Abstract
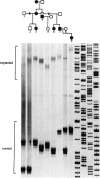
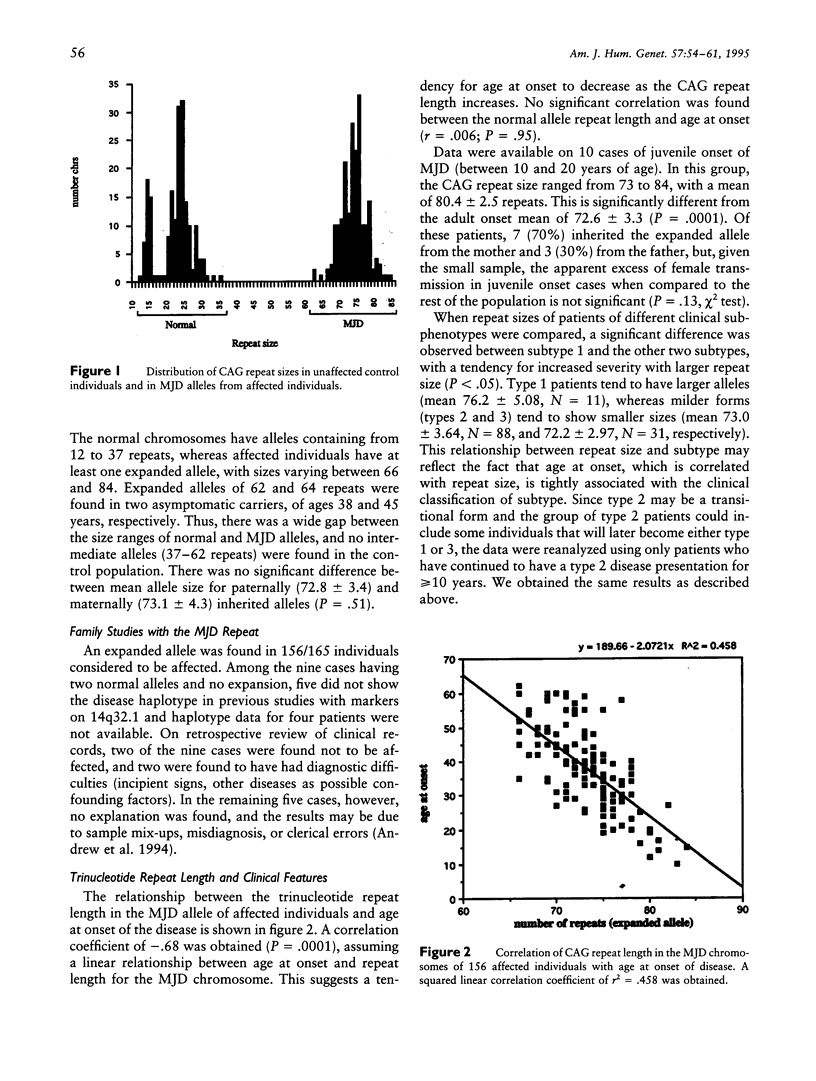
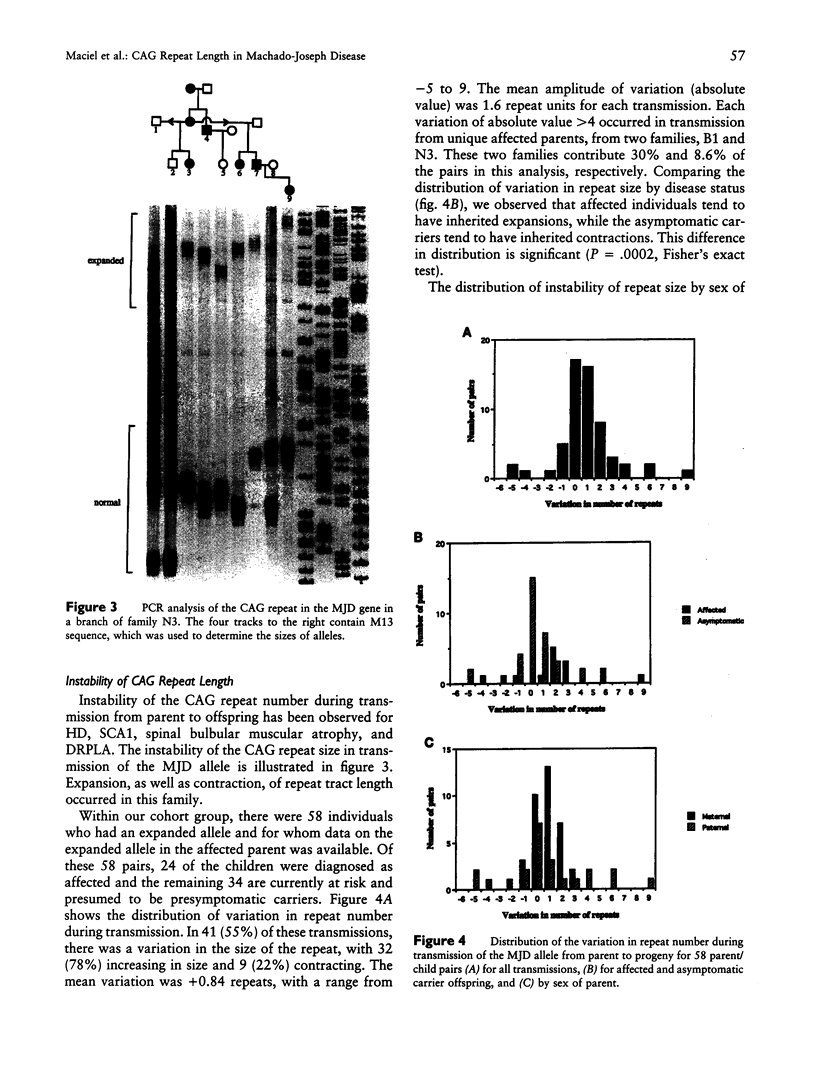
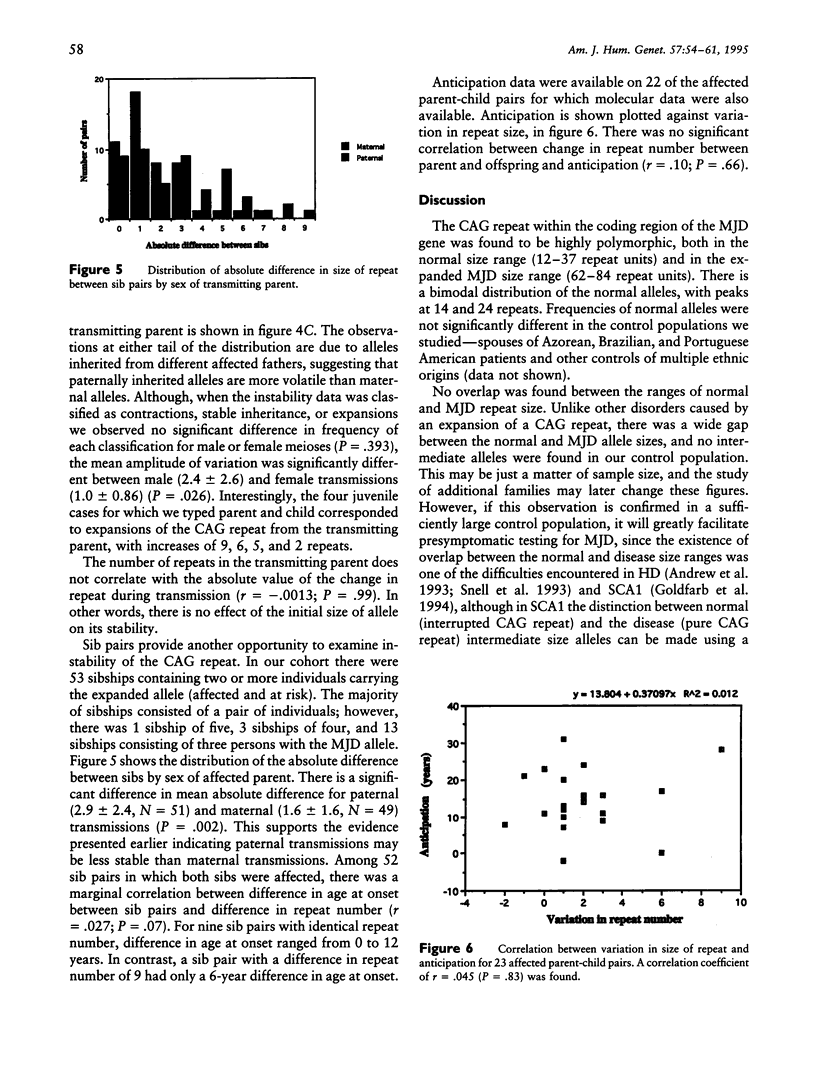
Machado-Joseph disease (MJD) is associated with the expansion of a CAG trinucleotide repeat in a novel gene on 14q32.1. We confirmed the presence of this expansion in 156 MJD patients from 33 families of different geographic origins: 15 Portuguese Azorean, 2 Brazilian, and 16 North American of Portuguese Azorean descent. Normal chromosomes contain between 12 and 37 CAG repeats in the MJD gene, whereas MJD gene carriers have alleles within the expanded range of 62–84 CAG units. The distribution of expanded alleles and the gap between normal and expanded allele sizes is either inconsistent with a premutation hypothesis or most (if not all) of the alleles we studied descend from a common ancestor. There is a strong correlation between the expanded repeat size and the age at onset of the disease as well as the clinical presentation. There is mild instability of the CAG tract length with transmission of the expanded alleles; both increase and decrease in size between parents and progeny occur, with larger variations in male than in female transmissions. Together, these effects can partly explain the variability of age at onset and of phenotypic features in MJD; however, other modifying factors must exist.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Andrew S. E., Goldberg Y. P., Kremer B., Squitieri F., Theilmann J., Zeisler J., Telenius H., Adam S., Almquist E., Anvret M. Huntington disease without CAG expansion: phenocopies or errors in assignment? Am J Hum Genet. 1994 May;54(5):852–863. [PMC free article] [PubMed] [Google Scholar]
- Andrew S. E., Goldberg Y. P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M. A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet. 1993 Aug;4(4):398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Barceló J. M., Mahadevan M. S., Tsilfidis C., MacKenzie A. E., Korneluk R. G. Intergenerational stability of the myotonic dystrophy protomutation. Hum Mol Genet. 1993 Jun;2(6):705–709. doi: 10.1093/hmg/2.6.705. [DOI] [PubMed] [Google Scholar]
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Coutinho P., Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978 Jul;28(7):703–709. doi: 10.1212/wnl.28.7.703. [DOI] [PubMed] [Google Scholar]
- Coutinho P., Guimarães A., Scaravilli F. The pathology of Machado-Joseph disease. Report of a possible homozygous case. Acta Neuropathol. 1982;58(1):48–54. doi: 10.1007/BF00692697. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993 Aug;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. P., Kremer B., Andrew S. E., Theilmann J., Graham R. K., Squitieri F., Telenius H., Adam S., Sajoo A., Starr E. Molecular analysis of new mutations for Huntington's disease: intermediate alleles and sex of origin effects. Nat Genet. 1993 Oct;5(2):174–179. doi: 10.1038/ng1093-174. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Rundle S. A., MacMillan J. C., Myring J., Brook J. D., Crow S., Reardon W., Fenton I., Shaw D. J., Harper P. S. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet. 1993 Jun;52(6):1164–1174. [PMC free article] [PubMed] [Google Scholar]
- Jodice C., Malaspina P., Persichetti F., Novelletto A., Spadaro M., Giunti P., Morocutti C., Terrenato L., Harding A. E., Frontali M. Effect of trinucleotide repeat length and parental sex on phenotypic variation in spinocerebellar ataxia I. Am J Hum Genet. 1994 Jun;54(6):959–965. [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Isozaki E., Kato S., Tanabe H., Oda M. Type III Machado-Joseph disease in a Japanese family: a clinicopathological study with special reference to the peripheral nervous system. Clin Neuropathol. 1989 May-Jun;8(3):134–141. [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Roling D. B., Harding A. E., Warner C. L., Spiegel R., Hausmanowa-Petrusewicz I., Yee W. C., Fischbeck K. H. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992 Dec;2(4):301–304. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- Lavedan C., Hofmann-Radvanyi H., Shelbourne P., Rabes J. P., Duros C., Savoy D., Dehaupas I., Luce S., Johnson K., Junien C. Myotonic dystrophy: size- and sex-dependent dynamics of CTG meiotic instability, and somatic mosaicism. Am J Hum Genet. 1993 May;52(5):875–883. [PMC free article] [PubMed] [Google Scholar]
- Lima L., Coutinho P. Clinical criteria for diagnosis of Machado-Joseph disease: report of a non-Azorena Portuguese family. Neurology. 1980 Mar;30(3):319–322. doi: 10.1212/wnl.30.3.319. [DOI] [PubMed] [Google Scholar]
- Ranum L. P., Chung M. Y., Banfi S., Bryer A., Schut L. J., Ramesar R., Duvick L. A., McCall A., Subramony S. H., Goldfarb L. Molecular and clinical correlations in spinocerebellar ataxia type I: evidence for familial effects on the age at onset. Am J Hum Genet. 1994 Aug;55(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. N., Nyhan W. L., Bay C., Shore P. Autosomal dominant striatonigral degeneration. A clinical, pathologic, and biochemical study of a new genetic disorder. Neurology. 1976 Aug;26(8):703–714. doi: 10.1212/wnl.26.8.703. [DOI] [PubMed] [Google Scholar]
- Rubinsztein D. C., Amos W., Leggo J., Goodburn S., Ramesar R. S., Old J., Bontrop R., McMahon R., Barton D. E., Ferguson-Smith M. A. Mutational bias provides a model for the evolution of Huntington's disease and predicts a general increase in disease prevalence. Nat Genet. 1994 Aug;7(4):525–530. doi: 10.1038/ng0894-525. [DOI] [PubMed] [Google Scholar]
- Sakai T., Ohta M., Ishino H. Joseph disease in a non-Portuguese family. Neurology. 1983 Jan;33(1):74–80. doi: 10.1212/wnl.33.1.74. [DOI] [PubMed] [Google Scholar]
- Sequeiros J., Coutinho P. Epidemiology and clinical aspects of Machado-Joseph disease. Adv Neurol. 1993;61:139–153. [PubMed] [Google Scholar]
- Sequeiros J., Silveira I., Maciel P., Coutinho P., Manaia A., Gaspar C., Burlet P., Loureiro L., Guimarães J., Tanaka H. Genetic linkage studies of Machado-Joseph disease with chromosome 14q STRPs in 16 Portuguese-Azorean kindreds. Genomics. 1994 Jun;21(3):645–648. doi: 10.1006/geno.1994.1327. [DOI] [PubMed] [Google Scholar]
- Snell R. G., MacMillan J. C., Cheadle J. P., Fenton I., Lazarou L. P., Davies P., MacDonald M. E., Gusella J. F., Harper P. S., Shaw D. J. Relationship between trinucleotide repeat expansion and phenotypic variation in Huntington's disease. Nat Genet. 1993 Aug;4(4):393–397. doi: 10.1038/ng0893-393. [DOI] [PubMed] [Google Scholar]
- Sudarsky L., Corwin L., Dawson D. M. Machado-Joseph disease in New England: clinical description and distinction from the olivopontocerebellar atrophies. Mov Disord. 1992;7(3):204–208. doi: 10.1002/mds.870070303. [DOI] [PubMed] [Google Scholar]
- Takiyama Y., Nishizawa M., Tanaka H., Kawashima S., Sakamoto H., Karube Y., Shimazaki H., Soutome M., Endo K., Ohta S. The gene for Machado-Joseph disease maps to human chromosome 14q. Nat Genet. 1993 Jul;4(3):300–304. doi: 10.1038/ng0793-300. [DOI] [PubMed] [Google Scholar]
- Telenius H., Kremer B., Goldberg Y. P., Theilmann J., Andrew S. E., Zeisler J., Adam S., Greenberg C., Ives E. J., Clarke L. A. Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat Genet. 1994 Apr;6(4):409–414. doi: 10.1038/ng0494-409. [DOI] [PubMed] [Google Scholar]