Abstract
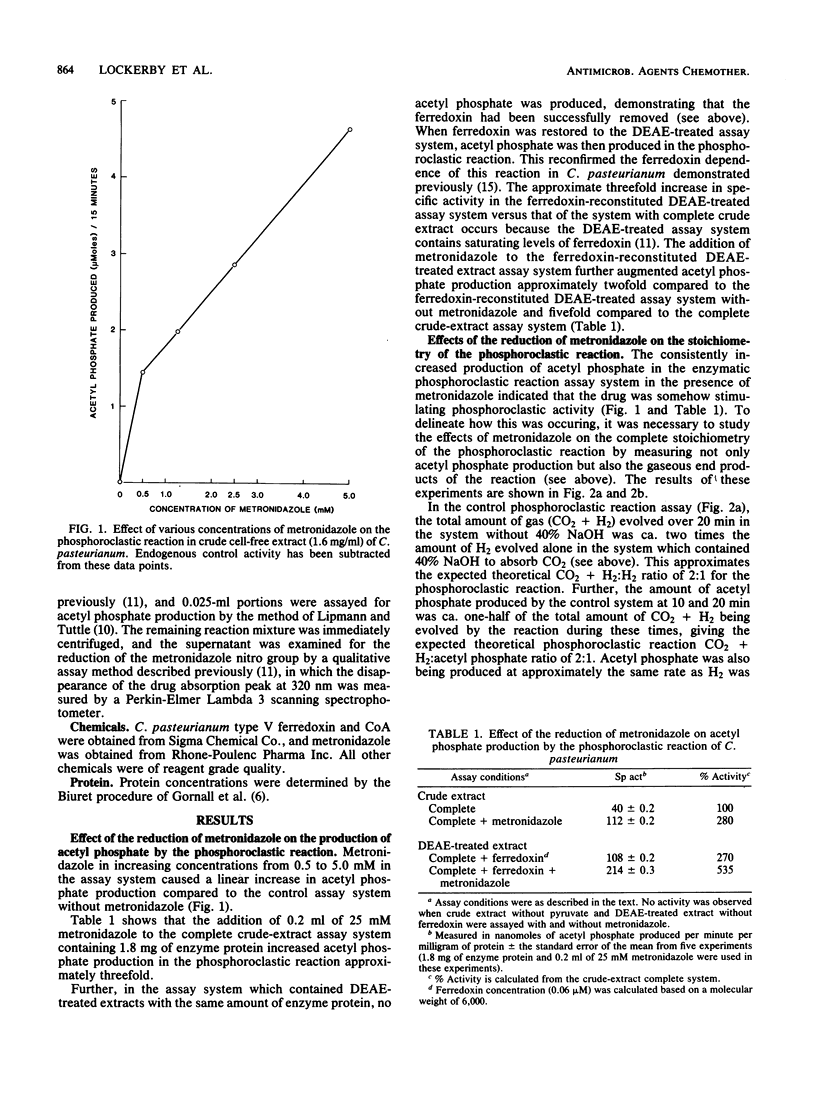
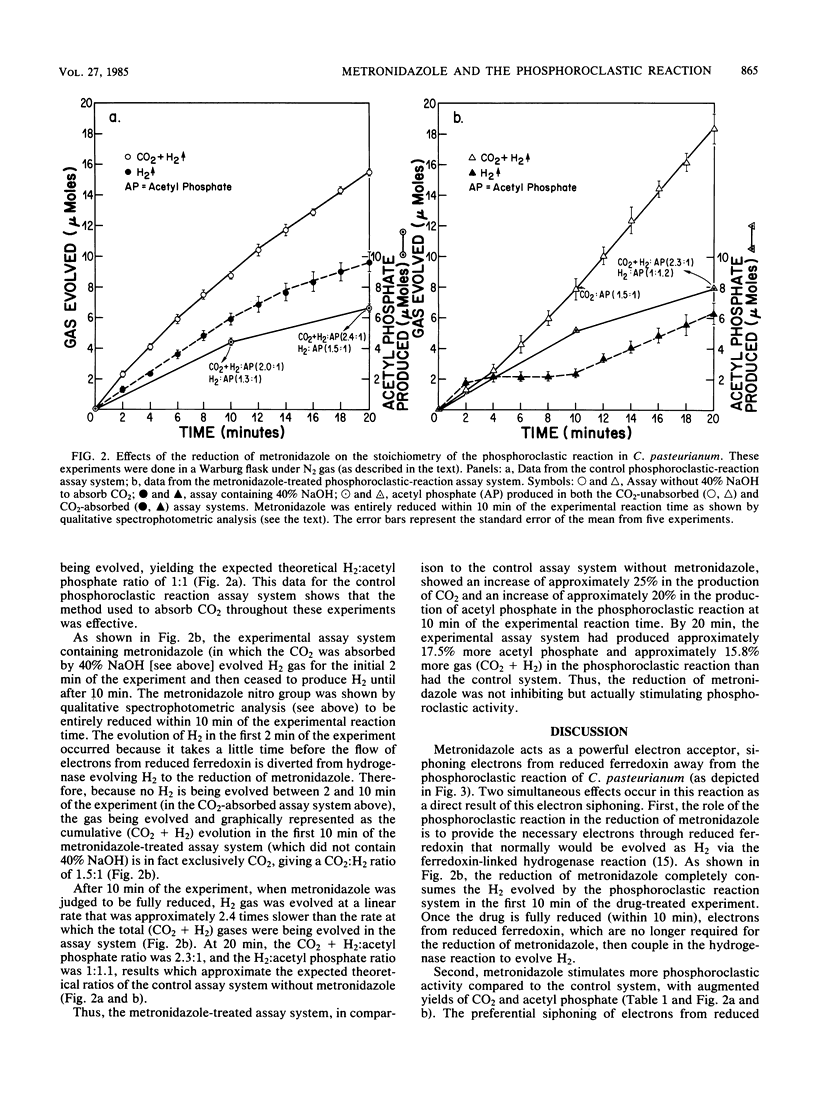
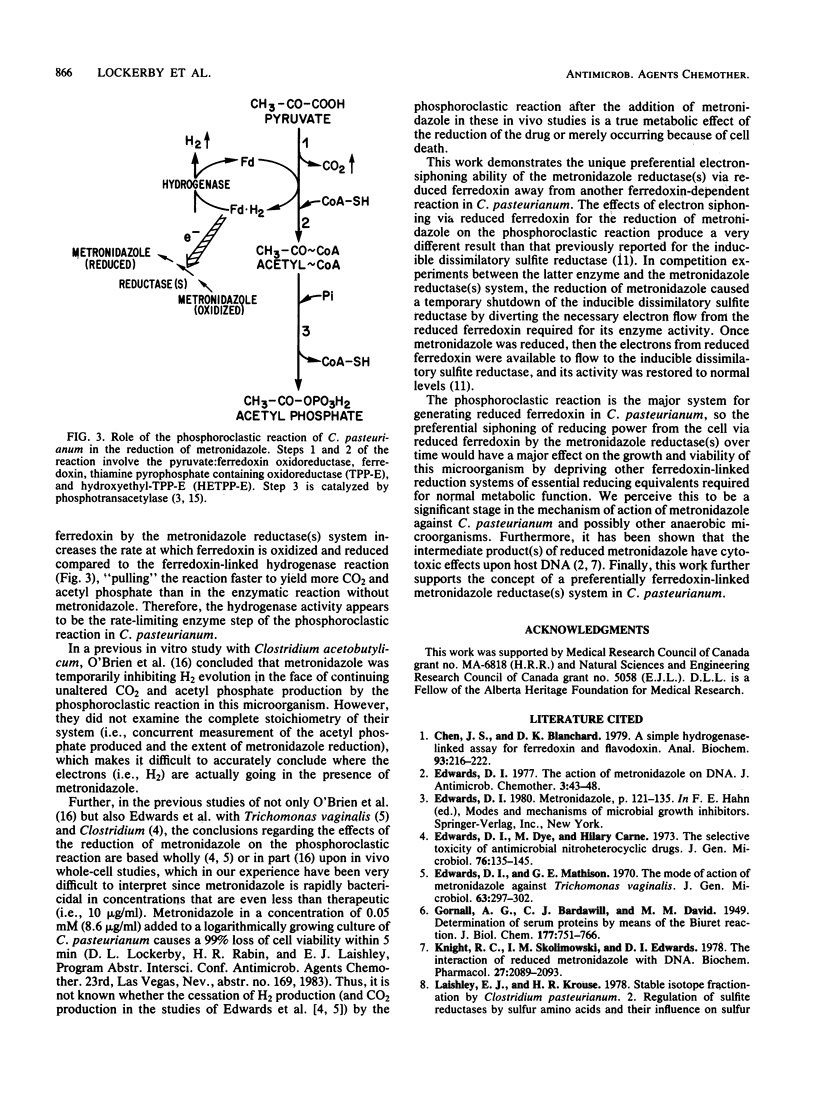
To demonstrate the importance of electron siphoning by the metronidazole reductase system from reduced ferredoxin to the mechanism of action of the drug in Clostridium pasteurianum, the effects of the reduction of metronidazole on the phosphoroclastic reaction were studied. Metronidazole concentrations between 0.5 and 5 mM caused a significant increase in acetyl phosphate production by the phosphoroclastic reaction compared to the control system without metronidazole. When this enzymatic reaction was assayed by standard manometric techniques under nitrogen gas, two simultaneous effects of electron siphoning were demonstrated: (i) the electrons from reduced ferredoxin were initially consumed for the reduction of metronidazole instead of being evolved as H2 via the ferredoxin-linked hydrogenase and (ii) phosphoroclastic activity was stimulated, with augmented production of CO2 and acetyl phosphate. This work further supports the notion of preferential scavenging of electrons away from ferredoxin-linked enzymatic reactions by metronidazole reductase(s) in C. pasteurianum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J. S., Blanchard D. K. A simple hydrogenase-linked assay for ferredoxin and flavodoxin. Anal Biochem. 1979 Feb;93(1):216–222. [PubMed] [Google Scholar]
- Edwards D. I., Dye M., Carne H. The selective toxicity of antimicrobial nitroheterocyclic drugs. J Gen Microbiol. 1973 May;76(1):135–145. doi: 10.1099/00221287-76-1-135. [DOI] [PubMed] [Google Scholar]
- Edwards D. I., Mathison G. E. The mode of action of metronidazole against Trichomonas vaginalis. J Gen Microbiol. 1970 Nov;63(3):297–302. doi: 10.1099/00221287-63-3-297. [DOI] [PubMed] [Google Scholar]
- Edwards D. I. The action of metronidazole on DNA. J Antimicrob Chemother. 1977 Jan;3(1):43–48. doi: 10.1093/jac/3.1.43. [DOI] [PubMed] [Google Scholar]
- Laishley E. J., Krouse H. R. Stable isotope fractionation by Clostridium pasteurianum. 2. Regulation of sulfite reductases by sulfur amino acids and their influence on sulfur isotope fractionation during SO32- and SO42- reduction. Can J Microbiol. 1978 Jun;24(6):716–724. doi: 10.1139/m78-120. [DOI] [PubMed] [Google Scholar]
- Laishley E. J., Lin P. M., Peck H. D., Jr A ferredoxin-linked sulfite reductase from Clostridium pasteurianum. Can J Microbiol. 1971 Jul;17(7):889–895. doi: 10.1139/m71-142. [DOI] [PubMed] [Google Scholar]
- Lockerby D. L., Rabin H. R., Bryan L. E., Laishley E. J. Ferredoxin-linked reduction of metronidazole in Clostridium pasteurianum. Antimicrob Agents Chemother. 1984 Nov;26(5):665–669. doi: 10.1128/aac.26.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTENSON L. E. FERREDOXIN REQUIREMENT FOR NITROGEN FIXATION BY EXTRACTS OF CLOSTRIDIUM PASTEURIANUM. Biochim Biophys Acta. 1964 Mar 9;81:473–478. doi: 10.1016/0926-6569(64)90132-4. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- Marczak R., Gorrell T. E., Müller M. Hydrogenosomal ferredoxin of the anaerobic protozoon, Tritrichomonas foetus. J Biol Chem. 1983 Oct 25;258(20):12427–12433. [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Effect of metronidazole on hydrogen production by Clostridium acetobutylicum. Arch Mikrobiol. 1972;84(3):225–233. doi: 10.1007/BF00425200. [DOI] [PubMed] [Google Scholar]


