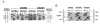Abstract
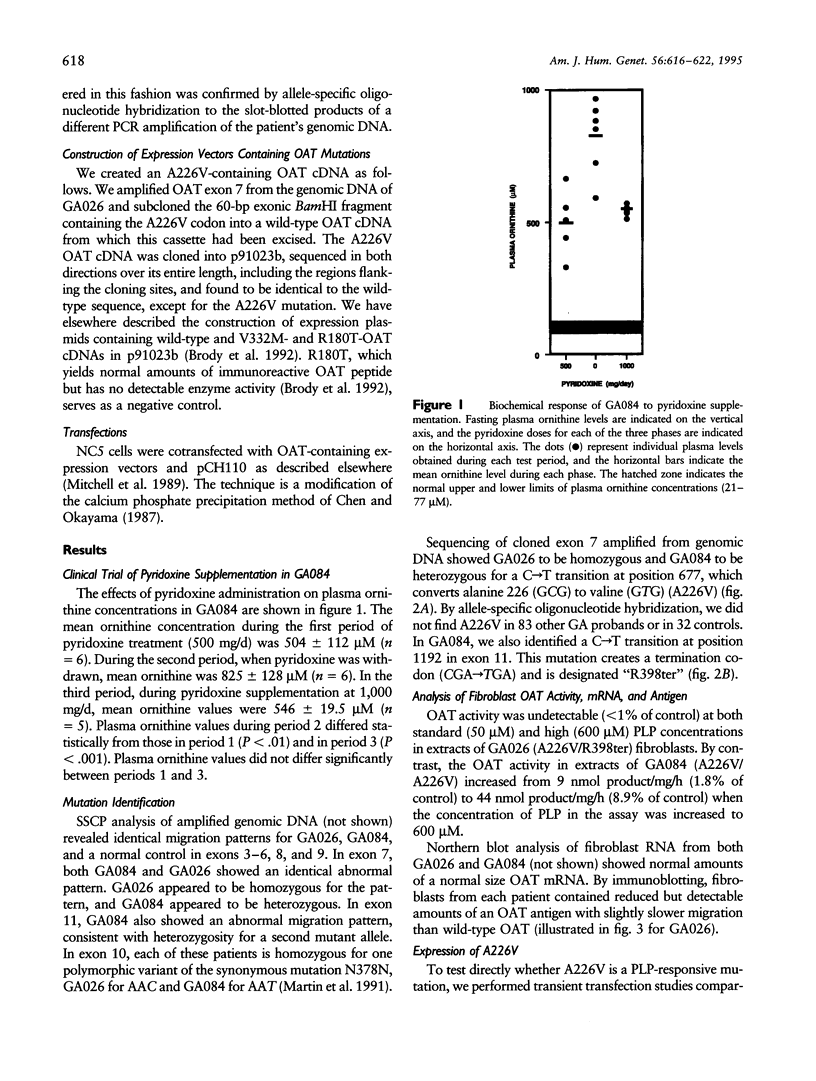
We discovered the missense mutation, A226V, in the ornithine-delta-aminotransferase (OAT) genes of two unrelated patients with gyrate atrophy of the choroid and retina (GA). One patient, who was a compound for A226V and for the premature termination allele R398ter, showed a significant (P < .01) decrease in mean plasma ornithine levels, following pyridoxine supplementation with a constant protein intake: 826 +/- 128 microM (n = 5; no pyridoxine supplementation) versus 504 +/- 112 microM (n = 6; 500 mg pyridoxine/d) and 546 +/- 19 microM (n = 6; 1,000 mg pyridoxine/d). In extracts of fibroblasts from a second GA patient homozygous for A226V and from Chinese hamster ovary cells expressing an OAT-cDNA-containing A226V, we found that OAT activity increased from undetectable levels to approximately 10% of normal when the concentration of pyridoxal phosphate was increased from 50 to 600 microM. A226V is the fourth disease-causing pyridoxine-responsive human mutation to be reported.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaki Y., Hotta Y., Mashima Y., Murakami A., Kennaway N. G., Weleber R. G., Inana G. A deletion in the ornithine aminotransferase gene in gyrate atrophy. J Biol Chem. 1992 Jun 25;267(18):12950–12954. [PubMed] [Google Scholar]
- Berson E. L., Schmidt S. Y., Shih V. E. Ocular and biochemical abnormalities in gyrate atrophy of the choroid and retina. Ophthalmology. 1978 Oct;85(10):1018–1027. doi: 10.1016/s0161-6420(78)35588-3. [DOI] [PubMed] [Google Scholar]
- Brody L. C., Mitchell G. A., Obie C., Michaud J., Steel G., Fontaine G., Robert M. F., Sipila I., Kaiser-Kupfer M., Valle D. Ornithine delta-aminotransferase mutations in gyrate atrophy. Allelic heterogeneity and functional consequences. J Biol Chem. 1992 Feb 15;267(5):3302–3307. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter P. D., Baumann M., Bishop D. F. Enzymatic defect in "X-linked" sideroblastic anemia: molecular evidence for erythroid delta-aminolevulinate synthase deficiency. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4028–4032. doi: 10.1073/pnas.89.9.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. C., Bottomley S. S., Wiley J. S., Bawden M. J., Matthews C. S., May B. K. X-linked pyridoxine-responsive sideroblastic anemia due to a Thr388-to-Ser substitution in erythroid 5-aminolevulinate synthase. N Engl J Med. 1994 Mar 10;330(10):675–679. doi: 10.1056/NEJM199403103301004. [DOI] [PubMed] [Google Scholar]
- Degols G., Jauniaux J. C., Wiame J. M. Molecular characterization of transposable-element-associated mutations that lead to constitutive L-ornithine aminotransferase expression in Saccharomyces cerevisiae. Eur J Biochem. 1987 Jun 1;165(2):289–296. doi: 10.1111/j.1432-1033.1987.tb11440.x. [DOI] [PubMed] [Google Scholar]
- Delauney A. J., Hu C. A., Kishor P. B., Verma D. P. Cloning of ornithine delta-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. J Biol Chem. 1993 Sep 5;268(25):18673–18678. [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hayasaka S., Saito T., Nakajima H., Takaku Y., Shiono T., Mizuno K., Ohmura K., Tada K. Gyrate atrophy with hyperornithinaemia: different types of responsiveness to vitamin B6. Br J Ophthalmol. 1981 Jul;65(7):478–483. doi: 10.1136/bjo.65.7.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F. L., Gu Z., Kozich V., Kraus J. P., Ramesh V., Shih V. E. Molecular basis of cystathionine beta-synthase deficiency in pyridoxine responsive and nonresponsive homocystinuria. Hum Mol Genet. 1993 Nov;2(11):1857–1860. doi: 10.1093/hmg/2.11.1857. [DOI] [PubMed] [Google Scholar]
- Inana G., Chambers C., Hotta Y., Inouye L., Filpula D., Pulford S., Shiono T. Point mutation affecting processing of the ornithine aminotransferase precursor protein in gyrate atrophy. J Biol Chem. 1989 Oct 15;264(29):17432–17436. [PubMed] [Google Scholar]
- Inana G., Totsuka S., Redmond M., Dougherty T., Nagle J., Shiono T., Ohura T., Kominami E., Katunuma N. Molecular cloning of human ornithine aminotransferase mRNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1203–1207. doi: 10.1073/pnas.83.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway N. G., Stankova L., Wirtz M. K., Weleber R. G. Gyrate atrophy of the choroid and retina: characterization of mutant ornithine aminotransferase and mechanism of response to vitamin B6. Am J Hum Genet. 1989 Mar;44(3):344–352. [PMC free article] [PubMed] [Google Scholar]
- Kennaway N. G., Weleber R. G., Buist N. R. Gyrate atrophy of the choroid and retina with hyperornithinemia: biochemical and histologic studies and response to vitamin B6. Am J Hum Genet. 1980 Jul;32(4):529–541. [PMC free article] [PubMed] [Google Scholar]
- Kozich V., de Franchis R., Kraus J. P. Molecular defect in a patient with pyridoxine-responsive homocystinuria. Hum Mol Genet. 1993 Jun;2(6):815–816. doi: 10.1093/hmg/2.6.815. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Le K., Swaroop M., Ohura T., Tahara T., Rosenberg L. E., Roper M. D., Kozich V. Human cystathionine beta-synthase cDNA: sequence, alternative splicing and expression in cultured cells. Hum Mol Genet. 1993 Oct;2(10):1633–1638. doi: 10.1093/hmg/2.10.1633. [DOI] [PubMed] [Google Scholar]
- Labuda D., Striker G. Sequence conservation in Alu evolution. Nucleic Acids Res. 1989 Apr 11;17(7):2477–2491. doi: 10.1093/nar/17.7.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marković-Housley Z., Kania M., Lustig A., Vincent M. G., Jansonius J. N., John R. A. Quaternary structure of ornithine aminotransferase in solution and preliminary crystallographic data. Eur J Biochem. 1987 Jan 15;162(2):345–350. doi: 10.1111/j.1432-1033.1987.tb10607.x. [DOI] [PubMed] [Google Scholar]
- Martin L. S., Mitchell G. A., Michaud J., Brody L. C., Valle D. A polymorphic synonymous mutation in human ornithine-delta-aminotransferase (N378N). Nucleic Acids Res. 1991 Apr 25;19(8):1962–1962. doi: 10.1093/nar/19.8.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima Y., Murakami A., Weleber R. G., Kennaway N. G., Clarke L., Shiono T., Inana G. Nonsense-codon mutations of the ornithine aminotransferase gene with decreased levels of mutant mRNA in gyrate atrophy. Am J Hum Genet. 1992 Jul;51(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., Kaufman D. L., Berson E. L., Tobin A. J., Shih V. E., Gusella J. F., Ramesh V. Splicing defect at the ornithine aminotransferase (OAT) locus in gyrate atrophy. Am J Hum Genet. 1990 Nov;47(5):790–794. [PMC free article] [PubMed] [Google Scholar]
- Michaud J., Brody L. C., Steel G., Fontaine G., Martin L. S., Valle D., Mitchell G. Strand-separating conformational polymorphism analysis: efficacy of detection of point mutations in the human ornithine delta-aminotransferase gene. Genomics. 1992 Jun;13(2):389–394. doi: 10.1016/0888-7543(92)90258-t. [DOI] [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Looney J., Steel G., Suchanek M., Dowling C., Der Kaloustian V., Kaiser-Kupfer M., Valle D. An initiator codon mutation in ornithine-delta-aminotransferase causing gyrate atrophy of the choroid and retina. J Clin Invest. 1988 Feb;81(2):630–633. doi: 10.1172/JCI113365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Brody L. C., Sipila I., Looney J. E., Wong C., Engelhardt J. F., Patel A. S., Steel G., Obie C., Kaiser-Kupfer M. At least two mutant alleles of ornithine delta-aminotransferase cause gyrate atrophy of the choroid and retina in Finns. Proc Natl Acad Sci U S A. 1989 Jan;86(1):197–201. doi: 10.1073/pnas.86.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Labuda D., Fontaine G., Saudubray J. M., Bonnefont J. P., Lyonnet S., Brody L. C., Steel G., Obie C., Valle D. Splice-mediated insertion of an Alu sequence inactivates ornithine delta-aminotransferase: a role for Alu elements in human mutation. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):815–819. doi: 10.1073/pnas.88.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. A., Looney J. E., Brody L. C., Steel G., Suchanek M., Engelhardt J. F., Willard H. F., Valle D. Human ornithine-delta-aminotransferase. cDNA cloning and analysis of the structural gene. J Biol Chem. 1988 Oct 5;263(28):14288–14295. [PubMed] [Google Scholar]
- Morino Y., Shimada K., Kagamiyama H. Mammalian aspartate aminotransferase isozymes. From DNA to protein. Ann N Y Acad Sci. 1990;585:32–47. doi: 10.1111/j.1749-6632.1990.tb28039.x. [DOI] [PubMed] [Google Scholar]
- Mueckler M. M., Pitot H. C. Sequence of the precursor to rat ornithine aminotransferase deduced from a cDNA clone. J Biol Chem. 1985 Oct 25;260(24):12993–12997. [PubMed] [Google Scholar]
- Oprian D. D., Molday R. S., Kaufman R. J., Khorana H. G. Expression of a synthetic bovine rhodopsin gene in monkey kidney cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8874–8878. doi: 10.1073/pnas.84.24.8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka A. J., Buoncristiani M. R., Howard P. K., Flamm J., Johnson C., Yamamoto R., Uchida K., Cook C., Ruppert J., Matsuzaki J. The Escherichia coli biotin biosynthetic enzyme sequences predicted from the nucleotide sequence of the bio operon. J Biol Chem. 1988 Dec 25;263(36):19577–19585. [PubMed] [Google Scholar]
- Park J. K., Herron B. J., O'Donnell J. J., Shih V. E., Ramesh V. Three novel mutations of the ornithine aminotransferase (OAT) gene in gyrate atrophy. Genomics. 1992 Oct;14(2):553–554. doi: 10.1016/s0888-7543(05)80271-x. [DOI] [PubMed] [Google Scholar]
- Ramesh V., McClatchey A. I., Ramesh N., Benoit L. A., Berson E. L., Shih V. E., Gusella J. F. Molecular basis of ornithine aminotransferase deficiency in B-6-responsive and -nonresponsive forms of gyrate atrophy. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3777–3780. doi: 10.1073/pnas.85.11.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh V., Shaffer M. M., Allaire J. M., Shih V. E., Gusella J. F. Investigation of gyrate atrophy using a cDNA clone for human ornithine aminotransferase. DNA. 1986 Dec;5(6):493–501. doi: 10.1089/dna.1.1986.5.493. [DOI] [PubMed] [Google Scholar]
- Richardson I. B., Hurley S. K., Hynes M. J. Cloning and molecular characterisation of the amdR controlled gatA gene of Aspergillus nidulans. Mol Gen Genet. 1989 May;217(1):118–125. doi: 10.1007/BF00330950. [DOI] [PubMed] [Google Scholar]
- Schmid S. R., Linder P., Reese R. T., Stanley H. A. Characterization of a putative ornithine aminotransferase gene of Plasmodium falciparum. Mol Biochem Parasitol. 1993 Oct;61(2):311–314. doi: 10.1016/0166-6851(93)90076-a. [DOI] [PubMed] [Google Scholar]
- Simmaco M., John R. A., Barra D., Bossa F. The primary structure of ornithine aminotransferase. Identification of active-site sequence and site of post-translational proteolysis. FEBS Lett. 1986 Apr 7;199(1):39–42. doi: 10.1016/0014-5793(86)81219-4. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Identification of conserved isotype-defining variable region sequences for four vertebrate beta tubulin polypeptide classes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4327–4331. doi: 10.1073/pnas.83.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonaha K., Nishie M., Aibara S. The primary structure of omega-amino acid:pyruvate aminotransferase. J Biol Chem. 1992 Jun 25;267(18):12506–12510. [PubMed] [Google Scholar]