Abstract
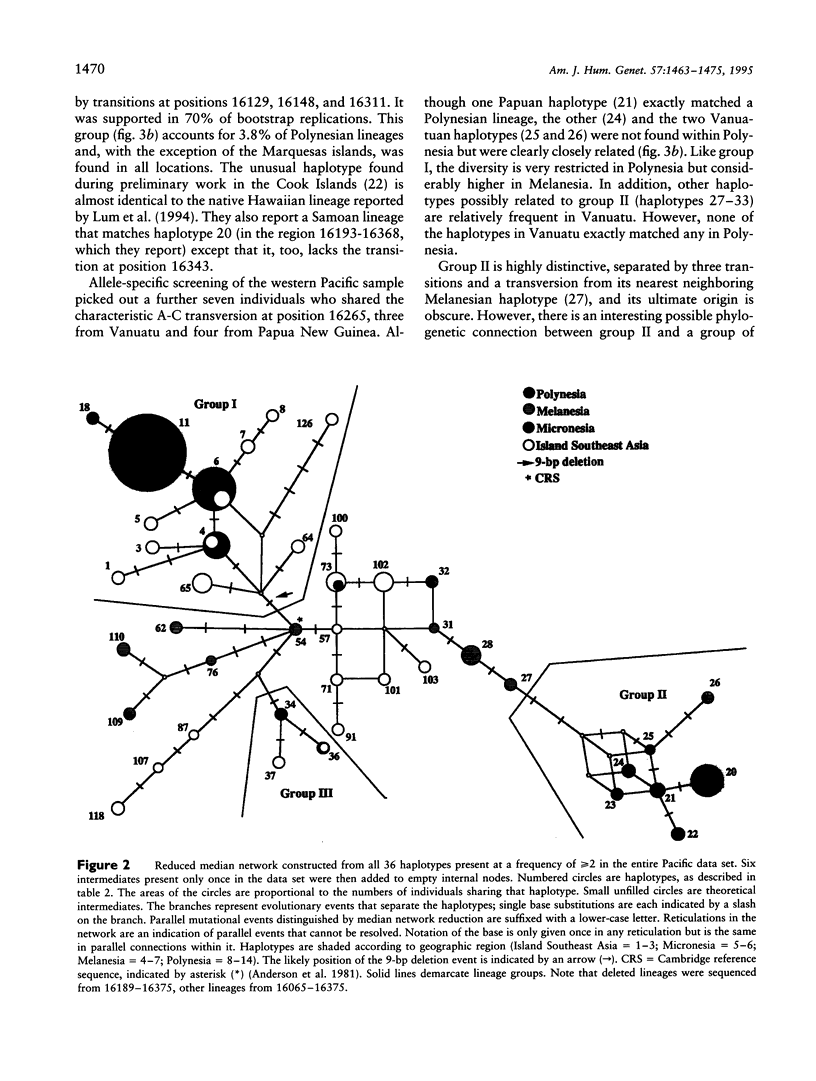
Using mitochondrial lineage analysis of 1,178 individuals from Polynesia, the western Pacific, and Taiwan, we show that the major prehistoric settlement of Polynesia was from the west and involved two or possibly three genetically distinct populations. The predominant lineage group, accounting for 94% of Polynesian mtDNA, shares a 9-bp COII/tRNA(Lys) intergenic deletion and characteristic control region transition variants, compared to the Cambridge reference sequence. In Polynesia, the diversity of this group is extremely restricted, while related lineages in Indonesia, the Philippines, and Taiwan are increasingly diverse. This suggests a relatively recent major eastward expansion into Polynesia, perhaps originating from Taiwan, in agreement with archeological and linguistic evidence, but which experienced one or more severe population bottlenecks. The second mitochondrial lineage group, accounting for 3.5% of Polynesian mtDNA haplotypes, does not have the 9-bp deletion and its characterized by an A-C transversional variant at nt position 16265. Specific oligonucleotides for this variant were used to select individuals from the population sample who, with other sequences, show that the Polynesian lineages were part of a diverse group in Vanuatu and Papua New Guinea. The very low overall diversity of both lineage groups in Polynesia suggests there was severe population restriction during the colonization of remote Oceania. A third group, represented by only four individuals (0.6%) in Polynesia but also present in the Philippines, shares variants at nt positions 16172 and 16304. Two Polynesians had unrelated haplotypes matching published sequences from native South Americans, which may be the first genetic evidence of prehistoric human contact between Polynesia and South America.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Avise J. C. Ten unorthodox perspectives on evolution prompted by comparative population genetic findings on mitochondrial DNA. Annu Rev Genet. 1991;25:45–69. doi: 10.1146/annurev.ge.25.120191.000401. [DOI] [PubMed] [Google Scholar]
- Bandelt H. J., Forster P., Sykes B. C., Richards M. B. Mitochondrial portraits of human populations using median networks. Genetics. 1995 Oct;141(2):743–753. doi: 10.1093/genetics/141.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertranpetit J., Sala J., Calafell F., Underhill P. A., Moral P., Comas D. Human mitochondrial DNA variation and the origin of Basques. Ann Hum Genet. 1995 Jan;59(Pt 1):63–81. doi: 10.1111/j.1469-1809.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Di Rienzo A., Wilson A. C. Branching pattern in the evolutionary tree for human mitochondrial DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1597–1601. doi: 10.1073/pnas.88.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginther C., Corach D., Penacino G. A., Rey J. A., Carnese F. R., Hutz M. H., Anderson A., Just J., Salzano F. M., King M. C. Genetic variation among the Mapuche Indians from the Patagonian region of Argentina: mitochondrial DNA sequence variation and allele frequencies of several nuclear genes. EXS. 1993;67:211–219. doi: 10.1007/978-3-0348-8583-6_17. [DOI] [PubMed] [Google Scholar]
- Hagelberg E., Clegg J. B. Genetic polymorphisms in prehistoric Pacific islanders determined by analysis of ancient bone DNA. Proc Biol Sci. 1993 May 22;252(1334):163–170. doi: 10.1098/rspb.1993.0061. [DOI] [PubMed] [Google Scholar]
- Hertzberg M., Mickleson K. N., Serjeantson S. W., Prior J. F., Trent R. J. An Asian-specific 9-bp deletion of mitochondrial DNA is frequently found in Polynesians. Am J Hum Genet. 1989 Apr;44(4):504–510. [PMC free article] [PubMed] [Google Scholar]
- Hewett D. R., Lynch J. R., Child A., Sykes B. C. A new missense mutation of fibrillin in a patient with Marfan syndrome. J Med Genet. 1994 Apr;31(4):338–339. doi: 10.1136/jmg.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V., Gentile B., Bonnardot J. M., Roux J., Weatherall D. J., Clegg J. B. Polynesian origins and affinities: globin gene variants in eastern Polynesia. Am J Hum Genet. 1987 May;40(5):453–463. [PMC free article] [PubMed] [Google Scholar]
- Horai S., Hayasaka K. Intraspecific nucleotide sequence differences in the major noncoding region of human mitochondrial DNA. Am J Hum Genet. 1990 Apr;46(4):828–842. [PMC free article] [PubMed] [Google Scholar]
- Lum J. K., Rickards O., Ching C., Cann R. L. Polynesian mitochondrial DNAs reveal three deep maternal lineage clusters. Hum Biol. 1994 Aug;66(4):567–590. [PubMed] [Google Scholar]
- Morin P. A., Moore J. J., Chakraborty R., Jin L., Goodall J., Woodruff D. S. Kin selection, social structure, gene flow, and the evolution of chimpanzees. Science. 1994 Aug 26;265(5176):1193–1201. doi: 10.1126/science.7915048. [DOI] [PubMed] [Google Scholar]
- Shields G. F., Schmiechen A. M., Frazier B. L., Redd A., Voevoda M. I., Reed J. K., Ward R. H. mtDNA sequences suggest a recent evolutionary divergence for Beringian and northern North American populations. Am J Hum Genet. 1993 Sep;53(3):549–562. [PMC free article] [PubMed] [Google Scholar]
- Stoneking M., Hedgecock D., Higuchi R. G., Vigilant L., Erlich H. A. Population variation of human mtDNA control region sequences detected by enzymatic amplification and sequence-specific oligonucleotide probes. Am J Hum Genet. 1991 Feb;48(2):370–382. [PMC free article] [PubMed] [Google Scholar]
- Stoneking M., Jorde L. B., Bhatia K., Wilson A. C. Geographic variation in human mitochondrial DNA from Papua New Guinea. Genetics. 1990 Mar;124(3):717–733. doi: 10.1093/genetics/124.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989 Nov;123(3):585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent R. J., Buchanan J. G., Webb A., Goundar R. P., Seruvatu L. M., Mickleson K. N. Globin genes are useful markers to identify genetic similarities between Fijians and Pacific Islanders from Polynesia and Melanesia. Am J Hum Genet. 1988 Apr;42(4):601–607. [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Pennington R., Harpending H., Kocher T. D., Wilson A. C. Mitochondrial DNA sequences in single hairs from a southern African population. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9350–9354. doi: 10.1073/pnas.86.23.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]


