Abstract
Plants can sense and respond to mechanical stimuli, like animals. An early mechanism of mechanosensing and response is speculated to be governed by as-yet-unidentified sensory complexes containing a Ca2+-permeable, stretch-activated (SA) channel. However, the components or regulators of such complexes are poorly understood at the molecular level in plants. Here, we report the molecular identification of a plasma membrane protein (designated Mca1) that correlates Ca2+ influx with mechanosensing in Arabidopsis thaliana. MCA1 cDNA was cloned by the functional complementation of lethality of a yeast mid1 mutant lacking a putative Ca2+-permeable SA channel component. Mca1 was localized to the yeast plasma membrane as an integral membrane protein and mediated Ca2+ influx. Mca1 also increased [Ca2+]cyt upon plasma membrane distortion in Arabidopsis. The growth of MCA1-overexpressing plants was impaired in a high-calcium but not a low-calcium medium. The primary roots of mca1-null plants failed to penetrate a harder agar medium from a softer one. These observations demonstrate that Mca1 plays a crucial role in a Ca2+-permeable SA channel system that leads to mechanosensing in Arabidopsis. We anticipate our findings to be a starting point for a deeper understanding of the molecular mechanisms of mechanotransduction in plants.
Keywords: calcium, calcium channel, calcium uptake, mechanosensing
Sensing and responding to mechanical stimuli, such as touch, gravity, flexure, and turgor, are fundamental characteristics of plants (1–3). Because the mechanical stimulation of plants immediately elicits a transient rise in the cytosolic Ca2+ concentration, [Ca2+]cyt, an early mechanism of mechanosensing and response is thought to be governed by mechanical sensors with a Ca2+-permeable, stretch-activated (SA) channel as an essential constituent (4–6). Thus, the SA channel is postulated to play a major role in the regulation of thigmotropism, gravitropism, the morphogenesis of organs including roots, and polarized growth of pollen tubes (7–9). However, the molecular identities of the channel and its regulators are unknown and their physiological functions are speculative, except for MscS-like proteins located in the plastid envelope in Arabidopsis thaliana (10).
The yeast Saccharomyces cerevisiae is a helpful eukaryote in isolating and characterizing plant membrane proteins. K+ channel cDNAs of Arabidopsis have been cloned by complementation of K+ uptake-deficient mutants of S. cerevisiae with Arabidopsis cDNA libraries (11, 12). Putative cyclic nucleotide-gated cation channels, found in the Arabidopsis genome, are successfully expressed in S. cerevisiae mutants defective in K+ uptake or Ca2+ uptake and their function and subcellular localization have been characterized (13, 14).
Here, we report the isolation and characterization of an Arabidopsis cDNA (designated MCA1) that complements the lethal phenotype of an S. cerevisiae mid1 mutant defective in a putative Ca2+-permeable SA channel component. We demonstrate that Mca1 is an integral plasma membrane protein that mediates Ca2+ uptake when expressed in S. cerevisiae. MCA1 mRNA is detected at varying levels in all Arabidopsis tissues examined, as revealed by Northern blotting. With the analyses of Arabidopsis mutants lacking or overexpressing MCA1, we suggest that Mca1 is an important constituent of a mechano-stimulated Ca2+ uptake mechanism in Arabidopsis.
Results
Isolation and Characterization of MCA1 cDNA.
To isolate a candidate gene encoding a component or regulator of a Ca2+-permeable SA channel from Arabidopsis, we used functional complementation of the lethal phenotype of the mid1 mutant of the yeast S. cerevisiae, which lacks a putative Ca2+-permeable SA channel component and becomes lethal because of a deficiency of Ca2+ influx during exposure to mating pheromone (15–17). The mid1 mutant was transformed with an Arabidopsis cDNA library constructed in a yeast expression vector (18). The transformants were screened for their viability after being exposed to mating pheromone. One viable transformant was obtained, and the full-length cDNA on the plasmid isolated from it was designated MCA1 for mid1-complementing activity 1. Quantitative assays showed that MCA1 indeed partially complemented the lethality of mid1 cells (Fig. 1A) and increased Ca2+ uptake activity (Fig. 1B). It should be noted that MCA1-expressing mid1 cells grew slightly slower than mid1 and wild-type cells. Therefore, the higher Ca2+ uptake in the MCA1-expressing mid1 cells is not due to their putative, higher growth rate.
Fig. 1.
Function in yeast cells and structural features of Mca1 and Mca2. (A) Complementation of lethality of the yeast mid1 mutant by MCA1. Cell viability of the mid1 mutant strain bearing pFL61-MCA1 or empty vector pFL61 and wild-type strain bearing pFL61 was determined after being exposed to the mating pheromone α-factor. Data are means ± SD from three independent experiments. ∗, P < 0.01 versus mid1/pFL61. (B) Ca2+ uptake activity. Exponentially growing cells of the above strains were suspended in Hepes/Tris buffer containing 74 kBq/ml 45CaCl2 (74 MBq/nmol) and aliquots of the suspension were taken at 1-min intervals over 8 min and filtered through a Millipore filter (type HA; 0.45 μm). The radioactivity retained on the filters was counted. Data are means ± SD from three independent experiments. ∗, P < 0.01 versus mid1/pFL61. (C) Genomic organization of the MCA1 and MCA2 genes. Boxes represent exons and their black areas show the ORF. T-DNA (drawn to an arbitrary size) is inserted into the site 28 bp upstream of the fourth exon, producing an mca1-null allele. (D) Multiple amino acid sequence alignment of the Mca1, Mca2, and rice OsMca1 with Clustal W (version 1.8). Amino acid sequence identity (and similarity) between Mca1 and Mca2 is 72.7% (89.4%), that between Mca1 and OsMca1 is 65.0% (90.3%), and that between Mca2 and OsMca1 is 57.2% (86.4%). Asterisk indicates identical amino acid; colon indicates amino acid with strong similarity; dot indicates amino acid with weak similarity. The ARPK domain (for amino-terminal domain of rice putative protein kinases) is boxed. The line under each sequence shows a potential transmembrane segment (TM) predicted by TopPred (19). The lines above the Mca1 sequence represent an EF-hand-like structure, a coiled-coil region, and a C-terminal, cysteine-rich region similar to the PLAC8 motif found in plant and animal proteins of unknown function. (E) Schema of Mca1 and the hydropathy profile of Mca1. The bars indicate the position of the potential transmembrane segments (TM) described in D. (F) MCA1 has the ability to increase Ca2+ accumulation even in the mid1 cch1 double mutant. MCA1 cDNA on a plasmid was expressed under the control of the TDH3 promoter in each yeast mutant (mid1, cch1, or mid1 cch1) as well as the parental strain (MID1 CCH1). The MID1 gene was expressed from the plasmid YEpMID1 (25). Exponentially growing cells were incubated for 2 h in the low Ca2+ medium SD.Ca100 (15) containing 185 kBq/ml 45CaCl2 (1.8 kBq/nmol) and aliquots of the culture were taken and filtered through a Millipore filter (type HA; 0.45 μm). ∗, P < 0.05 versus vector in each mutant.
Nucleotide sequencing of the MCA1 cDNA identified the corresponding hypothetical ORF At4g35920 on chromosome 4. The MCA1 gene (DDBJ accession no. AB196960) has 10 exons and was predicted to encode a polypeptide of 421 amino acid residues (Fig. 1 C and D). The overall amino acid sequence has only 10% identity and 41% similarity to that of the Mid1 protein and has no significant similarity to that of any protein characterized as an ion channel component. The TopPred (19), TMPred (20), and PredictProtein (21) programs suggested that Mca1 has at least two potential transmembrane segments (Fig. 1 D and E). The carboxyl-terminal half shows similarity to the PLAC8 (for human placenta-specific gene 8) region that is a cysteine-rich domain of unknown function found in 127 plant and animal proteins (22). The Mca1 amino-terminal half has an EF hand-like motif and is similar to a functionally unknown, amino-terminal domain found in many rice putative protein kinases [designated the ARPK domain for amino-terminal domain of rice putative protein kinases; see supporting information (SI) Fig. 5].
Previous studies have shown that Mid1 cooperates with Cch1, which is homologous to the α1 subunit of mammalian voltage-gated Ca2+ channels, to function as a putative Ca2+-permeable channel in yeast (23, 24). Thus, MID1 even on a multicopy plasmid is unable to complement the Ca2+-uptake defect of the mid1 cch1 double mutant (ref. 25; Fig. 1F). By contrast, MCA1 was able to do so (Fig. 1F). This Cch1-independent complementation by Mca1 suggests that Mca1 functions in a way different from Mid1.
A Blast search (BLASTP) (26) with the amino acid sequence of Mca1 showed 73% full-length identity to a hypothetical Arabidopsis ORF of unknown function (At2g17780) on chromosome 2. We designated its gene (DDBJ accession no. AB196961) MCA2. The features of the gene organization and protein structure are similar to those of Mca1 (Fig. 1 C and D), suggesting that the functions of these two proteins are similar. The Blast search (TBLASTN) (26) also identified a rice cDNA clone, J033126N07, which encodes an orthologue (designated OsMca1) of Mca1 with 65% full-length identity (Fig. 1D), suggesting that Mca1 constitutes a unique family in flowering plants.
Expression and Subcellular Localization of Mca1.
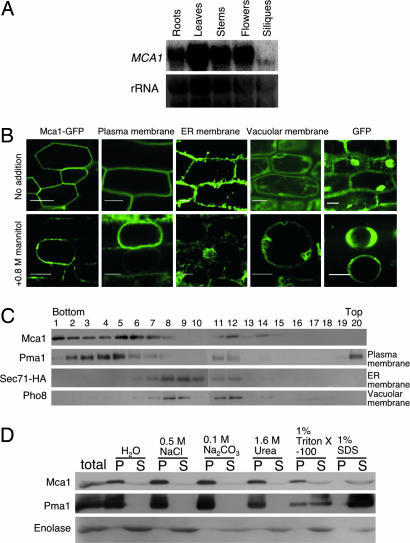
MCA1 mRNA was expressed in various organs of mature Arabidopsis examined, including roots, leaves, stems, flowers and siliques, with diverse expression levels (Fig. 2A). Fluorescence microscopy suggested that the Mca1-GFP fusion protein, produced under the control of the 35S promoter of the cauliflower mosaic virus (CaMV), was present in the plasma membrane of root cells (Fig. 2B), and this suggestion was strengthened by treatment with a high osmotic solution (0.8 M mannitol) that induced plasmolysis. The localization and behavior of Mca1-GFP were very similar to those of a plasma membrane marker protein (27) and different from those of marker proteins (27) for the endoplasmic reticulum and the vacuole as well as those of cytoplasmic GFP, all of which were also expressed under the control of the CaMV 35S promoter. We made anti-Mca1 antibodies that specifically recognize Mca1 expressed in yeast, but they did not detect Mca1 in Arabidopsis protein preparations by immunoblotting, probably because it is low in content or solubility. Therefore, we used yeast cells expressing Mca1 for examining its subcellular localization. Membrane fractionation experiments indicated that Mca1 is localized to the yeast plasma membrane as an integral membrane protein (Fig. 2 C and D). These results suggest that Mca1 is a plasma membrane protein responsible for Ca2+ influx in Arabidopsis.
Fig. 2.
Expression of MCA1. (A) Northern blotting of the MCA1 transcripts. Total RNA was isolated from roots, leaves, stems, flowers, and siliques of mature Arabidopsis plants and subjected to Northern blotting. rRNA was used for internal controls for the amount of RNA loaded. (B) GFP fluorescence images suggesting the localization of Mca1-GFP in the plasma membrane of root cells. The upper row represents intact roots and the bottom row those treated with 0.8 M mannitol for at least 10 min. Note that mannitol induced plasmolysis. The sample and membrane marker proteins used are as follows: Mca1-GFP, a GFP fusion to the C terminus of the full length Mca1 protein; Plasma membrane, a GFP fusion to the plasma membrane channel protein PIP2A expressed in line Q8 (27); ER membrane, a GFP fusion to an endoplasmic reticulum membrane protein expressed in line Q4 (27); Vacuolar membrane, a GFP fusion to the vacuolar membrane channel protein delta-TIP expressed in line Q5 (27); and cytoplasmic GFP. (Scale bars, 20 μm.) (C) Membrane fractionation by sucrose density gradient centrifugation and localization of Mca1 expressed in yeast. Pma1, plasma membrane H+-ATPase; Sec71-HA, an endoplasmic reticulum membrane protein tagged with hemagglutinin antigen (HA); Pho8, a vacuolar membrane protein. (D) Mca1 is an integral membrane protein in yeast. Note that Mca1 is not solubilized with NaCl, Na2CO3, and urea, all of which are known to solubilize peripheral membrane proteins. P, pellet after centrifugation at 100,000 × g for 1 h, containing membranes; S, supernatant.
Mca1 Promotes Ca2+ Influx upon Mechanical Stimulation in Planta.
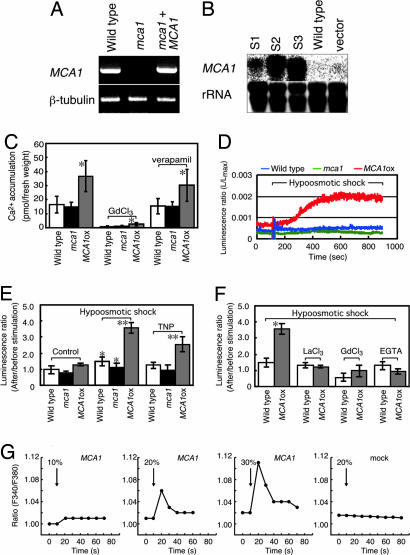
To determine the function of Mca1 in planta, we isolated an mca1-null mutant line from T-DNA insertion populations using PCR-based screening. In the null mutant, T-DNA was found to have inserted into a position 28 bp upstream of the fourth exon, corresponding to 28 bp upstream of the initiation codon, of the MCA1 locus (Fig. 1C), leading to no production of MCA1 mRNA, as revealed by RT-PCR (Fig. 3A). The mca1-null mutant grew like the wild type under ordinary growth conditions and on medium containing low or high CaCl2 (data not shown). We also made Arabidopsis transgenic lines (designated MCA1ox) that overexpress MCA1 cDNA under the control of the CaMV 35S promoter. Three independent, representative lines showed increased expression of MCA1 mRNA, although the expression levels varied from line to line, as revealed by Northern blotting (Fig. 3B). The development of the MCA1ox lines was abnormal and dependent on the expression levels (see below).
Fig. 3.
Mca1 protein enhances Ca2+ uptake activity in planta. (A) RT-PCR showing no detectable production of MCA1 transcripts in the mca1-null line. β-tubulin is a control. (B) Northern blotting, showing MCA1 mRNA levels in MCA1ox (S1, S2, and S3), wild-type, and vector-bearing wild-type lines. rRNA was used for control experiments. (C) Overexpression of Mca1 increases Ca2+ uptake in intact roots. The roots were incubated with 45CaCl2 for 20 min and washed five times with a washing solution containing LaCl3, which displaces 45Ca2+ nonspecifically bound to the cell wall (33). The uptake is inhibited by 1 mM Gd3+, but not by 20 μM verapamil. ∗, P < 0.01 versus wild type. (D) Hypoosmotic shock-induced [Ca2+]cyt changes, as revealed in aequorin luminescence. Wild-type, mca1-null, and MCA1ox seedlings with aequorin in MS medium (400 μl) were subjected to hypoosmotic shock with the addition of H2O (200 μl). Representative data are shown for each seedling. (E) Summary of hypoosmotic shock- and TNP-induced [Ca2+]cyt changes. As for hypoosmotic shock, experimental conditions were the same as those in D. For TNP stimulation and control experiments, 0.3 mM TNP (200 μl) and MS medium (200 μl) were added to the seedlings in MS medium (400 μl), respectively. The average of five independent experiments is shown for each sample. ∗, P < 0.05 versus each control; ∗∗, P < 0.005 versus wild type in each treatment. (F) Effect of channel blockers and a Ca2+ chelator on the hypoosmotic shock-induced [Ca2+]cyt increase. Ten minutes before hypoosmotic shock, 1 mM La3+, 1 mM Gd3+, or 5 mM EGTA (calcium chelator) was added to the medium. ∗, P < 0.005 versus wild-type and reagent-treated samples. (G) Stretch-activated Ca2+ response in MCA1-expressing and mock-transfected CHO cells. Cells cultured on elastic silicone membranes were loaded with 1 μM fura-2/AM and subjected to a uniaxial stretch pulse (10, 20, or 30% of the length for 1 s at room temperature). Fura-2 fluorescence intensities at excitation wavelengths of 340 and 380 nm were acquired and the ratio (F340/F380) was calculated. An increase in the ratio donates a [Ca2+]cyt increase. (Right) Mock-transfected cells. (Center and Left) MCA1-expressing cells.
Using the mca1-null mutant and MCA1ox lines as well as the wild type, we directly measured Ca2+ uptake in the intact roots of 5-week-old-plants using 45Ca2+ and found that MCA1ox roots incorporated Ca2+ to a greater extent than wild-type and mca1-null roots (Fig. 3C). This incorporation was almost completely inhibited by Gd3+, a blocker of nonselective cation channels, including SA channels, but not by verapamil, a blocker of voltage-gated Ca2+ channels.
To investigate the relationship between Mca1 and a mechanical stress-generated Ca2+ signal, seedlings of the wild-type, mca1-null, and MCA1ox lines having the Ca2+-indicator protein aequorin were exposed to hypoosmotic stress (6) or the anionic amphipath trinitrophenol (TNP), which preferentially penetrates the outer leaflet of the plasma membrane (28). Both treatments generate membrane distortion, resulting in an activation of SA channels (6, 28). The results showed that the degree of [Ca2+]cyt increase was markedly greater in the MCA1ox seedlings than in the wild-type ones and that there was no significant difference in [Ca2+]cyt changes between the mca1-null and wild-type seedlings (Fig. 3 D and E). The hypoosmotic stress-induced increase observed in the MCA1ox seedlings was inhibited by extracellulary applied La3+, Gd3+, or EGTA (Fig. 3F). These results suggest that Mca1 is involved in generating a Ca2+ signal upon plasma membrane distortion.
To examine this suggestion, we directly stretched MCA1-expressing mammalian cultured cells. MCA1 cDNA was placed under the control of the Zn2+-inducible human metallothionein IIa promoter in a plasmid and introduced into Chinese hamster ovary (CHO) cells as described (16, 29). The CHO cells were cultured on a fibronectin-coated silicone membrane, loaded with the Ca2+ indicator fura-2/AM, and subjected to uniaxial stretching for 1 s. As shown in Fig. 3G, although mock-transfected CHO cells treated with ZnCl2 showed no increase in the fura-2 fluorescence ratio (F340/F380) that represents [Ca2+]cyt (n = 808), MCA1-expressing CHO cells treated with ZnCl2 showed an increase in the ratio, depending on the degree of stretching (40 of 765 cells; 5.2%), where the mean increase was 2.6% ± 0.34 SE (n = 40; P < 0.01, Fisher–Behrens t test).
Taken together, these cumulative data obtained with three different approaches suggest that Mca1 works in a Ca2+-permeable SA channel system in the plasma membrane. The reason why there was no significant difference in the Ca2+ uptake and [Ca2+]cyt increase between the wild-type and mca1-null lines remains uncertain at present; the activation of a compensatory mechanism in the null mutants may make the difference obscure.
Phenotypes of MCA1ox and mca1-Null Mutants.
To characterize the physiological role of Mca1 in vivo, we investigated the phenotype of MCA1ox plants. Seedlings of one line (designated S3) expressing MCA1 mRNA at a relatively high level (Fig. 3B) showed browning of the hypocotyls in ≈9 days (Fig. 4A), and most of them eventually died within ≈20 days after germination. Another line (S1) expressing MCA1 mRNA at a relatively low level (Fig. 3B) developed milder symptoms than the S3 line, exhibiting developmental defects with short stems, small rosettes, no petals, shrunken siliques (Fig. 4B), and poor fertility (data not shown). Interestingly, leaf development was aggravated when the seedlings were grown on high-Ca2+ medium, but abrogated when they were grown on low (0.5 mM) Ca2+ medium (Fig. 4C). Essentially the same result was obtained with two other MCA1ox lines that are similar to the S1 line in MCA1 mRNA levels (data not shown). This result is consistent with the above findings that Mca1 is responsible for Ca2+ influx.
Fig. 4.
Phenotypes of mca1-null and MCA1ox lines. (A) A seedling of the MCA1ox line S3, showing browning of the hypocotyl. (Scale bars, 2.0 mm.) (B) An MCA1ox plant (line S1), showing a phenotype with no petals, shrunken siliques, short stems, and small rosettes. (White bars, 1.5 mm; black bars, 1.0 cm.) (C) Ca2+-dependent growth phenotypes of MCA1ox seedlings (line S1). Note that the ordinary MS medium contains 3.0 mM CaCl2. On this medium, MCA1ox seedlings exhibited growth deficiency. (Scale bar, 1.0 cm.) (D) Failure to penetrate the lower, harder agar (1.6%) medium of the primary roots of the mca1-null mutant. Seeds of various lines were placed on the surface of the upper, softer agar (0.8%) medium of the two-phase-agar medium, allowed to grow for 9 days, and then photographed. (Left) Wild type (side view). (Upper Center) mca1-null (side view). (Lower Center) mca1-null (oblique upper view), showing the root tips were coiled at the interface of the two agar media. (Right) mca1-null mutant complemented with wild-type MCA1. Arrowheads represent the interface of the two agar media. (Scale bar, 1.0 cm.) (E) Quantitative representation of the results shown in D. Number of seedlings examined: Wild type, n = 150; mca1-null, n = 150; mca1-null + MCA1, n = 80. ∗, P < 0.01 versus wild type and mca1-null + MCA1.
To assess a physiological relevance of the overexpression of MCA1, we performed a quantitative RT-PCR analysis and found that MCA1ox plants had a constitutively elevated expression of a touch gene, TCH3 (CML12), encoding a Ca2+-binding, calmodulin-related protein whose expression is known to be induced by touch and Ca2+ (ref. 30 and SI Fig. 6), suggesting that Mca1 stimulates the expression and activity of Tch3 through Ca2+ influx.
Because the root, where MCA1 mRNA is expressed, is a touch-sensitive organ that detects the hardness of soil during development (5), we examined whether the primary root of the mca1-null mutant failed to sense and adapt to the hardness of agar in medium. We thus developed a method, designated the two-phase-agar method, in which we used lower (harder) medium containing 1.6% agar covered with upper (softer) medium containing 0.8% agar, to observe root growth at the interface between the upper and lower media. Seeds were placed on the surface of the upper medium and allowed to develop into seedlings. It took both the primary roots of mca1-null and wild-type seedlings 5–7 days to reach the interface of the two-phase agar after sowing (n = 150 for each line). Although the primary root of the wild type was capable of penetrating the lower medium, that of a significant population of the mca1-null mutant was not (Fig. 4 D and E). Complementation of the mca1-null mutant with the extrinsic wild-type MCA1 gene confirmed that MCA1 is indeed responsible for this phenotype. Interestingly, when seeds were directly placed on 1.6% agar medium, the primary root of the mca1-null mutant was able to penetrate the medium, like that of the wild type. There was no difference in the length of the primary roots between the mca1-null and wild-type lines when they were grown in both 0.8 and 1.6% agar media (n > 65 primary roots for each medium).
Discussion
Arabidopsis Mca1 Mediates Ca2+ Uptake in Yeast.
The present study provides supporting evidence that Mca1 is a component of a plasma membrane Ca2+ influx system. Using a yeast screening system, we have cloned Arabidopsis MCA1 cDNA that complements both the lethality and low Ca2+ uptake activity of the yeast mid1 mutant defective in a putative Ca2+-permeable SA channel component. Ca2+ incorporation assays, computer analysis of the predicted amino acid sequence, and membrane fractionation experiments have demonstrated that Mca1 is localized to the yeast plasma membrane as an integral membrane protein that mediates Ca2+ uptake.
From structural and mechanistic viewpoints, however, Mca1 is very different from Mid1, although MCA1 complements the mid1 mutant. An amino acid sequence alignment suggests that Mca1 is not an orthologue of Mid1. Because Mid1 cooperates with Cch1 to function as a Ca2+ influx system, Mid1 is unable to complement the low Ca2+ uptake activity of the mid1 cch1 double mutant (25). By contrast, Mca1 can complement not only the mid1 mutant, but also the double mutant. This ability is particularly interesting, because the yeast S. cerevisiae has only one high-affinity Ca2+ influx system, which is composed of Mid1 and Cch1 and becomes functional when cells are incubated in the low-Ca2+ medium that we have used in this study (31). Thus, it is remarkable that Mca1 can mediate Ca2+ uptake in yeast cells lacking the high-affinity Ca2+ influx system. This evidence supports a hypothesis that Mca1 is a Ca2+-permeable transport protein. An alternative possibility that Mca1 activates another yeast endogenous transport system, however, cannot be completely ruled out.
Structural Features of Mca1.
Mca1 has no overall sequence similarity to all known ion channels. However, there are several structural features of interest, the importance of which should be addressed experimentally in further studies: First, Mca1 has at least two transmembrane segments. This finding is consistent with membrane fractionation experiments with yeast cells, showing that Mca1 is an integral membrane protein; Second, the amino-terminal region is similar in amino acid sequence to that of rice putative protein kinases whose carboxyl-terminal region is predicted to have a catalytic function, suggesting that the amino-terminal region has a regulatory role; Third, the amino-terminal half has an EF-hand-like sequence, suggesting that the function of Mca1 is regulated by Ca2+. Finally, the carboxyl-terminal half contains a region similar to the cysteine-rich PLAC8 domain of unknown function found in many plant and animal proteins (22).
Mca1 May Mediate Mechanically Stimulated Ca2+ Uptake in Arabidopsis.
Mca1 appears to be involved in Ca2+ uptake across the plasma membrane in response to mechanical stimulation in Arabidopsis. First, fluorescence microscopy has suggested that the Mca1-GFP fusion protein is localized to the plasma membrane, and this suggestion is reinforced by the aforementioned membrane fractionation experiments with yeast cells expressing Mca1. Second, the roots of MCA1ox plants accumulate 45Ca2+ more than those of wild-type and mca1-null plants. This accumulation is blocked by Gd3+, which is known to inhibit SA channels. Third, MCA1ox plants also show increasing [Ca2+]cyt in response to mild hypoosmotic shock and the amphipathic membrane crenator TNP, whereas wild-type and mca1-null plants do not. The [Ca2+]cyt increase is blocked by Gd3+, La3+, and EGTA added to the medium. Fourth, MCA1-expressing CHO cells show an increase in [Ca2+]cyt in response to cell stretching. In addition, our preliminary experiments with whole-cell patch-clamp recordings on mesophyll protoplasts have demonstrated that TNP-induced Ca2+ currents are statistically greater (P < 0.01) in MCA1ox protoplasts (2.3 ± 0.5 pA/pF at −80 mV; n = 5) than the wild-type and mca1-null protoplasts (0.5 ± 0.2 and 0.3 ± 0.2 pA/pF at −80 mV, respectively; n = 5 for each). These results suggest that Mca1 has a role in Ca2+ uptake in response to membrane distortion in Arabidopsis. It should be noted that this suggestion relies on the overexpression of MCA1, which may cause side effects. In addition, we have observed no significant difference in the foregoing phenotypes between wild-type and mca1-null plants. Thus, more decisive experiments are needed to verify this suggestion.
Physiological Relevance and Working Model.
Previous physiological studies have indicated that touch sensitivity is mediated by Ca2+ in the root tip. In the maize primary root, the root grows away from an agar block when the block is unilaterally applied to the root cap as a touch stimulus, and this response is enhanced when the agar contains Ca2+ (32). In the Arabidopsis primary root, touch induces a transient increase in [Ca2+]cyt in all regions of the root, including the cap and the meristematic, elongation, and differentiated zones (5). The Arabidopsis root plasma membrane has been electrophysiologically shown to have nonselective cation channels that mediate Ca2+ uptake (33). However, proteins or genes critically involved in these processes have remained to be uncovered.
We have found that the primary roots of the mca1-null seedlings are incapable of penetrating a lower (harder) agar surface (1.6%) after growth through an upper (softer) agar (0.8%) medium. Because only one T-DNA insertion allele is available, we have confirmed this finding by complementation with a wild-type copy of the MCA1 gene. It is unlikely that the primary roots of mca1-null seedlings merely grow weakly, because when the mca1-null seeds are directly sowed on 1.6% agar medium, the primary roots can penetrate it, like the wild-type primary roots. In addition, the primary roots of mca1-null and wild-type seedlings simultaneously reaches the interface of the upper and lower agar media in 5–7 days from the surface of the upper medium, and the length of the primary roots is essentially the same between the two seedlings, as described in Results, suggesting that the mca1-null mutation does not affect the growth rate of the primary root. Therefore, we suggest that Mca1 is required for sensing the hardness of agar or soil. Furthermore, these results suggest that conditioning or hardening of the primary roots for mechanosensing and response may be established in an early stage after germination.
Taken together, we propose a working model that Mca1 is crucial for mechano-stimulated Ca2+ uptake and mechanosensing in the primary root. Because the calmodulin-like protein Tch3 or Cml12, a cytosolic Ca2+-signaling protein known to be induced by touch and Ca2+ in many tissues of Arabidopsis, including roots (30), is overproduced in MCA1ox plants, Mca1 could have a physiologically relevant role in these processes. Further study on Mca1 and its family, such as Mca2 and OsMca1, should facilitate exploration of how plants expand their roots in highly complex but well organized ways.
Materials and Methods
Plant Growth Conditions.
The Columbia-0 (Col-0) ecotype of A. thaliana and its isogenic, transgenic lines were used. Seeds were sterilized with 70% ethanol for 10 s and then with 1% Antiformin for 10 min, and sown on MS/1% sucrose agar medium. MS medium contained Murashige and Skoog salts (34), 1× Gamborg's vitamin solution (Sigma Aldrich, Taufkichen, Germany), and 2.56 mM Mes-KOH, pH 5.7. Calcium-free Murashige and Skoog salts were made according to the formula of Murashige and Skoog (34) with the removal of CaCl2. For low-calcium medium, agarose was used instead of agar. Plants were grown at 22°C under 16-h light conditions at 40–60 μM m−2 s−1 light intensity.
Supporting Information.
For other methods, see SI Text.
Supplementary Material
Acknowledgments
We thank Dr. M. Minet (Centre de Génétique Moléculaire, Centre National de la Recherche Scientifique, Gif sur Yvette, France) for the A. thaliana cDNA library; Dr. K. Kuchitsu for valuable discussion; Dr. A. Sugino (Osaka University, Osaka, Japan) for plasmids; Dr. Y. Ohsumi (National Institute for Basic Biology, Okazaki, Japan) for anti-Pho8 antibodies; Ms. Y. Moriya for technical assistance; and Ms. Y. Higashi for secretarial assistance. This work was supported by Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology and CREST and ICORP/SORST, Japan Science and Technology Agency.
Abbreviations
- SA
stretch activated
- TNP
trinitrophenol.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607703104/DC1.
References
- 1.Darwin C. The Power of Movement in Plants. London: John Murray; 1880. [Google Scholar]
- 2.Trewavas A, Knight M. Plant Mol Biol. 1994;26:1329–1341. doi: 10.1007/BF00016478. [DOI] [PubMed] [Google Scholar]
- 3.Braam J. New Phytol. 2005;165:373–389. doi: 10.1111/j.1469-8137.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 4.Knight MR, Campbell AK, Smith SM, Trewavas AJ. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- 5.Legué V, Blancaflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi K, Isobe M, Knight MR, Trewavas AJ, Muto S. Plant Physiol. 1997;111:587–594. doi: 10.1104/pp.113.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove DJ, Hedrich R. Planta. 1991;186:143–153. doi: 10.1007/BF00201510. [DOI] [PubMed] [Google Scholar]
- 8.Pickard BG, Ding JP. Aust J Plant Physiol. 1993;20:439–459. doi: 10.1071/pp9930439. [DOI] [PubMed] [Google Scholar]
- 9.Taylor LP, Hepler PK. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- 10.Haswell ES, Meyerowitz EM. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JA, Huprikar SS, Kochian LV, Lucas WJ, Gaber RF. Proc Natl Acad Sci USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sentenac H, Bonneaud N, Minet M, Lacroute F, Salmon J-M, Gaymard F, Grignon C. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 13.Ali R, Zielinski RE, Berkowitz GA. J Exp Bot. 2006;57:125–138. doi: 10.1093/jxb/erj012. [DOI] [PubMed] [Google Scholar]
- 14.Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJ. J Exp Bot. 2006;57:791–800. doi: 10.1093/jxb/erj064. [DOI] [PubMed] [Google Scholar]
- 15.Iida H, Nakamura H, Ono T, Okumura MS, Anraku Y. Mol Cell Biol. 1994;14:8529–8271. doi: 10.1128/mcb.14.12.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H. Science. 1999;285:882–886. doi: 10.1126/science.285.5429.882. [DOI] [PubMed] [Google Scholar]
- 17.Kanzaki M, Nagasawa M, Kojima I, Sato C, Naruse K, Sokabe M, Iida H. Science. 2000;288:1347. doi: 10.1126/science.288.5470.1347. [DOI] [PubMed] [Google Scholar]
- 18.Minet M, Dufour M-E, Lacroute F. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann K, Stoffel W. Biol Chem Hoppe-Seyler. 1993;374:166. doi: 10.1515/bchm3.1993.374.7-12.507. [DOI] [PubMed] [Google Scholar]
- 20.Claros MG, von Heijne G. CABIOS. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 21.Rost B, Yachdav G, Liu J. Nucleic Acids Res. 2003;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchler-Bauer A, Anderson JB, Cherukuri PF, DeWeese-Scott C, Geer LY, Gwadz M, He S, Hurwitz DI, Jackson JD, Ke Z, et al. Nucleic Acids Res. 2005;33:D192–D196. doi: 10.1093/nar/gki069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer M, Schnell N, Chattaway J, Davies P, Dixon G, Sanders D. FEBS Lett. 1997;419:259–262. doi: 10.1016/s0014-5793(97)01466-x. [DOI] [PubMed] [Google Scholar]
- 24.Paidhungat M, Garrett S. Mol Cell Biol. 1997;17:6339–6347. doi: 10.1128/mcb.17.11.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iida K, Tada T, Iida H. FEBS Lett. 2004;576:291–296. doi: 10.1016/j.febslet.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutler SR, Ehrhardt DW, Griffitts JS, Somerville CR. Proc Natl Acad Sci USA. 2000;97:3718–3723. doi: 10.1073/pnas.97.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinac B, Adler J, Kung C. Nature. 1990;348:261–263. doi: 10.1038/348261a0. [DOI] [PubMed] [Google Scholar]
- 29.Naruse K, Yamada T, Sokabe M. Am J Physiol. 1998;274:H1532–H1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 30.Braam J. Proc Natl Acad Sci USA. 1992;89:3213–3216. doi: 10.1073/pnas.89.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller EM, Locke EG, Cunningham KW. Genetics. 2001;159:1527–1538. doi: 10.1093/genetics/159.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishikawa H, Evans ML. Plant Physiol. 1992;100:762–768. doi: 10.1104/pp.100.2.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demidchik V, Bowen HC, Maathuis FJM, Shabala SN, Tester MA, White PJ, Davies JM. Plant J. 2002;32:799–808. doi: 10.1046/j.1365-313x.2002.01467.x. [DOI] [PubMed] [Google Scholar]
- 34.Murashige T, Skoog S. Physiol Plant. 1962;15:473–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.