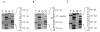Abstract
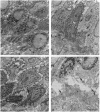
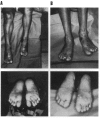
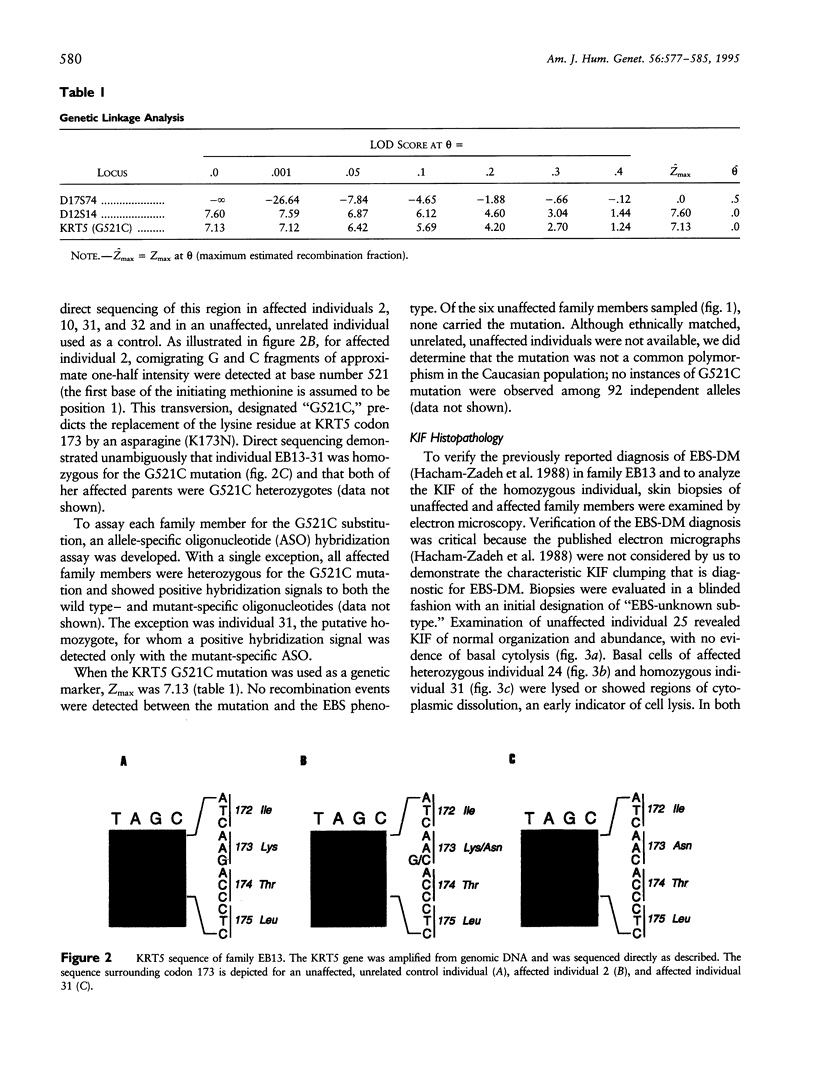
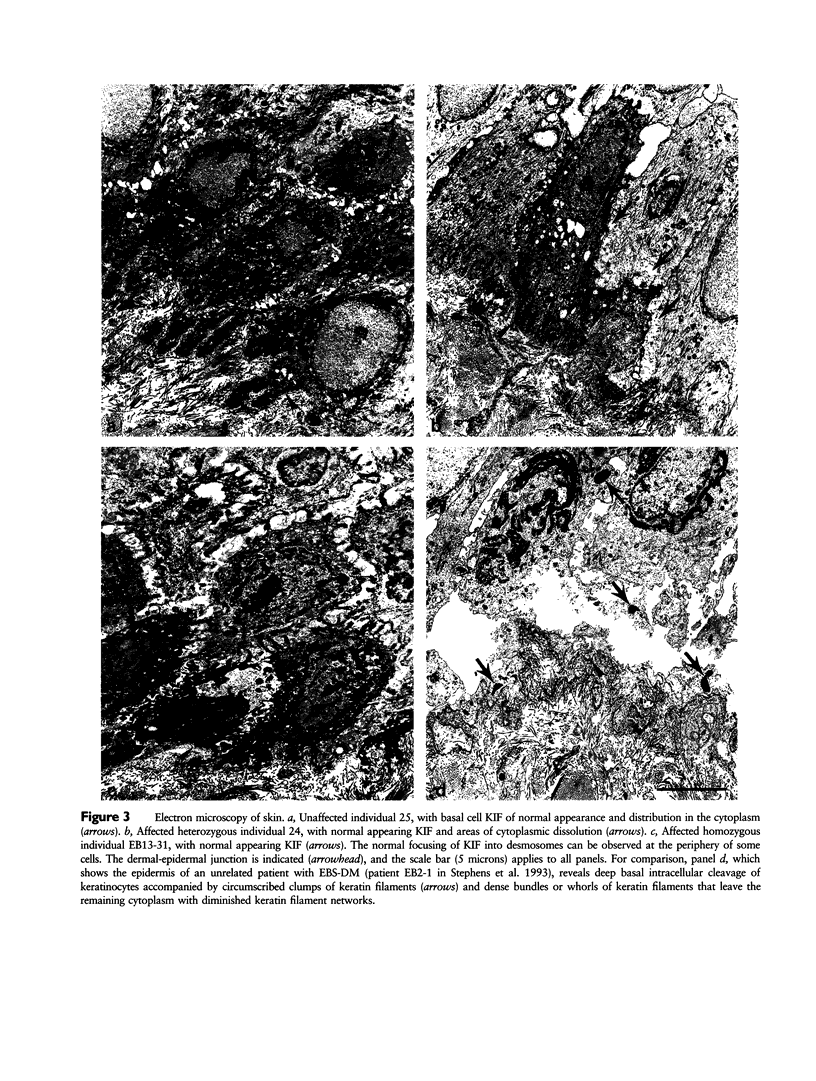
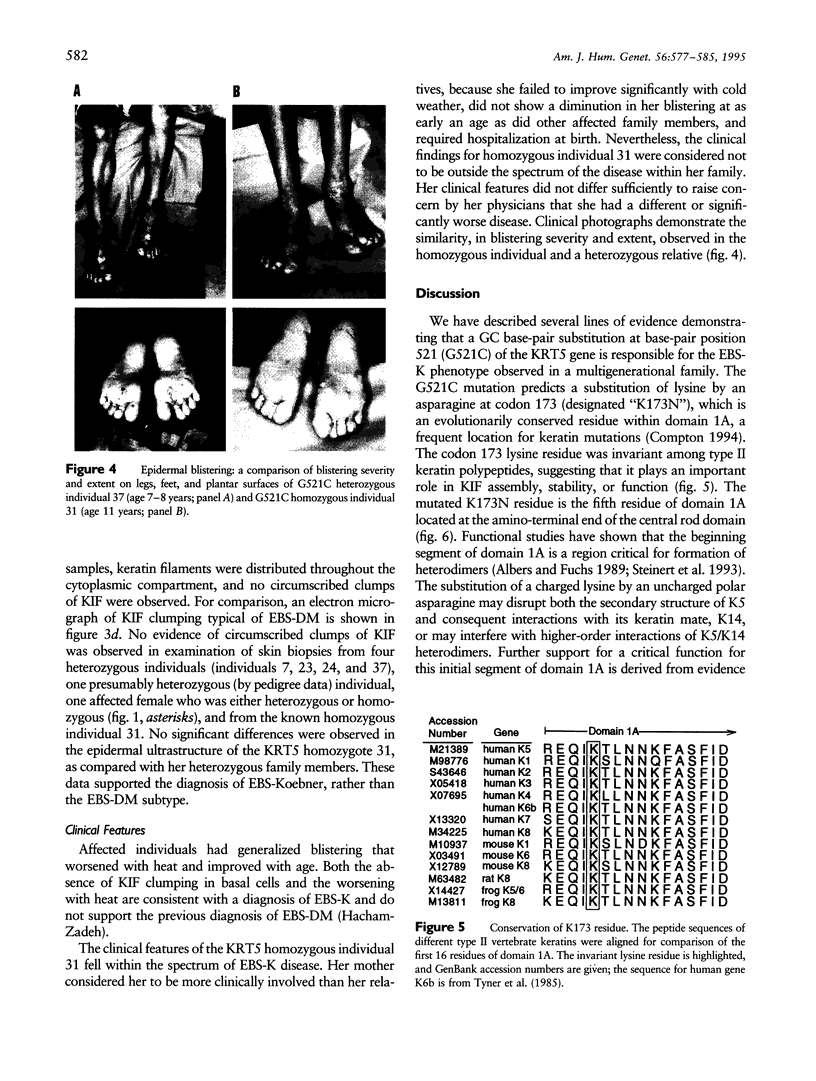
To explore the relationship between abnormal keratin molecules, 10-nm intermediate filament (IF) organization, and epidermal fragility and blistering, we sought to determine the functional consequences of homozygosity for a dominant keratin defect. We describe a family with an autosomal dominant skin-blistering disorder, epidermolysis bullosa simplex, Koebner subtype (EBS-K), that has a novel point mutation, occurring in the keratin 5 gene (KRT5), that predicts the substitution of an evolutionarily conserved lysine by an asparagine residue (K173N). Unlike previous heterozygous mutations located within the initial segment of domain 1A of keratin molecules, K173N heterozygosity did not result in severe disease or clumping of keratin filaments. One family member was found to be homozygous for the K173N allele, having inherited it from each of her affected first-cousin parents. Despite a lack of normal keratin 5 molecules, and an effective doubling of abnormal molecules, available for heterodimerization with keratin 14 during IF formation, there were no significant differences in the clinical severity or the ultrastructural organization of the keratin IF cytoskeleton of the homozygous individual. These data demonstrate that the K173N mutation behaves as a fully dominant allele and indicate that a limited number of abnormal keratin molecules are sufficient to impair cytoskeletal function and elicit epidermal fragility and blistering.
Full text
PDF

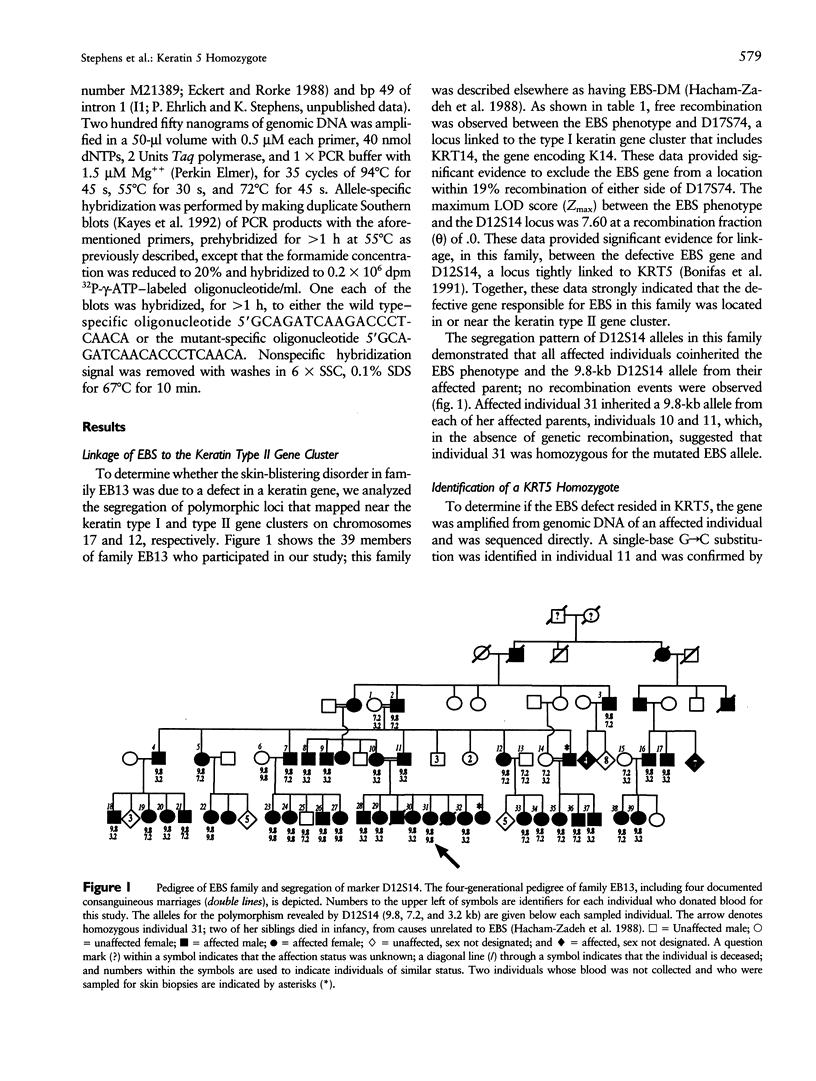



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers K., Fuchs E. Expression of mutant keratin cDNAs in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J Cell Biol. 1989 Apr;108(4):1477–1493. doi: 10.1083/jcb.108.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifas J. M., Rothman A. L., Epstein E. H., Jr Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991 Nov 22;254(5035):1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Brandi M. L., Weber G., Svensson A., Falchetti A., Tonelli F., Castello R., Furlani L., Scappaticci S., Fraccaro M., Larsson C. Homozygotes for the autosomal dominant neoplasia syndrome (MEN1). Am J Hum Genet. 1993 Dec;53(6):1167–1172. [PMC free article] [PubMed] [Google Scholar]
- Chan Y. M., Yu Q. C., LeBlanc-Straceski J., Christiano A., Pulkkinen L., Kucherlapati R. S., Uitto J., Fuchs E. Mutations in the non-helical linker segment L1-2 of keratin 5 in patients with Weber-Cockayne epidermolysis bullosa simplex. J Cell Sci. 1994 Apr;107(Pt 4):765–774. doi: 10.1242/jcs.107.4.765. [DOI] [PubMed] [Google Scholar]
- Chan Y., Anton-Lamprecht I., Yu Q. C., Jäckel A., Zabel B., Ernst J. P., Fuchs E. A human keratin 14 "knockout": the absence of K14 leads to severe epidermolysis bullosa simplex and a function for an intermediate filament protein. Genes Dev. 1994 Nov 1;8(21):2574–2587. doi: 10.1101/gad.8.21.2574. [DOI] [PubMed] [Google Scholar]
- Cohn D. H., Starman B. J., Blumberg B., Byers P. H. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1). Am J Hum Genet. 1990 Mar;46(3):591–601. [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Chan Y. M., Albers K., Fuchs E. Deletions in epidermal keratins leading to alterations in filament organization in vivo and in intermediate filament assembly in vitro. J Cell Biol. 1990 Dec;111(6 Pt 2):3049–3064. doi: 10.1083/jcb.111.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Dong W., Ryynänen M., Uitto J. Identification of a leucine-to-proline mutation in the keratin 5 gene in a family with the generalized Köbner type of epidermolysis bullosa simplex. Hum Mutat. 1993;2(2):94–102. doi: 10.1002/humu.1380020206. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Rorke E. A. The sequence of the human epidermal 58-kD (#5) type II keratin reveals an absence of 5' upstream sequence conservation between coexpressed epidermal keratins. DNA. 1988 Jun;7(5):337–345. doi: 10.1089/dna.1.1988.7.337. [DOI] [PubMed] [Google Scholar]
- Fine J. D., Bauer E. A., Briggaman R. A., Carter D. M., Eady R. A., Esterly N. B., Holbrook K. A., Hurwitz S., Johnson L., Lin A. Revised clinical and laboratory criteria for subtypes of inherited epidermolysis bullosa. A consensus report by the Subcommittee on Diagnosis and Classification of the National Epidermolysis Bullosa Registry. J Am Acad Dermatol. 1991 Jan;24(1):119–135. doi: 10.1016/0190-9622(91)70021-s. [DOI] [PubMed] [Google Scholar]
- Hacham-Zadeh S., Rappersberger K., Livshin R., Konrad K. Epidermolysis bullosa herpetiformis Dowling-Meara in a large family. J Am Acad Dermatol. 1988 Apr;18(4 Pt 1):702–706. doi: 10.1016/s0190-9622(88)70093-6. [DOI] [PubMed] [Google Scholar]
- Hobbs H. H., Brown M. S., Goldstein J. L. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1(6):445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- Holmgren G., Bergström S., Drugge U., Lundgren E., Nording-Sikström C., Sandgren O., Steen L. Homozygosity for the transthyretin-Met30-gene in seven individuals with familial amyloidosis with polyneuropathy detected by restriction enzyme analysis of amplified genomic DNA sequences. Clin Genet. 1992 Jan;41(1):39–41. doi: 10.1111/j.1399-0004.1992.tb03627.x. [DOI] [PubMed] [Google Scholar]
- Hovnanian A., Pollack E., Hilal L., Rochat A., Prost C., Barrandon Y., Goossens M. A missense mutation in the rod domain of keratin 14 associated with recessive epidermolysis bullosa simplex. Nat Genet. 1993 Apr;3(4):327–332. doi: 10.1038/ng0493-327. [DOI] [PubMed] [Google Scholar]
- Kayes L. M., Schroeder W. T., Marchuk D. A., Collins F. S., Riccardi V. M., Duvic M., Stephens K. The gene for a novel epidermal antigen maps near the neurofibromatosis 1 gene. Genomics. 1992 Oct;14(2):369–376. doi: 10.1016/s0888-7543(05)80228-9. [DOI] [PubMed] [Google Scholar]
- Kretz K. A., Carson G. S., O'Brien J. S. Direct sequencing from low-melt agarose with Sequenase. Nucleic Acids Res. 1989 Jul 25;17(14):5864–5864. doi: 10.1093/nar/17.14.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A., Coulombe P. A., McCormick M. B., Yu Q. C., Hutton E., Fuchs E. Disease severity correlates with position of keratin point mutations in patients with epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3197–3201. doi: 10.1073/pnas.90.8.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., de Oca-Luna R. M., Slaugenhaupt S., Pentao L., Guzzetta V., Trask B. J., Saucedo-Cardenas O., Barker D. F., Killian J. M., Garcia C. A. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991 Jul 26;66(2):219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986 Aug;73(4):320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- Ott J. A computer program for linkage analysis of general human pedigrees. Am J Hum Genet. 1976 Sep;28(5):528–529. [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Dickson L. A., Pope F. M., Korhonen V. R., Nicholls A., Prockop D. J., Myers J. C. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984 Nov 10;259(21):12941–12944. [PubMed] [Google Scholar]
- Reis A., Hennies H. C., Langbein L., Digweed M., Mischke D., Drechsler M., Schröck E., Royer-Pokora B., Franke W. W., Sperling K. Keratin 9 gene mutations in epidermolytic palmoplantar keratoderma (EPPK). Nat Genet. 1994 Feb;6(2):174–179. doi: 10.1038/ng0294-174. [DOI] [PubMed] [Google Scholar]
- Rothnagel J. A., Fisher M. P., Axtell S. M., Pittelkow M. R., Anton-Lamprecht I., Huber M., Hohl D., Roop D. R. A mutational hot spot in keratin 10 (KRT 10) in patients with epidermolytic hyperkeratosis. Hum Mol Genet. 1993 Dec;2(12):2147–2150. doi: 10.1093/hmg/2.12.2147. [DOI] [PubMed] [Google Scholar]
- Rothnagel J. A., Traupe H., Wojcik S., Huber M., Hohl D., Pittelkow M. R., Saeki H., Ishibashi Y., Roop D. R. Mutations in the rod domain of keratin 2e in patients with ichthyosis bullosa of Siemens. Nat Genet. 1994 Aug;7(4):485–490. doi: 10.1038/ng0894-485. [DOI] [PubMed] [Google Scholar]
- Rugg E. L., McLean W. H., Lane E. B., Pitera R., McMillan J. R., Dopping-Hepenstal P. J., Navsaria H. A., Leigh I. M., Eady R. A. A functional "knockout" of human keratin 14. Genes Dev. 1994 Nov 1;8(21):2563–2573. doi: 10.1101/gad.8.21.2563. [DOI] [PubMed] [Google Scholar]
- Rugg E. L., Morley S. M., Smith F. J., Boxer M., Tidman M. J., Navsaria H., Leigh I. M., Lane E. B. Missing links: Weber-Cockayne keratin mutations implicate the L12 linker domain in effective cytoskeleton function. Nat Genet. 1993 Nov;5(3):294–300. doi: 10.1038/ng1193-294. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Orr H. T., Anderson L., Nemens E., White J. A., Bonnycastle L., Weber J. L., Alonso M. E. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992 Oct 23;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Shiang R., Thompson L. M., Zhu Y. Z., Church D. M., Fielder T. J., Bocian M., Winokur S. T., Wasmuth J. J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994 Jul 29;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Sybert V. P. Intra-epidermal retention of type VII collagen in a patient with recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1990 Feb;94(2):261–264. doi: 10.1111/1523-1747.ep12874614. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Giebel L. B., Holmes S. A. Dominant negative and loss of function mutations of the c-kit (mast/stem cell growth factor receptor) proto-oncogene in human piebaldism. Am J Hum Genet. 1992 Feb;50(2):261–269. [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M. Structure, function, and dynamics of keratin intermediate filaments. J Invest Dermatol. 1993 Jun;100(6):729–734. doi: 10.1111/1523-1747.ep12475665. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Yang J. M., Bale S. J., Compton J. G. Concurrence between the molecular overlap regions in keratin intermediate filaments and the locations of keratin mutations in genodermatoses. Biochem Biophys Res Commun. 1993 Dec 15;197(2):840–848. doi: 10.1006/bbrc.1993.2555. [DOI] [PubMed] [Google Scholar]
- Stephens K., Sybert V. P., Wijsman E. M., Ehrlich P., Spencer A. A keratin 14 mutational hot spot for epidermolysis bullosa simplex, Dowling-Meara: implications for diagnosis. J Invest Dermatol. 1993 Aug;101(2):240–243. doi: 10.1111/1523-1747.ep12365079. [DOI] [PubMed] [Google Scholar]
- Torchard D., Blanchet-Bardon C., Serova O., Langbein L., Narod S., Janin N., Goguel A. F., Bernheim A., Franke W. W., Lenoir G. M. Epidermolytic palmoplantar keratoderma cosegregates with a keratin 9 mutation in a pedigree with breast and ovarian cancer. Nat Genet. 1994 Jan;6(1):106–110. doi: 10.1038/ng0194-106. [DOI] [PubMed] [Google Scholar]
- Vassar R., Coulombe P. A., Degenstein L., Albers K., Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991 Jan 25;64(2):365–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Wilkie A. O. The molecular basis of genetic dominance. J Med Genet. 1994 Feb;31(2):89–98. doi: 10.1136/jmg.31.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Byers P. H. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990 Jan;85(1):282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Pruchno C. J., Atkinson M., Byers P. H. Osteogenesis imperfecta type I is commonly due to a COL1A1 null allele of type I collagen. Am J Hum Genet. 1992 Sep;51(3):508–515. [PMC free article] [PubMed] [Google Scholar]
- Yamanishi K., Matsuki M., Konishi K., Yasuno H. A novel mutation of Leu122 to Phe at a highly conserved hydrophobic residue in the helix initiation motif of keratin 14 in epidermolysis bullosa simplex. Hum Mol Genet. 1994 Jul;3(7):1171–1172. doi: 10.1093/hmg/3.7.1171. [DOI] [PubMed] [Google Scholar]