Abstract
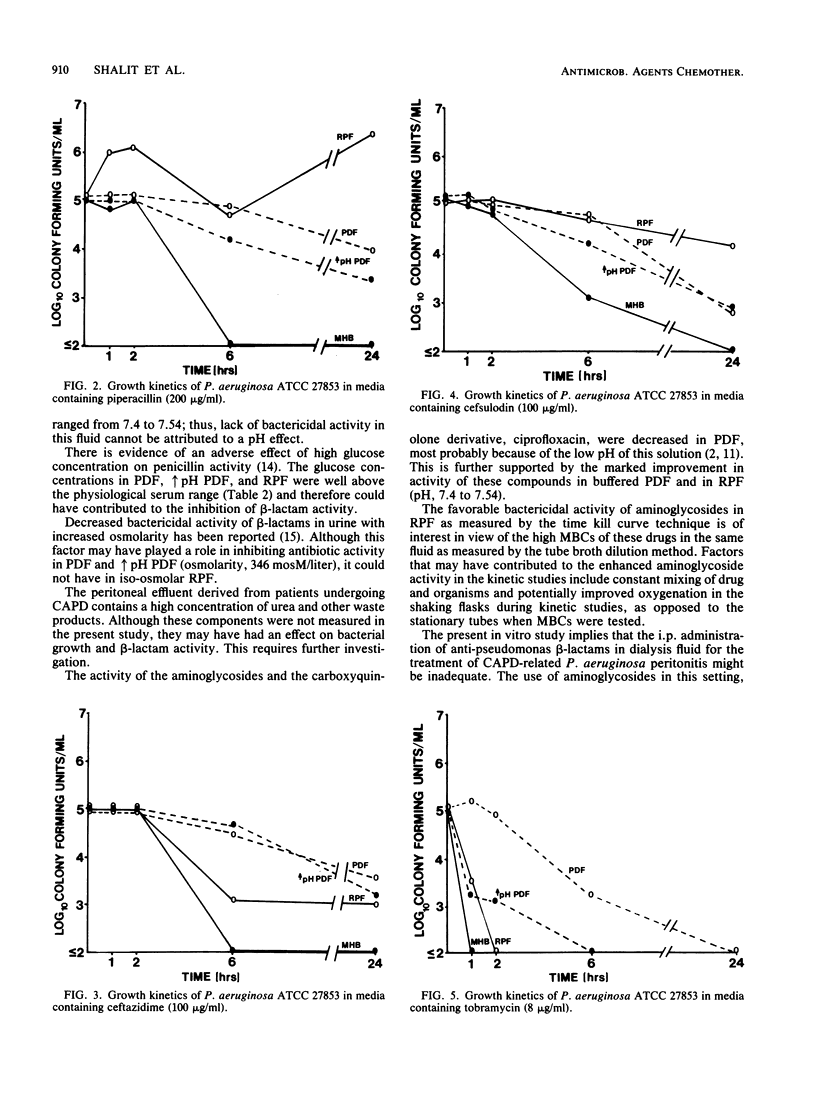
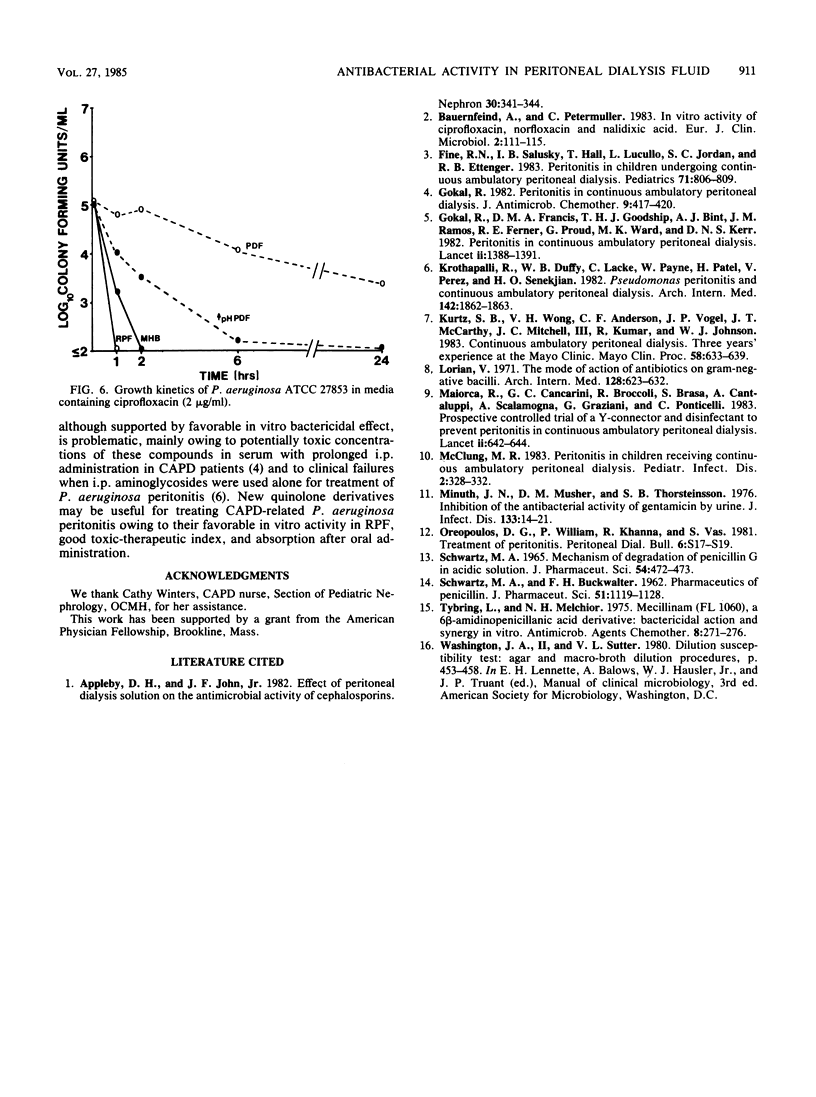
Intraperitoneal antibiotics are used to treat Pseudomonas aeruginosa peritonitis, a serious complication of continuous ambulatory peritoneal dialysis. However, P. aeruginosa killing is often inefficient despite low MBCs. Broth dilution MIC/MBC and time kill curves of tobramycin, amikacin, netilmicin, azlocillin, piperacillin, ceftazidime, cefsulodin, and ciprofloxacin were determined in peritoneal dialysis fluid (PDF), buffered PDF, fluid recovered from patients on continuous ambulatory peritoneal dialysis (RPF), and cation-supplemented Mueller-Hinton broth. MBCs of all antibiotics were 8 to 16 times greater in PDF and RPF than in Mueller-Hinton broth or buffered PDF. Use of the time kill curve technique and Mueller-Hinton broth showed that aminoglycosides killed greater than or equal to 99.9% of P. aeruginosa at 1 h, ciprofloxacin killed greater than or equal to 99.9% at 2 h, and beta-lactams killed greater than or equal to 99.9% at 6 h. In contrast, killing was not demonstrated in PDF by any drug at 6 h and by aminoglycosides only at 24 h. Bactericidal activity was optimal in RPF for ciprofloxacin at 1 h and for aminoglycosides at 2 h; bactericidal activity was not demonstrated in RPF with any beta-lactam (no kill by penicillins; less than 99% kill by cephalosporins). Slow bacterial growth, increased protein binding, and glucose concentrations and other inhibitors may interfere with beta-lactam activity in RPF. These considerations and reported clinical failures and toxicity of aminoglycoside therapy warrant further study of quinolones and drug combinations in P. aeruginosa peritonitis.
Full text
PDF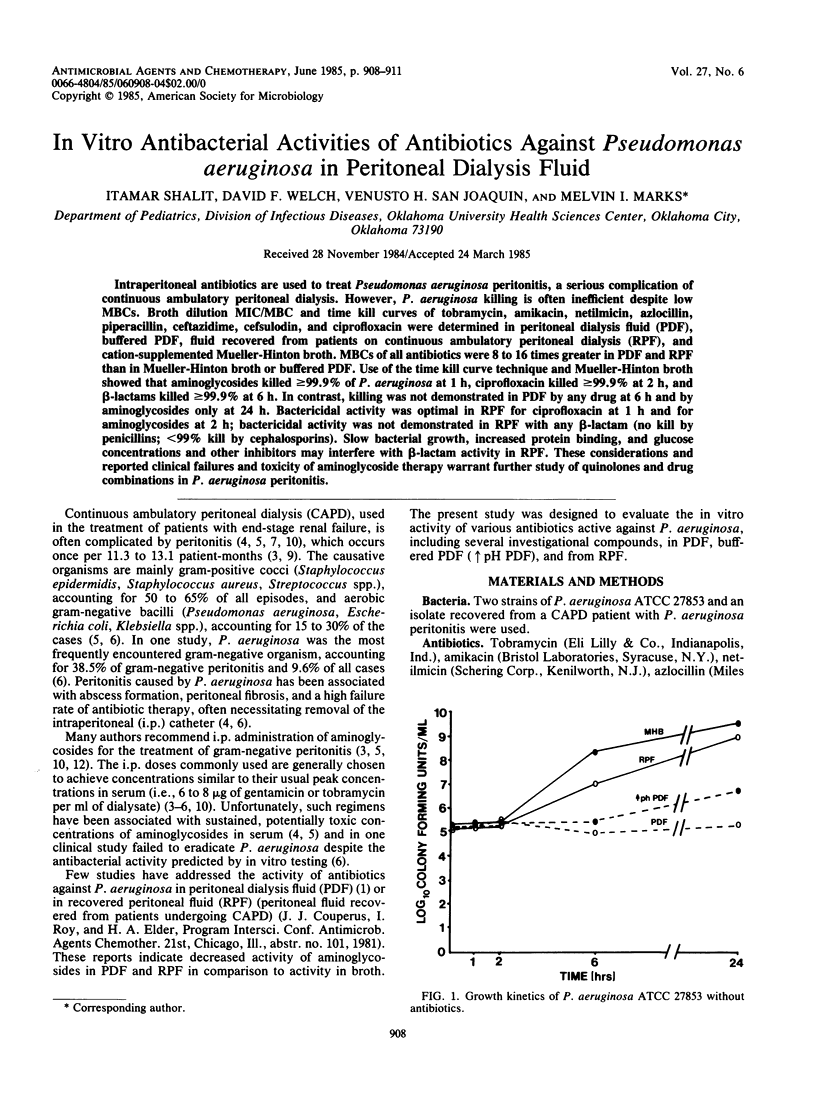

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby D. H., John J. F., Jr Effect of peritoneal dialysis solution on the antimicrobial activity of cephalosporins. Nephron. 1982;30(4):341–344. doi: 10.1159/000182513. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Petermüller C. In vitro activity of ciprofloxacin, norfloxacin and nalidixic acid. Eur J Clin Microbiol. 1983 Apr;2(2):111–115. doi: 10.1007/BF02001575. [DOI] [PubMed] [Google Scholar]
- Fine R. N., Salusky I. B., Hall T., Lucullo L., Jordan S. C., Ettenger R. B. Peritonitis in children undergoing continuous ambulatory peritoneal dialysis. Pediatrics. 1983 May;71(5):806–809. [PubMed] [Google Scholar]
- Gokal R. Peritonitis in continuous ambulatory peritoneal dialysis. J Antimicrob Chemother. 1982 Jun;9(6):417–420. doi: 10.1093/jac/9.6.417. [DOI] [PubMed] [Google Scholar]
- Gokal R., Ramos J. M., Francis D. M., Ferner R. E., Goodship T. H., Proud G., Bint A. J., Ward M. K., Kerr D. N. Peritonitis in continuous ambulatory peritoneal dialysis. Laboratory and clinical studies. Lancet. 1982 Dec 18;2(8312):1388–1391. doi: 10.1016/s0140-6736(82)91282-x. [DOI] [PubMed] [Google Scholar]
- Krothapalli R., Duffy W. B., Lacke C., Payne W., Patel H., Perez V., Senekjian H. O. Pseudomonas peritonitis and continuous ambulatory peritoneal dialysis. Arch Intern Med. 1982 Oct;142(10):1862–1863. [PubMed] [Google Scholar]
- Kurtz S. B., Wong V. H., Anderson C. F., Vogel J. P., McCarthy J. T., Mitchell J. C., 3rd, Kumar R., Johnson W. J. Continuous ambulatory peritoneal dialysis. Three years' experience at the Mayo Clinic. Mayo Clin Proc. 1983 Oct;58(10):633–639. [PubMed] [Google Scholar]
- Lorian V. The mode of action of antibiotics on gram-negative bacilli. Arch Intern Med. 1971 Oct;128(4):623–632. [PubMed] [Google Scholar]
- Maiorca R., Cantaluppi A., Cancarini G. C., Scalamogna A., Broccoli R., Graziani G., Brasa S., Ponticelli C. Prospective controlled trial of a Y-connector and disinfectant to prevent peritonitis in continuous ambulatory peritoneal dialysis. Lancet. 1983 Sep 17;2(8351):642–644. doi: 10.1016/s0140-6736(83)92528-x. [DOI] [PubMed] [Google Scholar]
- McClung M. R. Peritonitis in children receiving continuous ambulatory peritoneal dialysis. Pediatr Infect Dis. 1983 Jul-Aug;2(4):328–332. doi: 10.1097/00006454-198307000-00018. [DOI] [PubMed] [Google Scholar]
- Minuth J. N., Musher D. M., Thorsteinsson S. B. Inhibition of the antibacterial activity of gentamicin by urine. J Infect Dis. 1976 Jan;133(1):14–21. doi: 10.1093/infdis/133.1.14. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M. A., BUCKWALTER F. H. Pharmaceutics of penicillin. J Pharm Sci. 1962 Dec;51:1119–1128. doi: 10.1002/jps.2600511202. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ M. A. MECHANISM OF DEGRADATION OF PENICILLIN G IN ACIDIC SOLUTION. J Pharm Sci. 1965 Mar;54:472–473. doi: 10.1002/jps.2600540336. [DOI] [PubMed] [Google Scholar]
- Tybring L., Melchior N. H. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: bactericidal action and synergy in vitro. Antimicrob Agents Chemother. 1975 Sep;8(3):271–276. doi: 10.1128/aac.8.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]


