and recognition. Curr Opin Cell Biol. 1989 Oct;1(5):898–904. doi: 10.1016/0955-0674(89)90056-2. [DOI] [PubMed] [Google Scholar]
- Schrander-Stumpel C., Legius E., Fryns J. P., Cassiman J. J. MASA syndrome: new clinical features and linkage analysis using DNA probes. J Med Genet. 1990 Nov;27(11):688–692. doi: 10.1136/jmg.27.11.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonderegger P., Rathjen F. G. Regulation of axonal growth in the vertebrate nervous system by interactions between glycoproteins belonging to two subgroups of the immunoglobulin superfamily. J Cell Biol. 1992 Dec;119(6):1387–1394. doi: 10.1083/jcb.119.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp G., Vits L., Coucke P., Lyonnet S., Schrander-Stumpel C., Darby J., Holden J., Munnich A., Willems P. J. A duplication in the L1CAM gene associated with X-linked hydrocephalus. Nat Genet. 1993 Aug;4(4):421–425. doi: 10.1038/ng0893-421. [DOI] [PubMed] [Google Scholar]
- Vits L., Van Camp G., Coucke P., Fransen E., De Boulle K., Reyniers E., Korn B., Poustka A., Wilson G., Schrander-Stumpel C. MASA syndrome is due to mutations in the neural cell adhesion gene L1CAM. Nat Genet. 1994 Jul;7(3):408–413. doi: 10.1038/ng0794-408. [DOI] [PubMed] [Google Scholar]
- Willems P. J., Brouwer O. F., Dijkstra I., Wilmink J. X-linked hydrocephalus. Am J Med Genet. 1987 Aug;27(4):921–928. doi: 10.1002/ajmg.1320270419. [DOI] [PubMed] [Google Scholar]
- Williams E. J., Furness J., Walsh F. S., Doherty P. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron. 1994 Sep;13(3):583–594. doi: 10.1016/0896-6273(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Winter R. M., Davies K. E., Bell M. V., Huson S. M., Patterson M. N. MASA syndrome: further clinical delineation and chromosomal localisation. Hum Genet. 1989 Jul;82(4):367–370. doi: 10.1007/BF00273999. [DOI] [PubMed] [Google Scholar]
- Yeatman G. W. Mental retardation-clasped thumb syndrome. Am J Med Genet. 1984 Jan;17(1):339–344. doi: 10.1002/ajmg.1320170127. [DOI] [PubMed] [Google Scholar]