Abstract
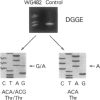
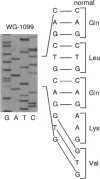
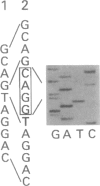
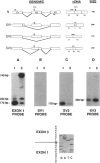
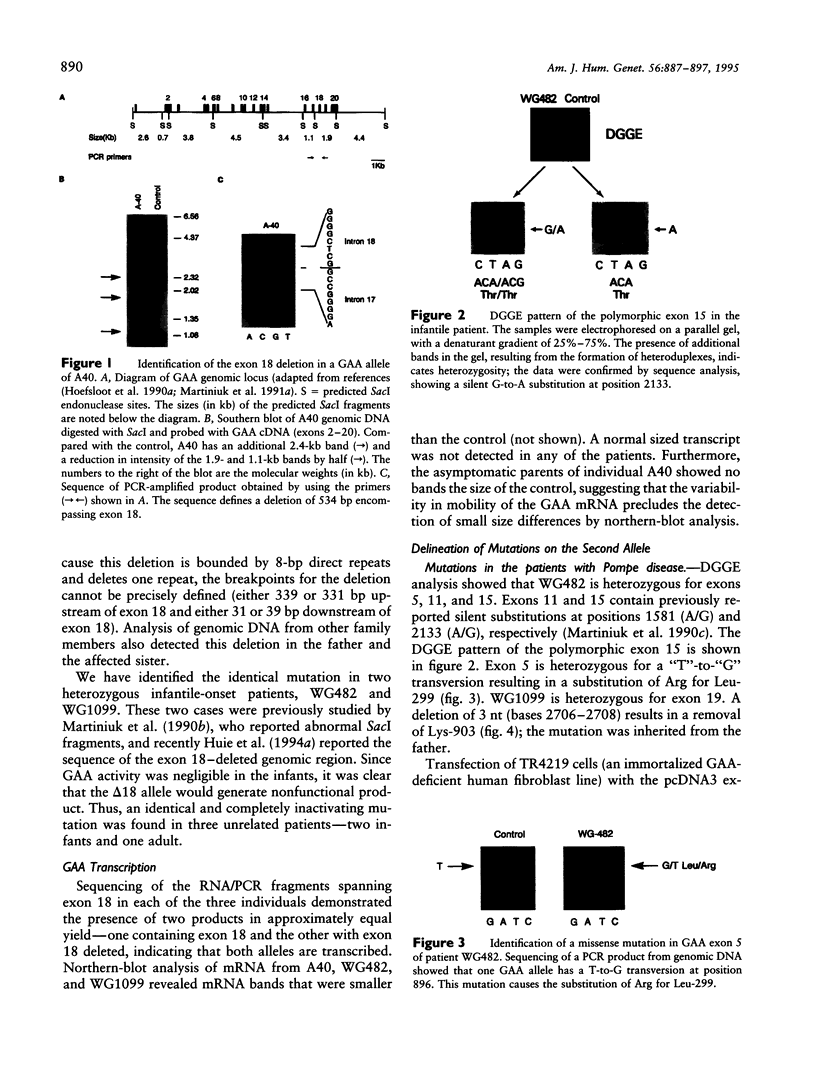
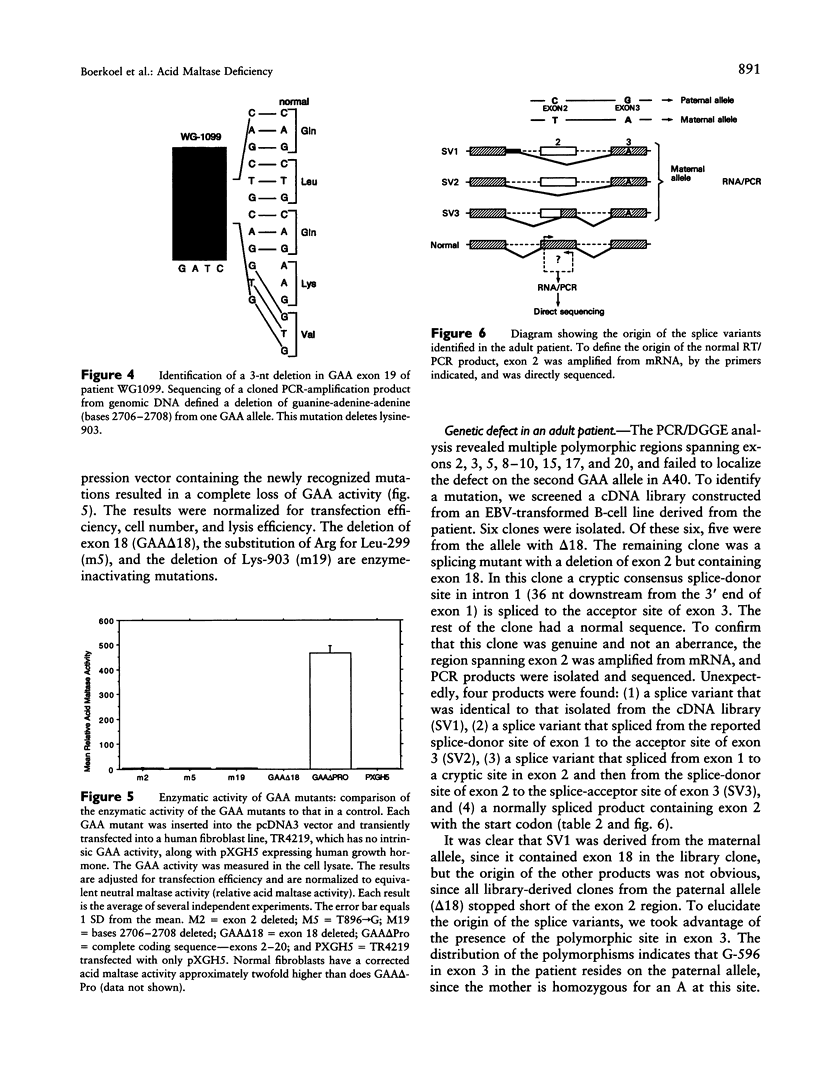
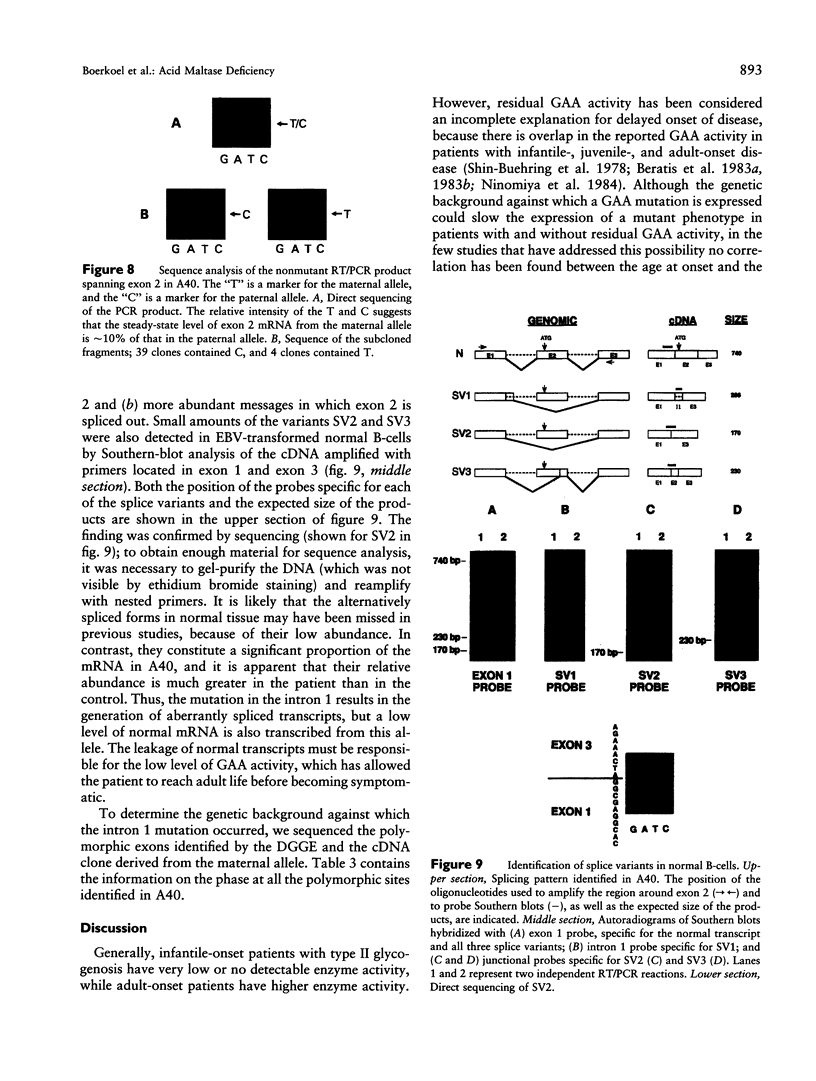
An autosomal recessive deficiency of acid alpha-glucosidase (GAA), type II glycogenosis, is genetically and clinically heterogeneous. The discovery of an enzyme-inactivating genomic deletion of exon 18 in three unrelated genetic compound patients--two infants and an adult--provided a rare opportunity to analyze the effect of the second mutation in patients who displayed dramatically different phenotypes. A deletion of Lys-903 in one patient and a substitution of Arg for Leu-299 in another resulted in the fatal infantile form. In the adult, a T-to-G base change at position -13 of intron 1 resulted in alternatively spliced transcripts with deletion of exon 2, the location of the start codon. The low level of active enzyme (12% of normal) generated from the leakage of normally spliced mRNA sustained the patient to adult life.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis N. G., LaBadie G. U., Hirschhorn K. Acid alpha-glucosidase: kinetic and immunologic properties of enzyme variants in health and disease. Isozymes Curr Top Biol Med Res. 1983;11:25–36. [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. I., Brown D. H., Jeffrey P. L. Simultaneous absence of alpha-1,4-glucosidase and alpha-1,6-glucosidase activities (pH 4) in tissues of children with type II glycogen storage disease. Biochemistry. 1970 Mar 17;9(6):1423–1428. doi: 10.1021/bi00808a017. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Courtecuissf V., Royer P., Habib R., Monnier C., Demos J. Glycogenose musculaire par deficit d'alpha-1-4-glucosidase simulant une dystrophie musculaire progressive. (Etude clinique et enzymatique. Microscopie optique electronique) Arch Fr Pediatr. 1965 Dec;22(10):1153–1164. [PubMed] [Google Scholar]
- DiMauro S., Stern L. Z., Mehler M., Nagle R. B., Payne C. Adult-onset acid maltase deficiency: a postmortem study. Muscle Nerve. 1978 Jan-Feb;1(1):27–36. doi: 10.1002/mus.880010105. [DOI] [PubMed] [Google Scholar]
- Engel A. G. Acid maltase deficiency in adults: studies in four cases of a syndrome which may mimic muscular dystrophy or other myopathies. Brain. 1970;93(3):599–616. doi: 10.1093/brain/93.3.599. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Dale A. J. Autophagic glycogenosis of late onset with mitochondrial abnormalities: light and electron microscopic observations. Mayo Clin Proc. 1968 Apr;43(4):233–279. [PubMed] [Google Scholar]
- Engel A. G., Gomez M. R., Seybold M. E., Lambert E. H. The spectrum and diagnosis of acid maltase deficiency. Neurology. 1973 Jan;23(1):95–106. doi: 10.1212/wnl.23.1.95. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Seybold M. E., Lambert E. H., Gomez M. R. Acid maltase deficiency: comparison of infantile, childhood, and adult types. Neurology. 1970 Apr;20(4):382–382. [PubMed] [Google Scholar]
- Galjaard H., Mekes M., Josselin de Jong JE D. E., Niermeijer M. F. A method for rapid prenatal diagnosis of glycogenosis II (Pompe's disease). Clin Chim Acta. 1973 Dec 27;49(3):361–375. doi: 10.1016/0009-8981(73)90234-9. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverstick D. M., Jeziorski M., Bannon M. J. Developmental profile of striatal preprotachykinin gene expression. J Neurochem. 1990 Sep;55(3):764–768. doi: 10.1111/j.1471-4159.1990.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Hermans M. M., Kroos M. A., de Graaff E., Oostra B. A., Reuser A. J. Two mutations affecting the transport and maturation of lysosomal alpha-glucosidase in an adult case of glycogen storage disease type II. Hum Mutat. 1993;2(4):268–273. doi: 10.1002/humu.1380020406. [DOI] [PubMed] [Google Scholar]
- Hermans M. M., Kroos M. A., van Beeumen J., Oostra B. A., Reuser A. J. Human lysosomal alpha-glucosidase. Characterization of the catalytic site. J Biol Chem. 1991 Jul 25;266(21):13507–13512. [PubMed] [Google Scholar]
- Hermans M. M., Wisselaar H. A., Kroos M. A., Oostra B. A., Reuser A. J. Human lysosomal alpha-glucosidase: functional characterization of the glycosylation sites. Biochem J. 1993 Feb 1;289(Pt 3):681–686. doi: 10.1042/bj2890681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans M. M., de Graaff E., Kroos M. A., Wisselaar H. A., Oostra B. A., Reuser A. J. Identification of a point mutation in the human lysosomal alpha-glucosidase gene causing infantile glycogenosis type II. Biochem Biophys Res Commun. 1991 Sep 16;179(2):919–926. doi: 10.1016/0006-291x(91)91906-s. [DOI] [PubMed] [Google Scholar]
- Hermans M. M., de Graaff E., Kroos M. A., Wisselaar H. A., Willemsen R., Oostra B. A., Reuser A. J. The conservative substitution Asp-645-->Glu in lysosomal alpha-glucosidase affects transport and phosphorylation of the enzyme in an adult patient with glycogen-storage disease type II. Biochem J. 1993 Feb 1;289(Pt 3):687–693. doi: 10.1042/bj2890687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Kroos M. A., van Beeumen J., Reuser A. J., Oostra B. A. Primary structure and processing of lysosomal alpha-glucosidase; homology with the intestinal sucrase-isomaltase complex. EMBO J. 1988 Jun;7(6):1697–1704. doi: 10.1002/j.1460-2075.1988.tb02998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot L. H., Hoogeveen-Westerveld M., Reuser A. J., Oostra B. A. Characterization of the human lysosomal alpha-glucosidase gene. Biochem J. 1990 Dec 1;272(2):493–497. doi: 10.1042/bj2720493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefsloot L. H., Willemsen R., Kroos M. A., Hoogeveen-Westerveld M., Hermans M. M., Van der Ploeg A. T., Oostra B. A., Reuser A. J. Expression and routeing of human lysosomal alpha-glucosidase in transiently transfected mammalian cells. Biochem J. 1990 Dec 1;272(2):485–492. doi: 10.1042/bj2720485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgson P., Gardner-Medwin D., Worsfold M., Pennington R. J., Walton J. N. Adult myopathy from glycogen storage disease due to acid maltase deficiency. Brain. 1968 Sep;91(3):435–462. doi: 10.1093/brain/91.3.435. [DOI] [PubMed] [Google Scholar]
- Huie M. L., Chen A. S., Brooks S. S., Grix A., Hirschhorn R. A de novo 13 nt deletion, a newly identified C647W missense mutation and a deletion of exon 18 in infantile onset glycogen storage disease type II (GSDII). Hum Mol Genet. 1994 Jul;3(7):1081–1087. doi: 10.1093/hmg/3.7.1081. [DOI] [PubMed] [Google Scholar]
- Huie M. L., Chen A. S., Tsujino S., Shanske S., DiMauro S., Engel A. G., Hirschhorn R. Aberrant splicing in adult onset glycogen storage disease type II (GSDII): molecular identification of an IVS1 (-13T-->G) mutation in a majority of patients and a novel IVS10 (+1GT-->CT) mutation. Hum Mol Genet. 1994 Dec;3(12):2231–2236. doi: 10.1093/hmg/3.12.2231. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. II. Kinetics of action of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1416–1422. doi: 10.1021/bi00808a016. [DOI] [PubMed] [Google Scholar]
- Jézéquel A. M., Arakawa K., Steiner J. W. The fine structure of the normal, neonatal mouse liver. Lab Invest. 1965 Nov;14(11):1894–1930. [PubMed] [Google Scholar]
- Martiniuk F., Bodkin M., Tzall S., Hirschhorn R. Identification of the base-pair substitution responsible for a human acid alpha glucosidase allele with lower "affinity" for glycogen (GAA 2) and transient gene expression in deficient cells. Am J Hum Genet. 1990 Sep;47(3):440–445. [PMC free article] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Bodkin M., Tzall S., Hirschhorn K., Zhong N., Hirschhorn R. Identification of a missense mutation in an adult-onset patient with glycogenosis type II expressing only one allele. DNA Cell Biol. 1991 Nov;10(9):681–687. doi: 10.1089/dna.1991.10.681. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Pellicer A., Tzall S., La Badie G., Hobart C., Ellenbogen A., Hirschhorn R. Isolation of a cDNA for human acid alpha-glucosidase and detection of genetic heterogeneity for mRNA in three alpha-glucosidase-deficient patients. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9641–9644. doi: 10.1073/pnas.83.24.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Tzall S., Meredith G., Hirschhorn R. Extensive genetic heterogeneity in patients with acid alpha glucosidase deficiency as detected by abnormalities of DNA and mRNA. Am J Hum Genet. 1990 Jul;47(1):73–78. [PMC free article] [PubMed] [Google Scholar]
- Mehler M., DiMauro S. Residual acid maltase activity in late-onset acid maltase deficiency. Neurology. 1977 Feb;27(2):178–184. doi: 10.1212/wnl.27.2.178. [DOI] [PubMed] [Google Scholar]
- Miranda A. F., Shanske S., Hays A. P., DiMauro S. Immunocytochemical analysis of normal and acid maltase-deficient muscle cultures. Arch Neurol. 1985 Apr;42(4):371–373. doi: 10.1001/archneur.1985.04060040081017. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols R. C., Raben N. Hints for direct sequencing of PCR-generated single-stranded DNA. Biotechniques. 1994 Sep;17(3):412–414. [PubMed] [Google Scholar]
- Ninomiya N., Matsuda I., Matsuoka T., Iwamasa T., Nonaka I. Demonstration of acid alpha-glucosidase in different types of Pompe disease by use of an immunochemical method. J Neurol Sci. 1984 Nov-Dec;66(2-3):129–139. doi: 10.1016/0022-510x(84)90001-7. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Koster J. F., Hoogeveen A., Galjaard H. Biochemical, immunological, and cell genetic studies in glycogenosis type II. Am J Hum Genet. 1978 Mar;30(2):132–143. [PMC free article] [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Oude Elferink R. P., Tager J. M. Defects in synthesis, phosphorylation, and maturation of acid alpha-glucosidase in glycogenosis type II. J Biol Chem. 1985 Jul 15;260(14):8336–8341. [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Willemsen R., Swallow D., Tager J. M., Galjaard H. Clinical diversity in glycogenosis type II. Biosynthesis and in situ localization of acid alpha-glucosidase in mutant fibroblasts. J Clin Invest. 1987 Jun;79(6):1689–1699. doi: 10.1172/JCI113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenow E. C., 3rd, Engel A. G. Acid maltase deficiency in adults presenting as respiratory failure. Am J Med. 1978 Mar;64(3):485–491. doi: 10.1016/0002-9343(78)90235-8. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanske S., Bresolin N., DiMauro S. Multiple neutral maltase activities in normal and acid maltase-deficient human muscle. Exp Neurol. 1984 Jun;84(3):565–578. doi: 10.1016/0014-4886(84)90204-8. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin-Buehring Y. S., Drefers M., Kroenner H., Osang M., Schaub J. Separation of acid and neutral alpha-glucosidase isoenzymes from fetal and adult tissues, cultivated fibroblasts and amniotic fluid cells by DEAE-cellulose and sephadex G-100 column chromatography. Clin Chim Acta. 1978 Nov 1;89(3):393–404. doi: 10.1016/0009-8981(78)90401-1. [DOI] [PubMed] [Google Scholar]
- Soyama K., Ono E., Shimada N., Tanaka K., Oya N. Properties of the alpha-glucosidase from various human tissues in relation to glycogenosis type II (Pompe's disease). Clin Chim Acta. 1977 Aug 1;78(3):473–478. doi: 10.1016/0009-8981(77)90080-8. [DOI] [PubMed] [Google Scholar]
- Steckel F., Gieselmann V., Waheed A., Hasilik A., von Figura K., Oude Elferink R., Kalsbeek R., Tager J. M. Biosynthesis of acid alpha-glucosidase in late-onset forms of glycogenosis type II (Pompe's disease). FEBS Lett. 1982 Dec 13;150(1):69–76. doi: 10.1016/0014-5793(82)81306-9. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Liu L., Kimura J., Shiojiri S., Takahashi Y., Kitaguchi N., Nakamura S., Ueda K. Age-related changes in the proportion of amyloid precursor protein mRNAs in Alzheimer's disease and other neurological disorders. Brain Res Mol Brain Res. 1992 Oct;15(3-4):303–310. doi: 10.1016/0169-328x(92)90122-r. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Nakamura S., Kimura J., Ueda K. Age-related change in the proportion of amyloid precursor protein mRNAs in the gray matter of cerebral cortex. Neurosci Lett. 1993 Nov 26;163(1):19–21. doi: 10.1016/0304-3940(93)90219-b. [DOI] [PubMed] [Google Scholar]
- Van der Kraan M., Kroos M. A., Joosse M., Bijvoet A. G., Verbeet M. P., Kleijer W. J., Reuser A. J. Deletion of exon 18 is a frequent mutation in glycogen storage disease type II. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1535–1541. doi: 10.1006/bbrc.1994.2360. [DOI] [PubMed] [Google Scholar]
- Wisselaar H. A., Kroos M. A., Hermans M. M., van Beeumen J., Reuser A. J. Structural and functional changes of lysosomal acid alpha-glucosidase during intracellular transport and maturation. J Biol Chem. 1993 Jan 25;268(3):2223–2231. [PubMed] [Google Scholar]
- Zhong N., Martiniuk F., Tzall S., Hirschhorn R. Identification of a missense mutation in one allele of a patient with Pompe disease, and use of endonuclease digestion of PCR-amplified RNA to demonstrate lack of mRNA expression from the second allele. Am J Hum Genet. 1991 Sep;49(3):635–645. [PMC free article] [PubMed] [Google Scholar]
- de Barsy T., Jacquemin P., Devos P., Hers H. G. Rodent and human acid -glucosidase. Purification, properties and inhibition by antibodies. Investigation in type II glycogenosis. Eur J Biochem. 1972 Nov 21;31(1):156–165. doi: 10.1111/j.1432-1033.1972.tb02514.x. [DOI] [PubMed] [Google Scholar]