Abstract
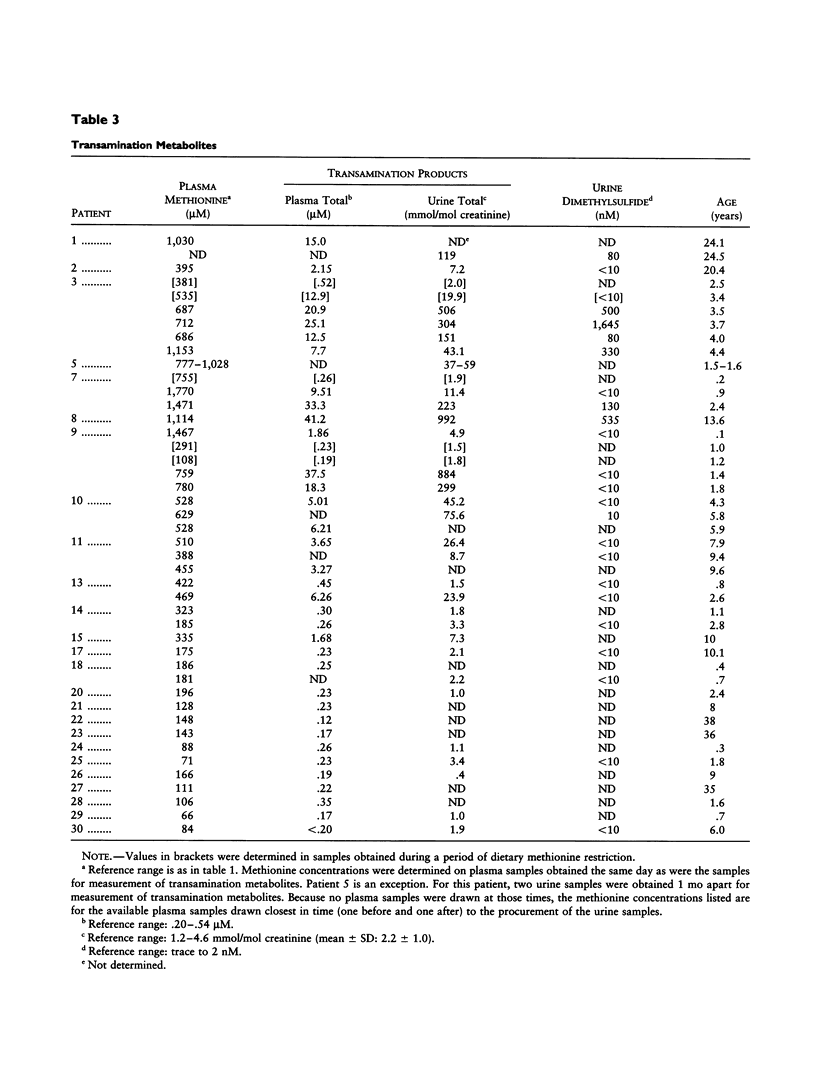
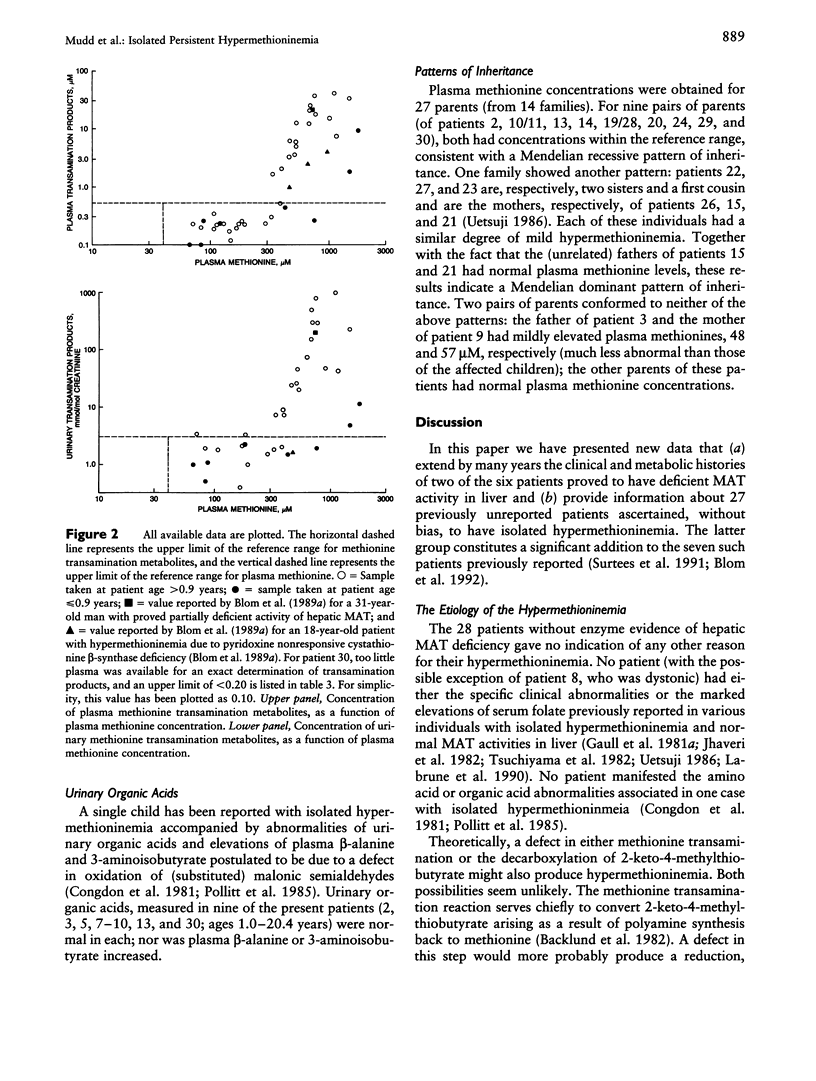
New information has been obtained on 30 patients with isolated persistent hypermethioninemia, most of them previously unreported. Biopsies to confirm the presumptive diagnosis of partially deficient activity of ATP: L-methionine S-adenosyltransferase (MAT; E.C.2.5.1.6) in liver were not performed on most of these patients. However, none showed the clinical findings or the extreme elevations of serum folate previously described in other patients with isolated hypermethioninemia considered not to have hepatic MAT deficiency. Patients ascertained on biochemical grounds had no neurological abnormalities, and 27/30 had IQs or Bayley development-index scores within normal limits or were judged to have normal mental development. Methionine transamination metabolites accumulated abnormally only when plasma methionine concentrations exceeded 300-350 microM and did so more markedly after 0.9 years of age. Data were obtained on urinary organic acids as well as plasma creatinine concentrations. Patterns of inheritance of isolated hypermethioninemia were variable. Considerations as to the optimal management of this group of patients are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG M. D., BINKLEY E. L., Jr Studies on phenylketonuria. V. Observations on a newborn infant with phenylketonuria. Proc Soc Exp Biol Med. 1956 Dec;93(3):418–420. doi: 10.3181/00379727-93-22775. [DOI] [PubMed] [Google Scholar]
- Al Mardini H., Leonard J., Bartlett K., Lloyd S., Record C. O. Effect of methionine loading and endogenous hypermethioninaemia on blood mercaptans in man. Clin Chim Acta. 1988 Aug 15;176(1):83–89. doi: 10.1016/0009-8981(88)90177-5. [DOI] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Chang C. P., Smith R. A. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5'-methylthioadenosine. J Biol Chem. 1982 Apr 25;257(8):4196–4202. [PubMed] [Google Scholar]
- Blom H. J., Boers G. H., Trijbels J. M., van Roessel J. J., Tangerman A. Cystathionine-synthase-deficient patients do not use the transamination pathway of methionine to reduce hypermethioninemia and homocystinemia. Metabolism. 1989 Jun;38(6):577–582. doi: 10.1016/0026-0495(89)90220-5. [DOI] [PubMed] [Google Scholar]
- Blom H. J., Boers G. H., van den Elzen J. P., Gahl W. A., Tangerman A. Transamination of methionine in humans. Clin Sci (Lond) 1989 Jan;76(1):43–49. doi: 10.1042/cs0760043. [DOI] [PubMed] [Google Scholar]
- Blom H. J., Davidson A. J., Finkelstein J. D., Luder A. S., Bernardini I., Martin J. J., Tangerman A., Trijbels J. M., Mudd S. H., Goodman S. I. Persistent hypermethioninaemia with dominant inheritance. J Inherit Metab Dis. 1992;15(2):188–197. doi: 10.1007/BF01799629. [DOI] [PubMed] [Google Scholar]
- Congdon P. J., Haigh D., Smith R., Green A., Pollitt R. J. Hypermethioninaemia and 3-hydroxyisobutyric aciduria in an apparently healthy baby. J Inherit Metab Dis. 1981;4(2):79–80. doi: 10.1007/BF02263600. [DOI] [PubMed] [Google Scholar]
- Diamond A., Ciaramitaro V., Donner E., Djali S., Robinson M. B. An animal model of early-treated PKU. J Neurosci. 1994 May;14(5 Pt 2):3072–3082. doi: 10.1523/JNEUROSCI.14-05-03072.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J. D., Kyle W. E., Martin J. J. Abnormal methionine adenosyltransferase in hypermethioninemia. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1491–1497. doi: 10.1016/0006-291x(75)90527-6. [DOI] [PubMed] [Google Scholar]
- Gahl W. A., Bernardini I., Finkelstein J. D., Tangerman A., Martin J. J., Blom H. J., Mullen K. D., Mudd S. H. Transsulfuration in an adult with hepatic methionine adenosyltransferase deficiency. J Clin Invest. 1988 Feb;81(2):390–397. doi: 10.1172/JCI113331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl W. A., Finkelstein J. D., Mullen K. D., Bernardini I., Martin J. J., Backlund P., Ishak K. G., Hoofnagle J. H., Mudd S. H. Hepatic methionine adenosyltransferase deficiency in a 31-year-old man. Am J Hum Genet. 1987 Jan;40(1):39–49. [PMC free article] [PubMed] [Google Scholar]
- Gaull G. E., Tallan H. H., Lonsdale D., Przyrembel H., Schaffner F., von Bassewitz D. B. Hypermethioninemia associated with methionine adenosyltransferase deficiency: clinical, morphologic, and biochemical observations on four patients. J Pediatr. 1981 May;98(5):734–741. doi: 10.1016/s0022-3476(81)80833-5. [DOI] [PubMed] [Google Scholar]
- Gaull G. E., Tallan H. H. Methionine adenosyltransferase deficiency: new enzymatic defect associated with hypermethioninemia. Science. 1974 Oct 4;186(4158):59–60. doi: 10.1126/science.186.4158.59. [DOI] [PubMed] [Google Scholar]
- Gout J. P., Serre J. C., Dieterlen M., Antener I., Frappat P., Bost M., Beaudoing A. Une nouvelle cause d'hyperméthioninémie de l'enfant: le deficit en S-adenosyl-méthionine-synthétase. Arch Fr Pediatr. 1977 May;34(5):416–423. [PubMed] [Google Scholar]
- Guízar Vázquez J., Sánchez Aguilar G., Velázquez A., Fragoso R., Rostenberg I., Alejandre I. Hipermetioninemia. A propósito de un caso en un matrimonio consanguíneo. Bol Med Hosp Infant Mex. 1980 Nov-Dec;37(6):1237–1244. [PubMed] [Google Scholar]
- Jhaveri B. M., Buist N. R., Gaull G. E., Tallan H. H. Intermittent hypermethioninaemia associated with normal hepatic methionine adenosyltransferase activity: report of a case. J Inherit Metab Dis. 1982;5(2):101–105. doi: 10.1007/BF01800001. [DOI] [PubMed] [Google Scholar]
- Jones S. M., Yeaman S. J. Oxidative decarboxylation of 4-methylthio-2-oxobutyrate by branched-chain 2-oxo acid dehydrogenase complex. Biochem J. 1986 Jul 15;237(2):621–623. doi: 10.1042/bj2370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb M., Geller A. M. Methionine adenosyltransferase: structure and function. Pharmacol Ther. 1993 Aug;59(2):125–143. doi: 10.1016/0163-7258(93)90042-c. [DOI] [PubMed] [Google Scholar]
- Labrune P., Perignon J. L., Rault M., Brunet C., Lutun H., Charpentier C., Saudubray J. M., Odievre M. Familial hypermethioninemia partially responsive to dietary restriction. J Pediatr. 1990 Aug;117(2 Pt 1):220–226. doi: 10.1016/s0022-3476(05)80533-5. [DOI] [PubMed] [Google Scholar]
- Lane J. D., Neuhoff V. Phenylketonuria: clinical and experimental considerations revealed by the use of animal models. Naturwissenschaften. 1980 May;67(5):227–233. doi: 10.1007/BF01054531. [DOI] [PubMed] [Google Scholar]
- Levy H. L., Madigan P. M., Peneva P. Evidence for delayed histidine transamination in neonates with histidinemia. Pediatrics. 1971 Jan;47(1):128–132. [PubMed] [Google Scholar]
- Mudd S. H., Ebert M. H., Scriver C. R. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980 Aug;29(8):707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Poole J. R. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975 Jun;24(6):721–735. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- Pollitt R. J., Green A., Smith R. Excessive excretion of beta-alanine and of 3-hydroxypropionic, R- and S-3-aminoisobutyric, R- and S-3-hydroxyisobutyric and S-2-(hydroxymethyl)butyric acids probably due to a defect in the metabolism of the corresponding malonic semialdehydes. J Inherit Metab Dis. 1985;8(2):75–79. doi: 10.1007/BF01801669. [DOI] [PubMed] [Google Scholar]
- Rey F., Pellié C., Sivy M., Blandin-Savoja F., Rey J., Frézal J. Influence of age on ortho-hydroxyphenylacetic acid excretion in phenylketonuria and its genetic variants. Pediatr Res. 1974 May;8(5):540–545. doi: 10.1203/00006450-197405000-00002. [DOI] [PubMed] [Google Scholar]
- Smith I., Beasley M. G., Ades A. E. Intelligence and quality of dietary treatment in phenylketonuria. Arch Dis Child. 1990 May;65(5):472–478. doi: 10.1136/adc.65.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Q. R., Momma S., Aoyagi M., Rapoport S. I. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987 Nov;49(5):1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Stöckler S., Holzbach U., Hanefeld F., Marquardt I., Helms G., Requart M., Hänicke W., Frahm J. Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res. 1994 Sep;36(3):409–413. doi: 10.1203/00006450-199409000-00023. [DOI] [PubMed] [Google Scholar]
- Surtees R., Leonard J., Austin S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. 1991 Dec 21;338(8782-8783):1550–1554. doi: 10.1016/0140-6736(91)92373-a. [DOI] [PubMed] [Google Scholar]
- Tallan H. H., Cohen P. A. Methionine adenosyltransferase: kinetic properties of human and rat liver enzymes. Biochem Med. 1976 Dec;16(3):234–250. doi: 10.1016/0006-2944(76)90029-6. [DOI] [PubMed] [Google Scholar]
- Tangerman A., Meuwese-Arends M. T., van Tongeren J. H. New methods for the release of volatile sulfur compounds from human serum: its determination by Tenax trapping and gas chromatography and its application in liver diseases. J Lab Clin Med. 1985 Aug;106(2):175–182. [PubMed] [Google Scholar]
- Tsuchiyama A., Oyanagi K., Nakata F., Uetsuji N., Tsugawa S., Nakao T., Mori M. A new type of hypermethioninemia in neonates. Tohoku J Exp Med. 1982 Nov;138(3):281–288. doi: 10.1620/tjem.138.281. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Pinkus L. M., Jakoby W. B. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol. 1980 Oct 15;29(20):2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]


