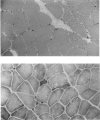
Abstract
We have studied a family segregating a form of autosomal dominant distal myopathy (MIM 160500) and containing nine living affected individuals. The myopathy in this family is closest in clinical phenotype to that first described by Gowers in 1902. A search for linkage was conducted using microsatellite, VNTR, and RFLP markers. In total, 92 markers on all 22 autosomes were run. Positive linkage was obtained with 14 of 15 markers tested on chromosome 14, with little indication of linkage elsewhere in the genome. Maximum two-point LOD scores of 2.60 at recombination fraction .00 were obtained for the markers MYH7 and D14S64--the family structure precludes a two-point LOD score > or = 3. Recombinations with D14S72 and D14S49 indicate that this distal myopathy locus, MPD1, should lie between these markers. A multipoint analysis assuming 100% penetrance and using the markers D14S72, D14S50, MYH7, D14S64, D14S54, and D14S49 gave a LOD score of exactly 3 at MYH7. Analysis at a penetrance of 80% gave a LOD score of 2.8 at this marker. This probable localization of a gene for distal myopathy, MPD1, on chromosome 14 should allow other investigators studying distal myopathy families to test this region for linkage in other types of the disease, to confirm linkage or to demonstrate the likely genetic heterogeneity.
Full text
PDF
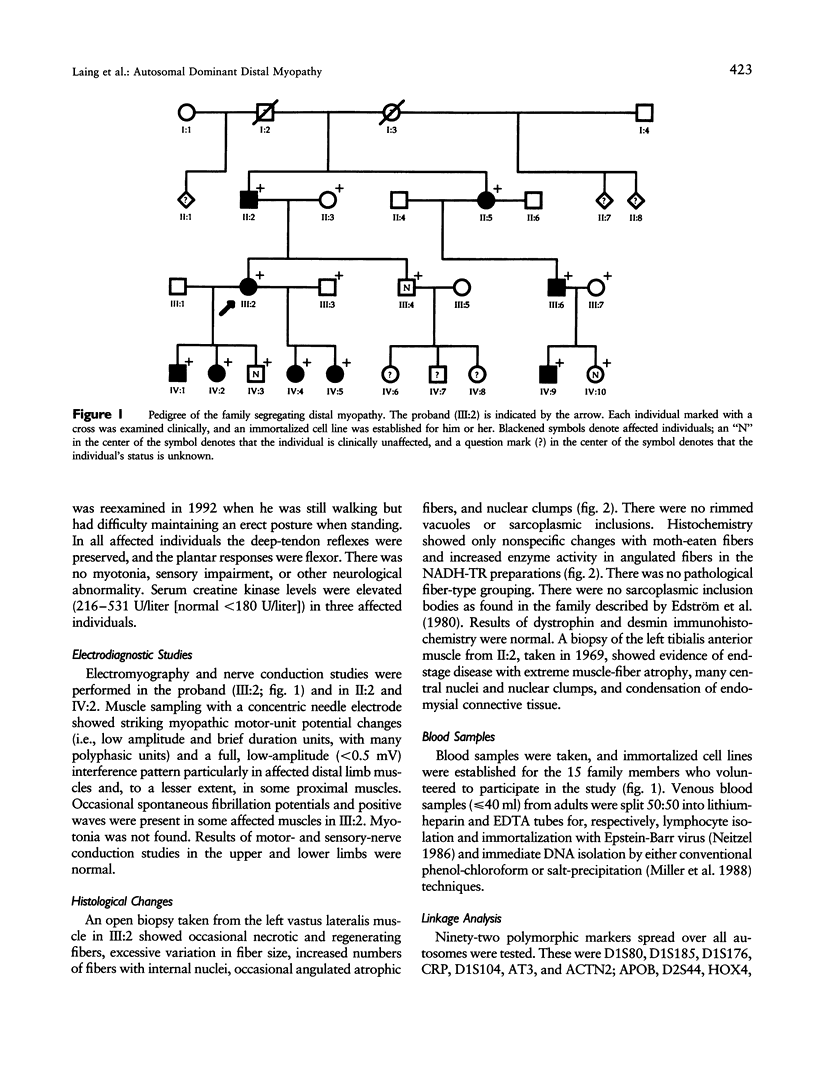

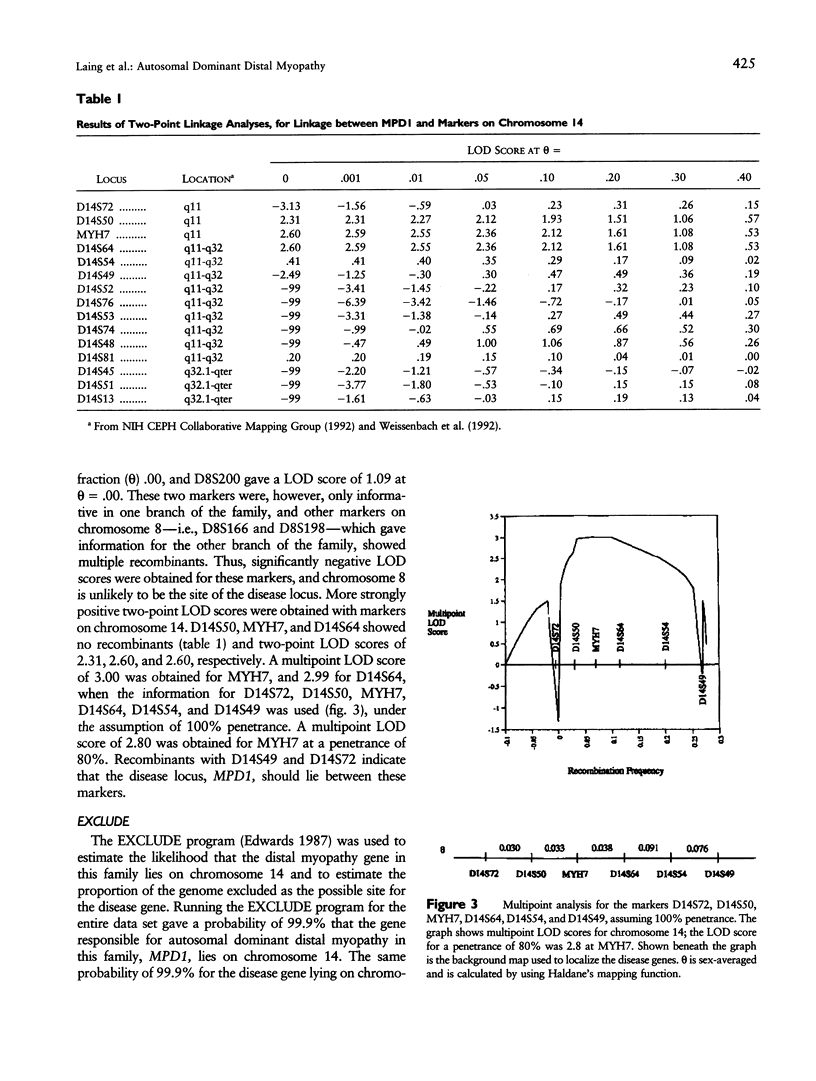


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edström L., Thornell L. E., Eriksson A. A new type of hereditary distal myopathy with characteristic sarcoplasmic bodies and intermediate (skeletin) filaments. J Neurol Sci. 1980 Aug;47(2):171–190. doi: 10.1016/0022-510x(80)90002-7. [DOI] [PubMed] [Google Scholar]
- Edwards J. H. Exclusion mapping. J Med Genet. 1987 Sep;24(9):539–543. doi: 10.1136/jmg.24.9.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandreski M. A., Sole M. J., Liew C. C. Two different forms of beta myosin heavy chain are expressed in human striated muscle. Hum Genet. 1987 Oct;77(2):127–131. doi: 10.1007/BF00272378. [DOI] [PubMed] [Google Scholar]
- MORTON N. E. Sequential tests for the detection of linkage. Am J Hum Genet. 1955 Sep;7(3):277–318. [PMC free article] [PubMed] [Google Scholar]
- Markesbery W. R., Griggs R. C., Leach R. P., Lapham L. W. Late onset hereditary distal myopathy. Neurology. 1974 Feb;24(2):127–134. doi: 10.1212/wnl.24.2.127. [DOI] [PubMed] [Google Scholar]
- Matsuoka R., Chambers A., Kimura M., Kanda N., Bruns G., Yoshida M., Takao A. Molecular cloning and chromosomal localization of a gene coding for human cardiac myosin heavy-chain. Am J Med Genet. 1988 Feb;29(2):369–376. doi: 10.1002/ajmg.1320290217. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulley J. C., Kozman H. M., Phillips H. A., Gedeon A. K., McCure J. A., Iles D. E., Gregg R. G., Hogan K., Couch F. J., MacLennan D. H. Refined genetic localization for central core disease. Am J Hum Genet. 1993 Feb;52(2):398–405. [PMC free article] [PubMed] [Google Scholar]
- Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986 Aug;73(4):320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- Nonaka I., Sunohara N., Ishiura S., Satoyoshi E. Familial distal myopathy with rimmed vacuole and lamellar (myeloid) body formation. J Neurol Sci. 1981 Jul;51(1):141–155. doi: 10.1016/0022-510x(81)90067-8. [DOI] [PubMed] [Google Scholar]
- Ott J. Estimation of the recombination fraction in human pedigrees: efficient computation of the likelihood for human linkage studies. Am J Hum Genet. 1974 Sep;26(5):588–597. [PMC free article] [PubMed] [Google Scholar]
- Sarfarazi M., Upadhyaya M., Padberg G., Pericak-Vance M., Siddique T., Lucotte G., Lunt P. An exclusion map for facioscapulohumeral (Landouzy-Déjérine) disease. J Med Genet. 1989 Aug;26(8):481–484. doi: 10.1136/jmg.26.8.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELANDER L. Myopathia distalis tarda hereditaria; 249 examined cases in 72 pedigrees. Acta Med Scand Suppl. 1951;265:1–124. [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Weiffenbach B., Dubois J., Storvick D., Tawil R., Jacobsen S. J., Gilbert J., Wijmenga C., Mendell J. R., Winokur S., Altherr M. R. Mapping the facioscapulohumeral muscular dystrophy gene is complicated by chromsome 4q35 recombination events. Nat Genet. 1993 Jun;4(2):165–169. doi: 10.1038/ng0693-165. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Hewitt J. E., Sandkuijl L. A., Clark L. N., Wright T. J., Dauwerse H. G., Gruter A. M., Hofker M. H., Moerer P., Williamson R. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet. 1992 Sep;2(1):26–30. doi: 10.1038/ng0992-26. [DOI] [PubMed] [Google Scholar]