Abstract
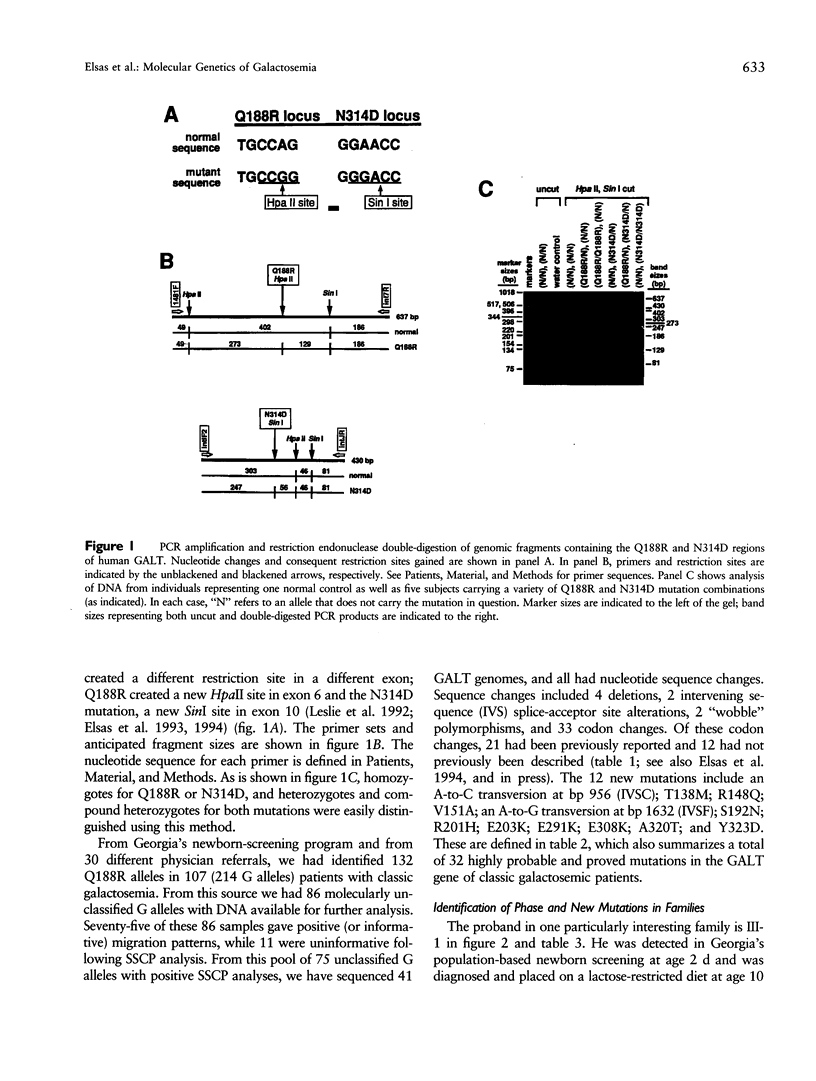
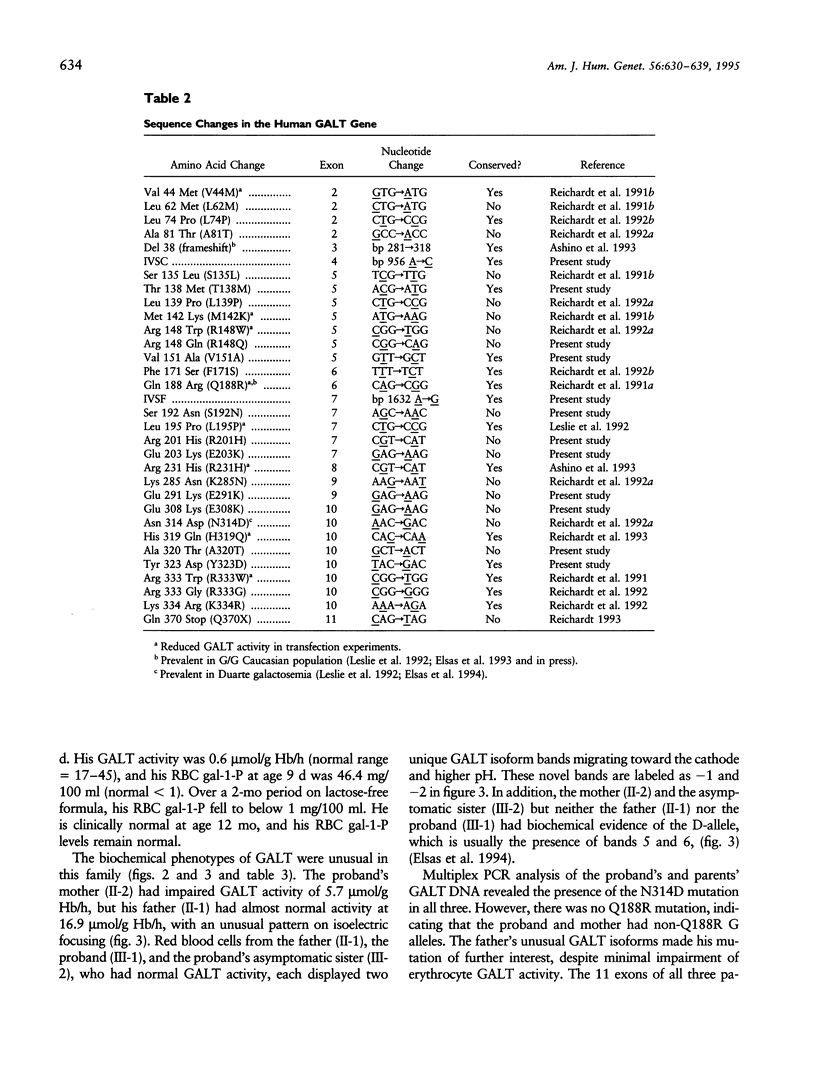
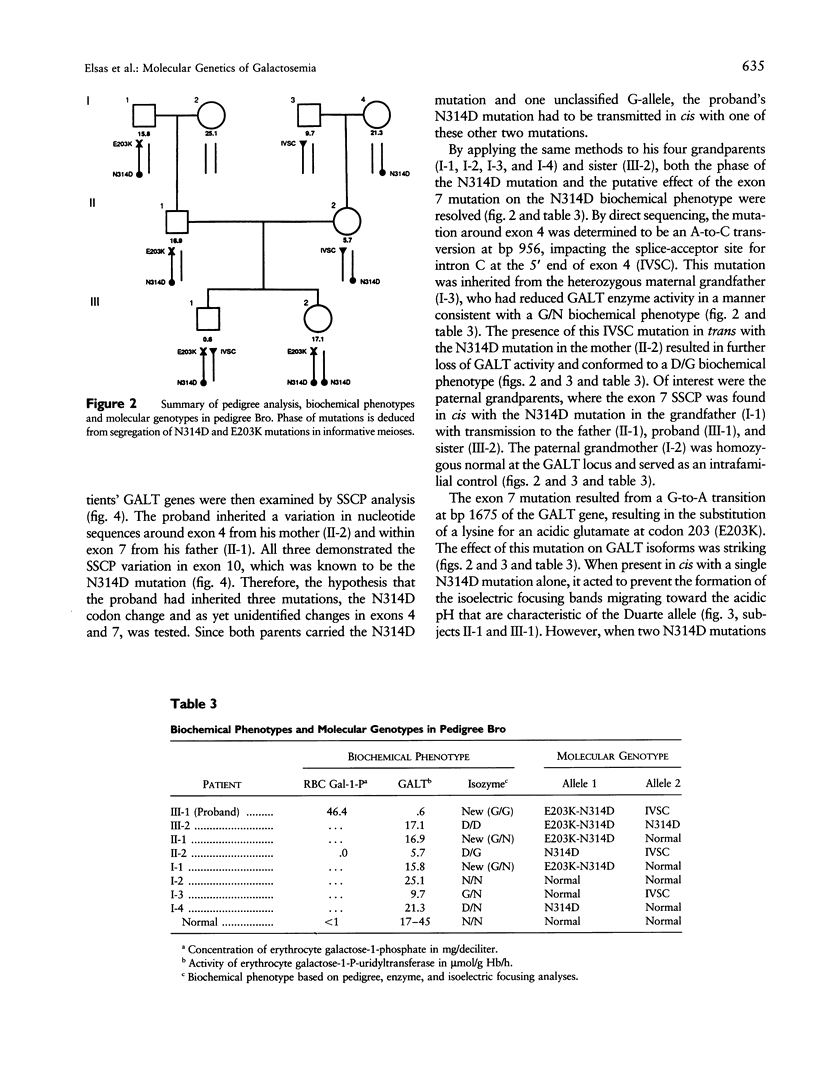
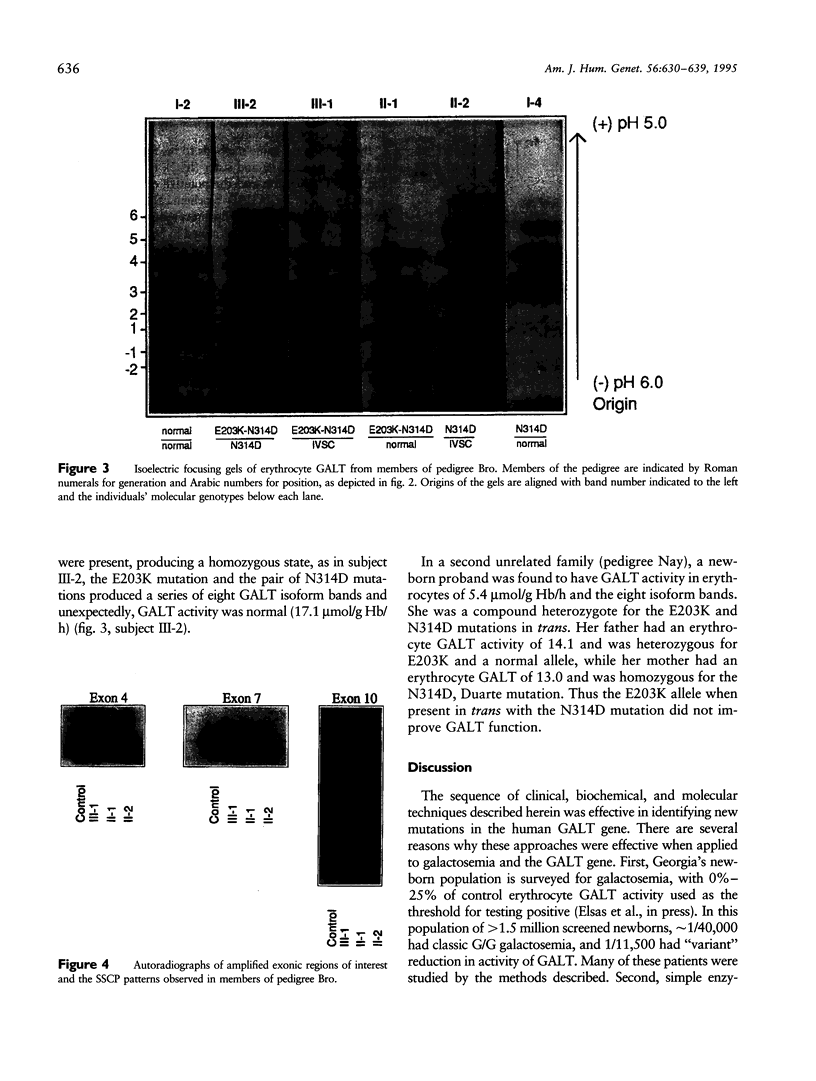
We describe a stratagem for identifying new mutations in the galactose-1-phosphate uridyl transferase (GALT) gene. GALT enzyme activity and isoforms were defined in erythrocytes from probands and their first-degree relatives. If the biochemical phenotypes segregated in an autosomal recessive pattern, we screened for common mutations by using multiplex PCR and restriction endonuclease digestions. If common mutant alleles were not present, the 11 exons of the GALT gene were amplified by PCR, and variations from the normal nucleotide sequences were identified by SSCP. The suspected region(s) was then analyzed by direct DNA sequencing. We identified 86 mutant GALT alleles that reduced erythrocyte GALT activity. Seventy-five of these GALT genomes had abnormal SSCP patterns, of which 41 were sequenced, yielding 12 new and 21 previously reported, rare mutations. Among the novel group of 12 new mutations, an unusual biochemical phenotype was found in a family whose newborn proband has classical galactosemia. He had inherited two mutations in cis (N314D-E203K) from his father, whose GALT activity was near normal, and an additional GALT mutation in the splice-acceptor site of intron C (IVSC) from his mother. The substitution of a positively charged E203K mutation created a unique isoform-banding pattern. An asymptomatic sister's GALT genes carries three mutations (E203K-N314D/N314D) with eight distinct isoform bands. Surprisingly, her erythrocytes have normal GALT activity. We conclude that the synergism of pedigree, biochemical, SSCP, and direct GALT gene analyses is an efficient protocol for identifying new mutations and speculate that E203K and N314D codon changes produce intraallelic complementation when in cis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen M. W., Williams V. P., Helmer G. R., Jr, Fried C., Popják G. Transferase-deficiency galactosemia: evidence for the lack of a transferase protein in galactosemic red cells. Arch Biochem Biophys. 1983 Apr 1;222(1):326–331. doi: 10.1016/0003-9861(83)90530-1. [DOI] [PubMed] [Google Scholar]
- Andersen M. W., Williams V. P., Sparkes M. C., Sparkes R. S. Transferase-deficiency galactosemia: immunochemical studies of the Duarte and Los Angeles variants. Hum Genet. 1984;65(3):287–290. doi: 10.1007/BF00286519. [DOI] [PubMed] [Google Scholar]
- Beutler E., Baluda M. C., Sturgeon P., Day R. W. The genetics of galactose-1-phosphate uridyl transferase deficiency. J Lab Clin Med. 1966 Oct;68(4):646–658. [PubMed] [Google Scholar]
- Beutler E. Screening for galactosemia. Studies of the gene frequencies for galactosemia and the Duarte variant. Isr J Med Sci. 1973 Sep-Oct;9(9):1323–1329. [PubMed] [Google Scholar]
- Elsas L. J., Dembure P. P., Langley S., Paulk E. M., Hjelm L. N., Fridovich-Keil J. A common mutation associated with the Duarte galactosemia allele. Am J Hum Genet. 1994 Jun;54(6):1030–1036. [PMC free article] [PubMed] [Google Scholar]
- Flach J. E., Reichardt J. K., Elsas L. J., 2nd Sequence of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1990 Aug;7(4):365–369. [PubMed] [Google Scholar]
- Fox J. G. Experience of the Manitoba Perinatal Screening Program, 1965-85. CMAJ. 1987 Nov 15;137(10):883–888. [PMC free article] [PubMed] [Google Scholar]
- Frey P. A., Wong L. J., Sheu K. F., Yang S. L. Galactose-1-phosphate uridylyltransferase: detection, isolation, and characterization of the uridylyl enzyme. Methods Enzymol. 1982;87:20–36. doi: 10.1016/s0076-6879(82)87004-3. [DOI] [PubMed] [Google Scholar]
- Fridovich-Keil J. L., Jinks-Robertson S. A yeast expression system for human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):398–402. doi: 10.1073/pnas.90.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. I., Harris H., Mellman W. J. Characterization of normal and abnormal variants of galactose-1-phosphate uridylyltransferase (EC 2.7.7.12) by isoelectric focusing. Hum Genet. 1983;63(3):274–279. doi: 10.1007/BF00284663. [DOI] [PubMed] [Google Scholar]
- Kelley R. I., Segal S. Evaluation of reduced activity galactose-1-phosphate uridyl transferase by combined radioisotopic assay and high-resolution isoelectric focusing. J Lab Clin Med. 1989 Aug;114(2):152–156. [PubMed] [Google Scholar]
- Kühnl P., Nowicki L., Spielmann W. Untersuchungen zum Polymorphismus der Galaktose-1-Phosphat-Uridyltransferase (EC: 2.7.7.12) mittels Agarosegelelektrophorese. Humangenetik. 1974;24(3):227–230. [PubMed] [Google Scholar]
- LELOIR L. F. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch Biochem Biophys. 1951 Sep;33(2):186–190. doi: 10.1016/0003-9861(51)90096-3. [DOI] [PubMed] [Google Scholar]
- Lee J. E., Ng W. G. Semi-micro techniques for the genotyping of galactokinase and galactose-1-phosphate uridyltransferase. Clin Chim Acta. 1982 Sep 30;124(3):351–356. doi: 10.1016/0009-8981(82)90429-6. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Müller-Hill B. Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res. 1986 Oct 10;14(19):7705–7711. doi: 10.1093/nar/14.19.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie N. D., Immerman E. B., Flach J. E., Florez M., Fridovich-Keil J. L., Elsas L. J. The human galactose-1-phosphate uridyltransferase gene. Genomics. 1992 Oct;14(2):474–480. doi: 10.1016/s0888-7543(05)80244-7. [DOI] [PubMed] [Google Scholar]
- Longo N., Langley S. D., Griffin L. D., Elsas L. J. Activation of glucose transport by a natural mutation in the human insulin receptor. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):60–64. doi: 10.1073/pnas.90.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo N., Shuster R. C., Griffin L. D., Langley S. D., Elsas L. J. Activation of insulin receptor signaling by a single amino acid substitution in the transmembrane domain. J Biol Chem. 1992 Jun 25;267(18):12416–12419. [PubMed] [Google Scholar]
- Mellman W. J., Tedesco T. A. An improved assay of erythrocyte and leukocyte galactose-1-phosphate uridyl transferase: stabilization of the enzyme by a thiol protective reagent. J Lab Clin Med. 1965 Dec;66(6):980–986. [PubMed] [Google Scholar]
- Reichardt J. K., Belmont J. W., Levy H. L., Woo S. L. Characterization of two missense mutations in human galactose-1-phosphate uridyltransferase: different molecular mechanisms for galactosemia. Genomics. 1992 Mar;12(3):596–600. doi: 10.1016/0888-7543(92)90453-y. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Berg P. Cloning and characterization of a cDNA encoding human galactose-1-phosphate uridyl transferase. Mol Biol Med. 1988 Apr;5(2):107–122. [PubMed] [Google Scholar]
- Reichardt J. K., Levy H. L., Woo S. L. Molecular characterization of two galactosemia mutations and one polymorphism: implications for structure-function analysis of human galactose-1-phosphate uridyltransferase. Biochemistry. 1992 Jun 23;31(24):5430–5433. doi: 10.1021/bi00139a002. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Novelli G., Dallapiccola B. Molecular characterization of the H319Q galactosemia mutation. Hum Mol Genet. 1993 Mar;2(3):325–326. doi: 10.1093/hmg/2.3.325. [DOI] [PubMed] [Google Scholar]
- Reichardt J. K., Packman S., Woo S. L. Molecular characterization of two galactosemia mutations: correlation of mutations with highly conserved domains in galactose-1-phosphate uridyl transferase. Am J Hum Genet. 1991 Oct;49(4):860–867. [PMC free article] [PubMed] [Google Scholar]
- Reichardt J. K., Woo S. L. Molecular basis of galactosemia: mutations and polymorphisms in the gene encoding human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2633–2637. doi: 10.1073/pnas.88.7.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. S., Niedermeier H. P., Endres W., Schaub J., Weidinger S. Agarose gel isoelectrofocusing of UDP-galactose pyrophosphorylase and galactose-1-phosphate uridyltransferase. Developmental aspect of UDP-galactose pyrophosphorylase. Clin Chim Acta. 1987 Jun 30;166(1):27–35. doi: 10.1016/0009-8981(87)90191-4. [DOI] [PubMed] [Google Scholar]
- Sparkes M. C., Crist M., Sparkes R. S. Improved technique for electrophoresis of human galactose-1-p uridyl transferase (EC 2.7.7.12). Hum Genet. 1977 Dec 29;40(1):93–97. doi: 10.1007/BF00280835. [DOI] [PubMed] [Google Scholar]
- Tajima M., Nogi Y., Fukasawa T. Primary structure of the Saccharomyces cerevisiae GAL7 gene. Yeast. 1985 Sep;1(1):67–77. doi: 10.1002/yea.320010108. [DOI] [PubMed] [Google Scholar]
- Tedesco T. A. Human galactose 1-phosphate uridyltransferase. Purification, antibody production, and comparison of the wild type, Duarte variant, and galactosemic gene products. J Biol Chem. 1972 Oct 25;247(20):6631–6636. [PubMed] [Google Scholar]





