Abstract
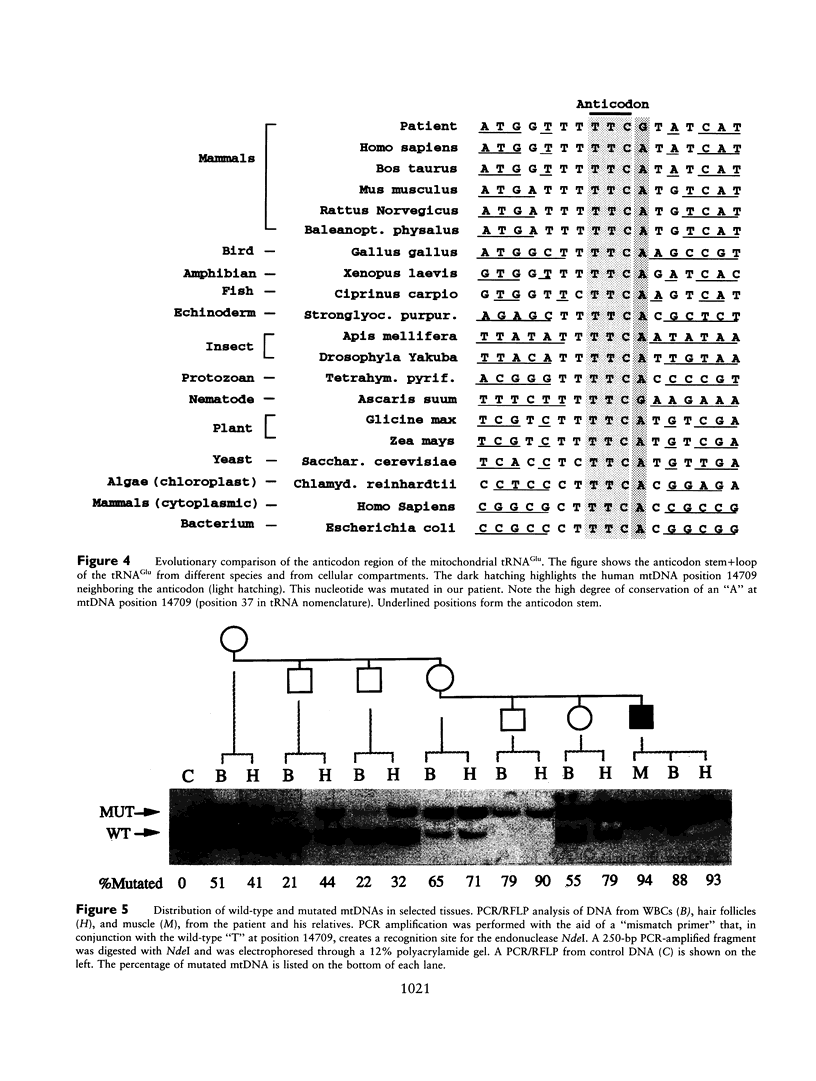
We have identified a novel mtDNA mutation in a 29-year-old man with myopathy and diabetes mellitus. This T-->C transition at mtDNA position 14709 alters an evolutionarily conserved nucleotide in the region specifying for the anticodon loop of the mitochondrial tRNA(Glu). The nt-14709 mutation was heteroplasmic but present at very high levels in the patient's muscle, white blood cells (WBCs), and hair follicles; lower proportions of mutated mtDNA were observed in WBCs and hair follicles of all examined maternal relatives. In the patient's muscle, abnormal fibers showed mitochondrial proliferation, severe focal defects in cytochrome c oxidase activity, and absence of cross-reacting material for mitochondrially synthesized polypeptides. These fibers had higher levels of mutated mtDNA than did surrounding "normal" fibers. Although the percentage of mutated mtDNA in WBCs from family members were distributed around the percentage observed in the mothers, the pattern was different in hair follicles, where the mutated population tended to increase in subsequent generations. PCR/RFLP analysis of single hairs showed that the intercellular variations in the percentage of mutated mtDNA differed among family members, with younger generations having a more homogeneous distribution of mutated mtDNA in different hair follicles. These results suggest that the intercellular distribution of the mutated and wild-type mtDNA populations may drift toward homogeneity in subsequent generations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Shoffner J. M., Hedaya E. V., Trounce I., Polak M. A., Koontz D. A., Wallace D. C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992 Apr;1(1):11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Wikström P. M., Byström A. S. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science. 1989 May 26;244(4907):986–989. doi: 10.1126/science.2471265. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Martinuzzi A., Yoneda M., Daga A., Hurko O., Johns D., Lai S. T., Nonaka I., Angelini C., Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Hagervall T. G., Ericson J. U., Esberg K. B., Li J. N., Björk G. R. Role of tRNA modification in translational fidelity. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):263–266. doi: 10.1016/0167-4781(90)90178-5. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan C., Lach B., Shoubridge E. A. Variable distribution of mutant mitochondrial DNAs (tRNA(Leu[3243])) in tissues of symptomatic relatives with MELAS: the role of mitotic segregation. Neurology. 1993 Aug;43(8):1586–1590. doi: 10.1212/wnl.43.8.1586. [DOI] [PubMed] [Google Scholar]
- Mitochondrial encephalomyopathies: gene mutation. Neuromuscul Disord. 1994 May;4(3):285–287. doi: 10.1016/0960-8966(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Ionasescu V., Schon E. A., DiMauro S. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nat Genet. 1993 Jul;4(3):284–288. doi: 10.1038/ng0793-284. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Jansen C., Hirano M., Rao N., Lovelace R. E., Rowland L. P., Schon E. A., DiMauro S. Two novel pathogenic mitochondrial DNA mutations affecting organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest. 1993 Dec;92(6):2906–2915. doi: 10.1172/JCI116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Silvestri G., Shanske S., Sciacco M., Hirano M., Schon E. A., Bonilla E., DiMauro S. Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul Disord. 1993 Jan;3(1):43–50. doi: 10.1016/0960-8966(93)90040-q. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ricci E., Bonilla E., DiMauro S., Schon E. A. The mitochondrial tRNA(Leu(UUR)) mutation in mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes (MELAS): genetic, biochemical, and morphological correlations in skeletal muscle. Am J Hum Genet. 1992 May;50(5):934–949. [PMC free article] [PubMed] [Google Scholar]
- Pena S. D., Barreto G., Vago A. R., De Marco L., Reinach F. C., Dias Neto E., Simpson A. J. Sequence-specific "gene signatures" can be obtained by PCR with single specific primers at low stringency. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1946–1949. doi: 10.1073/pnas.91.5.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzella V., Moraes C. T., Sano M. C., Bonilla E., DiMauro S., Schon E. A. Extremely high levels of mutant mtDNAs co-localize with cytochrome c oxidase-negative ragged-red fibers in patients harboring a point mutation at nt 3243. Hum Mol Genet. 1994 Mar;3(3):449–454. doi: 10.1093/hmg/3.3.449. [DOI] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Bindoff L., Morten K., Land J., Brown G. Families of mtDNA re-arrangements can be detected in patients with mtDNA deletions: duplications may be a transient intermediate form. Hum Mol Genet. 1993 Jan;2(1):23–30. doi: 10.1093/hmg/2.1.23. [DOI] [PubMed] [Google Scholar]
- Sciacco M., Bonilla E., Schon E. A., DiMauro S., Moraes C. T. Distribution of wild-type and common deletion forms of mtDNA in normal and respiration-deficient muscle fibers from patients with mitochondrial myopathy. Hum Mol Genet. 1994 Jan;3(1):13–19. doi: 10.1093/hmg/3.1.13. [DOI] [PubMed] [Google Scholar]
- Tritschler H. J., Bonilla E., Lombes A., Andreetta F., Servidei S., Schneyder B., Miranda A. F., Schon E. A., Kadenbach B., DiMauro S. Differential diagnosis of fatal and benign cytochrome c oxidase-deficient myopathies of infancy: an immunohistochemical approach. Neurology. 1991 Feb;41(2 ):300–305. doi: 10.1212/wnl.41.2_part_1.300. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Macfarlane J. L., Okimoto R., Clary D. O., Wahleithner J. A. Bizarre tRNAs inferred from DNA sequences of mitochondrial genomes of nematode worms. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1324–1328. doi: 10.1073/pnas.84.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ouweland J. M., Lemkes H. H., Ruitenbeek W., Sandkuijl L. A., de Vijlder M. F., Struyvenberg P. A., van de Kamp J. J., Maassen J. A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992 Aug;1(5):368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]






